Effect of Probiotic Bacillus coagulans on Performance and Blood Metabolites of Dairy Cows with Subclinical Mastitis
Research Article
Effect of Probiotic Bacillus coagulans on Performance and Blood Metabolites of Dairy Cows with Subclinical Mastitis
Sudeb Saha1,2*, Jay Prakash Ray3, Apurbo Kumar Mondal4, Md. Irtija Ahsan5, Marina Debnath6, Dilruba Afrin7, Syed Sayeem Uddin Ahmed5, Jayanta Datta Gupta3, Nivedita Datta8, Hugo O. Toledo-Alvarado9, Haruki Kitazawa1
1Food and Feed Immunology Group, Laboratory of Animal Food Function, Graduate School of Agricultural Science, Tohoku University, Sendai 980-8576, Japan; 2Department of Dairy Science, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet-3100, Bangladesh; 3Square Pharmaceuticals PLC., Dhaka-1212, Bangladesh; 4Department of of Physiology and Pharmacology, Faculty of Veterinary Medicine and Animal Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur-1706, Bangladesh; 5Department of Epidemiology and Public Health, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet-3100, Bangladesh; 6Ministry of Public Administration, Government of the People’s Republic of Bangladesh, Dhaka-1000, Bangladesh; 7Department of Animal and Fish Biotechnology, Faculty of Biotechnology and Genetic Engineering, Sylhet Agricultural University, Sylhet-3100, Bangladesh; 8Associate Editor, International Journal of Dairy Technology, Wiley-Blackwell, UK; 9Department of Genetics and Biostatistics, Faculty of Veterinary Medicine and Zootechnics, National Autonomous University of Mexico, Mexico City, Mexico.
Abstract | Subclinical mastitis is almost asymptomatic in nature, with no visible signs detected on the udder and most prevalent disease in dairy cows incuring a huge economic loss in the dairy industry. The treatment and prevention of this disease employing antibiotics are not always effective and have adverse effects in public health. The present study aims to measure the effectiveness of application of a probiotic (Bacillus coagulans MTCC 25250) feed additive in subclinical mastitis cows on improvement of body condition, blood parameters, milk yields and milk composition apart from mitigation of the disease. A total of 20 subclinical mastitis cows were randomly assigned into four groups with various probiotic doses comprising T1 (15 g/d/cow), T2 (30 g/d/cow), T3 (45 g/d/cow) and control (without probiotic) in a trial of 60 days. All four groups received a total mixed diet. Cows’ body conditions and their bloods, milk yields and composition were investigated on the day of 0, 30 and 60. The results demonstrated that body conditions score, milk production, and compositions were improved in the T2 and T3 groups. Additionally, T2 and T3 groups showed a lower somatic cell and total bacterial counts in milk. The blood analyses showed that red blood cell, hemoglobin, lymphocyte, and monocyte counts were significantly higher for T1, T2 and T3 groups, however, white blood cell and neutrophil counts were decreased. The data suggest that application of a probiotic Bacillus coagulans MTCC 25250 as a feed supplement might be beneficial in milk yields and composition in subclinical mastitis cows. This potential probiotic strain would be useful for the mitigation of subclinical mastitis and improves the productivity of dairy cows.
Keywords | Probiotic, Bacillus coagulans MTCC 25250, Biochemical indices, Dairy industry, Blood parameters, Milk traits, Subclinical mastitis
Received | September 11, 2023; Accepted | December 30, 2023; Published | January 26, 2024
*Correspondence | Sudeb Saha, Food and Feed Immunology Group, Laboratory of Animal Animal Food Function, Graduate School of Agricultural Science, Tohoku University, Sendai 980-8576, Japan; Email: [email protected]
Citation | Saha S, Ray JP, Mondal AK, Ahsan MI, Debnath M, Afrin D, Ahmed SSU, Gupta JD, Datta N, Toledo-Alvarado HO, Kitazawa H (2024). Effect of probiotic Bacillus coagulans on performance and blood metabolites of dairy cows with subclinical mastitis.
Adv. Anim. Vet. Sci., 12(2):318-326.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.2.318.326
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Bovine mastitis is a widespread and critical problem in the dairy industry, negatively impacting the economic profitability and the well-being of dairy cattle (Cheng and Han, 2020; Kober et al., 2022). Mastitis is generally indicated as an inflammation of the mammary gland in either clinical or subclinical form. A devastating feature of mastitis is its recurrent nature (Jamali et al., 2018). The recurrent rate of mastitis is approximately 50%, causing poor milk quality, reducing milk yields, increasing the culling rate, and shrinking the animal’s longevity in the farm (Wente et al., 2020). Mastitis is caused by various microorganisms such as bacteria, virus, protozoa, and fungi (Dalanezi et al., 2020). These pathogens most frequently affect the mammary gland of animals during the dry off and transition period and in the immunosuppression conditions of animals, resulting in progression of inflammation during early lactation phase (Cobirka et al., 2000). This infection starts in the subclinical form and can progress into clinical mastitis, which continues to the early stage of lactation (Bradley and Green, 2000). A study by Rajala-Schultz et al. (2011) revealed that the subclinical mastitis of animals in the dry period were progressed in to clinical mastitis in the early lactation in 50% cases.
The conventional treatment for controlling mastitis is based on antibiotic therapy (Cheng et al., 2008). However, suceess of the mastitis treatment relies on the pathogen specific therapy, and any irrational use of antibiotics can establish the development of antimicrobial resistance, resulting in a major threat to the well-being of cattle and public health (Dalton, 2006; Kober et al., 2022). In recent years, some countries have been using the vaccine for the control of bovine mastitis. However, the efficacy of vaccination is a questionable as well because multietiological microorganisms are involved to cause mastitis (Tashakkori et al., 2020; Urakawa et al., 2022). However, the low frequency of cure rate and high probability of antimicrobial resistance, and composition dependent of the target of vaccine application resulted in for exploration of an innovative and sustainable approach for treating mastitis in animals.
Recent studies suggested employment of probiotics seems to be a good choice for the treatment/control of mastitis (Shkromada et al., 2022; Spaniol et al., 2015). Bacillus coagulans, a gram-positive, spore-forming, lactic acid-producing bacteria, has been proved as a probiotic (Özüsağlam, 2010). After the administration, of the Bacillus coagulans, it can survive in gastrointestinal conditions and exert health benefits on the host. As a result, Bacillus coagulans have been widely used as a probiotic feed in animal husbandry worldwide (Zhou et al., 2020). Literature showed that supplementation of Bacillus coagulans can improve growth performance and reduce diarrhea in piglets (Zhang et al., 2018). Additionally, Bacillus coagulans exhibits a growth enhancing effect in broiler and in aquatics. e.g. shrimp (Zhou et al., 2020). However, so far, very limited numer of researches have been conducted to evaluate the effect of Bacillus coagulans on dairy farming. Thus, the present study was undertaken to evaluate the effects of Bacillus coagulans MTCC 25250 on the body reserves, blood metabolites, yield, and milk composition of cows with subclinical mastitis.
The care and treatment of the experimental animals were performed by the guidelines and regulations of Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Bangladesh. The animal experiment protocol was approved by the ethics committee, Sylhet Agricultural University, Bangladesh (SAU/Ethical committee/AUP/22/19).
Farms and animals
A positive test using the California Mastitis Test (CMT; DeLaval, Tumba, Sweden) to detect subclinical mastitis was used as the criteria to select cows for their the mastitis history during the previous lactation period. A total of 73 milking cows were present in a dairy farm located in the Sylhet region of Bangladesh. Machine milking was performed twice a day (08:00 h and 17:00 h). All milking cows were tested for California Mastitis test. Twenty four cows were showed positive results in California Mastitis test. We considered twenty (20) multiparous (2-4 lactations) crossbred cows (Local × Holstein) for this study, and the rest four cows were excluded due to different crossbred cows. The cows in this experiment had an average parity of 2.9 and days in milk (DIM) were 135 days, respectively. All animals were randomly allocated to four (4) treatment groups, and 5 cows were included in each treatment. Four dietary treatments were used in this trial and the description of the diet is shown as:
- Control: Animals were fed a total mixed ration without probiotic supplementation;
- T1: Animals were fed a total mixed ration with 15 g/d/cow probiotic supplementation;
- T2: Animals were fed a total mixed ration with 30 g/d/cow probiotic supplementation;
- T3: Animals were fed a total mixed ration with 45 g/d/cow probiotic supplementation. Probiotics (Bacilllus coagulans MTCC 25250 6.0 × 109 cfu/g) was supplied by the Square Pharmaceuticals, PLC., Bangladesh. The cows were fed adlibitum access to feed and water at 7.30 and 16.00 hours daily. Total mixed rations were prepared by mixing of concentrate mixture (38%), green grass (20.5%), and rice straw (41.5%). Diets were prepared according to the NRC recommendations (NRC, 2001). The cows were housed in a tie-stall barn and remained in an identical manner through out the entire experimental period.
Sample collection and laboratory analysis
The total study period was 67 days with 7 days of the adjustment period. The following data were recorded, and sample collection was performed three times (start, 0 day; mid, 30 day; and end, 60 day) during the entire experiment period.
- Individual daily milk yields (MY, kg/d).
- Milk samples (100 ml per cow) were collected from individual cows at the evening milking and immediately transferred to the laboratory of Dairy Science, Sylhet Agricultural University, Bangladesh, and refrigerated at 4o C without preservative.
- Live body weight (BW), were measured using digital cattle weighing scale (Model: NVK-SCS-A12, NVK Electric Weighing Scale, China).
- Body Condition score (BCS), were measured by an experienced operator on the same day of milk sample collection based on the method described by Edmonson et al. (1989), using a 1 to 5 scale with 0.25 increments.
Detection of subclinical mastitis using California mastitis test
Milk samples were tested with the California Mastitis Test for showing the presence of subclinical mastitis, using the techniques described by Dingwell et al. (2003). This test was performed twice, at the start (0 d) and end (60 d) points.
Milk quality parameters
Milk samples were analysed to investigate the composition (fat, protein, lactose, and total solids) using an ultrasonic milk analyzer (MT-25, Wincom company Ltd, China). The somatic cell count (SCC) was determined using Eko-milk Scan (Somatic Cell Analyzer, Bulgaria) and the data were transformed into the logarithmic somatic cell score (SCS), according to Ali and Shook (1980) method.
Microbial analysis of milk samples
The standard plate count (SPC) and Coliform counts were determined in the collected milk samples, on the same test day of milk quality traits using the method of American Public Health Association (Saha and Ara, 2012), The plate count agar for SPC and EMB agar for the Coliform count were used and the methods described by Saha et al. (2022) were utilized for this microbial study.
Blood collection and analysis
Blood samples (50 ml) were collected at the start (0 days) and end (60 days) date from the jugular vein of cows into tubes containing sodium heparin. To determine blood parameters, blood samples were quickly shifted to the laboratory of Physiology, Sylhet Agricultural University, Bangladesh. Red blood cells (RBC) and White blood cells (WBC) were counted using Neubauer haemocytometer method. The Microhaematocrit technique were used to determine the packed cell volume according to Islam et al. (2014). Differential leukocyte test was performed using thin blood films stained with Giemsa stain by counting 100 white cells from each slide, and the relative abundance in percent of each white cell type was calculated.
Statistical analysis
The somatic cell score was calculated as SCS= (log)2 (SCC⁄100000) + 3. The statistical analyses were conducted using R software (R Core Team, 2016). Data were analyzed using a linear mixed model using the following equation:
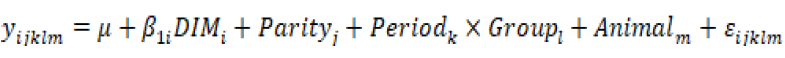
Where yijkl is the response trait (BW, BCS, MY, Fat, Protein, Lactose, Total solids, Fat: Protein, SCC, SCS, SPC, Coliform, WBC, RBC, Hb, PCV, Neutrophil, Eosinophil, Lympocyte, Monocyte); μ is the general mean; β1i is the partial regression coefficient on DIMi; Parityi is the effect of parity number j(j= 2,3,4); (Period)k× (Group)l is the effect of the interaction between the Period k(k=start, middle, end) and the Group j(j=C, T1, T2, T3); Animalm is the random effect of the animal; and εijklm~NID(0,σe2) is the residual. An analysis of variance of the linear mixed model was performed, and then multiple comparisons of means were made using a Tukey test. In addition, the estimated marginal means of the periods and groups were calculated, and their 95% confidence intervals were obtained for the data analysis.
Results
Table 1 present the descriptive statistics for body weight (BW), body condition score (BCS), blood parameters, yields, traits, and microbial parameters of milk produced from treatment group cows during the experimental period. On average, the BW and BCS of treatment group animals were 420 kg and 3.17, respectively. The average milk yields was 9.6 kg/d, containing 4.18% fat, 3.57% protein, 4.97% lactose, and 13.0% total solids. The mean value of SCS was 5.06. The average amount of standard plate count (SPC) and coliform counts were 94.03 × 103 CFU/ml and 30.17 CFU/ml, respectively. Results from blood samples indicated that the mean value of White blood cell (WBC), Red blood cell (RBC), and hemoglobin (Hb) were 161.8 × 109/l, 6.26× 1012/l, and 11.26 g/100 ml, respectively. The observed packed cell volume (PCV), neutrophil, eosinophil, lymphocyte, and monocyte were 23%, 35.98%, 0%, 33.39%, and 4.29%, respectively.
Table 1: Descriptive statistics for body weight (BW), body condition score (BCS), blood parameters, yield, traits, and microbial parameters of milk.
|
Traits1 |
n |
Mean |
SD |
Median |
Minimum |
Maximum |
|
BW, Kg |
60 |
420 |
71.75 |
438 |
260 |
521 |
|
BCS |
60 |
3.17 |
0.21 |
3.25 |
2.75 |
3.75 |
|
MY, Kg |
60 |
9.57 |
2.11 |
9.45 |
5.6 |
15.5 |
|
Fat, % |
60 |
4.18 |
0.47 |
4.12 |
3.47 |
5.51 |
|
Protein, % |
60 |
3.57 |
0.22 |
3.58 |
3.03 |
4.08 |
|
Lactose, % |
60 |
4.97 |
0.22 |
4.96 |
4.51 |
5.59 |
|
Total solids, % |
60 |
13 |
0.37 |
13 |
12.23 |
14.21 |
|
Fat:Protein |
60 |
1.17 |
0.14 |
1.19 |
0.91 |
1.46 |
|
SCC thous./ml |
60 |
442.4 |
137.1 |
473 |
78 |
661 |
|
SCS |
60 |
5.05 |
0.58 |
5.24 |
2.64 |
5.72 |
|
SPC, CFU/ml × 103 |
60 |
94.03 |
165.6 |
93.4 |
66.7 |
123.1 |
|
Coliform, CFU/ml |
60 |
30.17 |
10.7 |
28.5 |
12 |
69 |
|
WBC, 109/L |
60 |
161.76 |
28.28 |
156.7 |
121.6 |
231.9 |
|
RBC, 1012/L |
60 |
6.26 |
0.73 |
6.18 |
5.11 |
7.69 |
|
Hb, g/100ML |
60 |
11.26 |
1.67 |
11.75 |
6.75 |
13.4 |
|
PCV, % |
60 |
23 |
4.0 |
23 |
17 |
40 |
|
Neutrophil, % |
60 |
35.98 |
11.19 |
36.17 |
18.75 |
57.31 |
|
Eosonophil, % |
60 |
0 |
0 |
0 |
0 |
0 |
|
Lympocyte, % |
60 |
33.39 |
8.15 |
33.33 |
17.67 |
47.29 |
|
Monocyte, % |
60 |
4.29 |
0.82 |
4 |
3.23 |
6.21 |
1BW, Body weight; BCS, Body condition score; MY, Milk yield; WBC, White blood cell; RBC, Red blood cell; Hb, Hemoglobin; PCV, Packed cell volume; SPC, Standard plate count; SCC, Somatic cell count; SCS, Somatic cell score [SCS = 3 + log2 (SCC/100,000)].
The sources of variation in the statistical model are presented in Table 2. Days in milk (DIM) and parity exerted a smaller effect than the tested period, group, and period × group. DIM affected the milk-related traits (fat, fat: protein, SCS, and SPC), while no effects were found on blood parameters except RBC. Similarly, parity effect was significant for milk-related traits (fat, fat: protein, SCS, Total solids, and SPC), whereas a little effect observed on blood parameters (RBC, WBC, and hemoglobin). The variation included period, group, and period × group affected most of the studied traits are displayed in Table 2.
Effect of probiotic feeding on body and milk traits
The addition of Bacillus coagulans showed no effect on BW (Figure 1). On the other hand, the groups had received probiotic showed apparent beneficial effect on BCS and it is more pronounced for T2 and T3 groups. There was a trend towards increasing for the T2 group (p < 0.05) after 30 days of supplement and was better prominent after 60 days. The T3 group had little effect on BCS but did not significant different from the control and other treatment groups.
Table 2: Results from ANOVA (F-value and significance) for body weight (BW), body condition score (BCS), blood parameters, yield, traits, and microbial parameters of milk.
|
Traits1 |
DIM2 |
Parity |
Period |
Group |
Period × Group |
|
BW, Kg |
0.20 |
4.04 * |
0.00 |
0.04 |
0.02 |
|
BCS |
1.36 |
2.50 |
0.46 |
3.98 * |
4.57 ** |
|
MY, Kg |
9.48 ** |
5.44 ** |
25.83*** |
2.78 |
0.95 |
|
Fat, % |
5.25 * |
12.66*** |
3.94 * |
1.21 |
1.72 |
|
Protein, % |
0.00 |
2.09 |
2.27 |
12.04*** |
2.71 * |
|
Lactose, % |
006 |
0.50 |
4.66 * |
1.28 |
2.48 * |
|
Total solids, % |
1.23 |
2.75 † |
1.46 |
1.81 |
1.76 |
|
Fat:Protein |
3.06 † |
7.78 ** |
5.57 ** |
1.84 |
0.61 |
|
SCC |
3.01 † |
0.89 |
41.21*** |
20.89*** |
6.22 *** |
|
SCS |
5.11† |
1.72 |
29.9*** |
12.77*** |
6.48*** |
|
SPC, CFU/ml × 103 |
15.07*** |
14.99*** |
95.34*** |
40.39*** |
18.57*** |
|
Coliform, CFU/ml |
0.16 |
1.36 |
14.67*** |
1.16 |
2.44 * |
|
WBC, 109/L |
0.4 |
5.83 ** |
2.58 † |
18.61*** |
6.18 *** |
|
RBC, 1012/L |
8.65 ** |
10.89*** |
22.20*** |
19.51*** |
6.04 *** |
|
Hb, g/100ML |
0.35 |
4.88 * |
4.29 * |
11.79*** |
3.51 ** |
|
PCV, % |
1.07 |
1.45 |
0.15 |
2.26 † |
0.78 |
|
Neutrophil, % |
0.09 |
0.83 |
13.63*** |
12.81*** |
2.67 * |
|
Eosonophil, % |
1.60 |
1.93 |
2.56 † |
2.30 † |
2.56 * |
|
Lympocyte, % |
0.27 |
2.88 † |
2.02 |
28.16*** |
8.18 *** |
|
Monocyte, % |
2.82 |
1.02 |
21.93*** |
8.65 *** |
3.78 ** |
1BW, Body weight; BCS, Body condition score; MY, Milk yield; WBC, White blood cell; RBC, Red blood cell; Hb, Hemoglobin; PCV, Packed cell volume; SPC, Standard plate count. 2DIM: Days in milk. Significant codes: †= 0.05; *= 0.01; **= 0.001; ***= 0; SCC, Somatic cell count; SCS, Somatic cell score [SCS = 3 + log2 (SCC/100,000)].
As expected, milk yield was decreased over the experimental period due to the advancement of lactation, whereas the decreasing rate was lower for the T3 group than other groups and indicated a significant difference (p < 0.05) after 60 days of supplementation (Figure 1). Fat percentage in milk did not differ among treatment groups, but protein percentage in milk was higher for T2 and T3 groups at the end of 60 days of supplementation. The supplementation of probiotic did not influence the other milk components (lactose, total solids, and fat: protein). Somatic cells are considered one of the major defense components of mammary gland found in milk. Our findings showed that supplementation of probiotic reduced the SCC on day 30 of this study (p<0.05) and showed more pronounced reduction on day 60 (Figure 1). Regarding microbiological characteristics, probiotic supplementations decreased the total bacterial count in milk compared to the control (Figure 2). There was a decrease of bacterial count observed after 30 days of usage and more noticeable after 60 days of probitic usage.
Effect of probiotic feeding on blood metbolites
The results of blood metabolites are presented in Figure 3. Our study reveals a significant improvement of RBC and Hb in blood by adding probiotics in the diet. There was a tendency to increase RBC and Hb after 30 days of spplementation, as wll as the increasing trend was observed after 60 days of the study. However, the probiotic suplement groups showed decrease in WBC counts after 60 days of the trial. In the context of differential WBC count parameters (neutrophil, lymphocyte, and monocyte), a significant decrease in neutrophil count was observed after 60 days of supplementation for all probiotic-treated groups, whereas lymphocyte and monocyte were increased significantly in probiotic treated groups, which highlights that probiotic supplement improves the immune system. Thus, probiotic supplement in the subclinical mastitis of cow diet can be considered as a critical component for mitigating the mastitis.
Discussion
Dairy cattle health, milk yields, and quality are the significant factors for sustainable and profitable dairy farming. During the last two decades, several studies have focused on improving the cattle health, milk quality, and disease control by adding probiotics to the dairy ration (Lambo et al., 2021). However, studies related with the efficacy of probiotic against subclinical mastitis in cow are scarce. In the present study showed probiotic supplementation particularly use of Bacillus coagulans had a positive effect on the body condition score and similar to our study Fredebeul-Krein (2022) found that supplementation of some Bacillus spp. can improve the body condition, and assist in reducing the clinical mastitis in dairy cows. In addition, some researchers have observed improved body condition of dairy cows that have received Bacillus spp. as a single or multispecies probiotic (Choonkham et al., 2021; Merati and Tawhidi, 2022). Dietary probiotic supplement may help improve animal health status by improving nutrient digestion and absorption through the gastrointestinal tract (Mahesh et al., 2021).
The probiotic supplement has notable effects on daily milk yields and composition (Merati and Tawhidi, 2022). In the current study, with the advancement of lactation, the reduction of daily milk yields was as expected, but reduced the decreased rate for T3 probiotic supplement group as compared to the control. Souza et al. (2017) corroborated a positive response to milk yields treated with Bacillus subtilis spores. In order to illucidate this beneficial effect of probiotics on milk production, Spaniol et al. (2015) reported that probiotics may influence milk yields by improving the digestion, preventing ruminal acidosis, boosting the immune system and reducing the somatic cell count. Therefore, the supplementation with probiotics similarly influencing the protein content of milk from T2 and T3 group cows after 60 days of the study. On the other hand, the probiotic supplementation did not influence the fat and lactose percentage in milk. Like our study, Souza et al. (2017) reported that B. subtilis spores supplementation affected the protein metabolism in the rumen resulted an increased protein content in milk, whereas fat and lactose contents were not altered. Several literatures suggest that ruminal ammonia accumulation increased by the supplementation of B. subtilis in the diet which may contribute to the increase of protein content in milk (Qiao et al., 2010; Peng et al., 2012).
Generally, milk SCC (SCS) was increased in subclinical mastitis cows. This higher SCC not only detrimental to udder but also produces severe affects in the quality of raw milk which are prepared with the high SCC raw milk by enhanced protease and lipase enzyme concentrations, which are highly heat resistant and had a detrimental effect in the processing of milk and other milk products (Barbano et al., 2006). Milk SCC (SCS) was decreased in the current study by probiotic supplementation which indicating a beneficial effect. Sun et al. (2013) reported that supplementation of Bacillus subtili in natto reduced SCC in milk, but a crystal clear mechanism remained unclear, and their observation was supported by our findings.
The present study showed that the feeding of probiotic decreased the total bacterial count in milk, but coliform counts were comparable. There is no sufficient evidence in the literature on Bacillus spp. supplementation lowering the bacterial counts in milk in in subclinical studies. In the current investigation, a possible explanation of reduction numer of bacterial count in milk withthe probiotic suplement could be include the inhibitory effects of probiotics on the growth of pathogenic bacteria. The probiotics have exerted the inhibitory effects by the production of acetic, lactic and other acids, bacteriocin, nisin and other antimicrobial compounds for destroying pathogenic and spoilage bacteria present in raw milk.
In general, the health status of the animals could be reflected by blood parameters. The supplementation of probiotic may have the capacity to act on the blood parameters of animal (Choonkham et al., 2021). In this study, probiotic supplementation showed the increament of Hb concentration and RBC count, similar results were obtained by Kabir et al. (2022) and Ghazanfar et al. (2015) in fattening cattle and dairy heifers fed probiotic-supplemented diet, respectively. The probable reasons of elevated Hb concentration and RBC count in blood is that supplementation of probiotics can increase the absorption of iron salt and vitamins B from the small intestine which exerted a positive effect in the blood cells formation process (Ghazanfar et al., 2015). On the otherhand, control group recorded a higher leukocyte (WBC) count compared to probiotic supplement groups, however the presence of normal range of WBC in the blood reflecting absence of negative effects of immunity by WBC which also ensuring providing the regular immunity by WBC. Sarvesha et al. (2017) reported that circulating leukocytes content were increased in the blood and its value reached above the reference value in cows with clinical and subclinical mastitis. In this investigation, a decreased trend for the neutrophils counts was observed for probiotic supplementation groups. Neutrophils are the first cells to act as a defense against any inflammation in the body, and the high neutrophil count is an indication of inflammation. A decrease in blood neutrophils in probiotic supplemented group may be indicated by the diminished mastitis inflammation in the animals which enhancing the beneficiary list. Additionally, a probiotic supplementation causes an increased in the lymphocyte and monocyte counts in blood, which might indicate gut microbiota stimulating intestinal immune system responses. Our findings corroborated with Mousa et al. (2019) results, who reported that supplement of Bacillus spp. to lamb significantly improved leukocytes and monocytes counts in the blood. Elevated lymphocyte and monocyte counts can play an essential role by improving body immune system with eventual destroying invading disease-producing agents.
Conclusions
In conclusion, our study reveals that addition of a probiotic (Bacillus coagulans MTCC 25250) in the diet of dairy cows had positive effects to mitigate subclinical mastitis in cows, influencing body condition score, daily milk yields, protein percentage and decrease the SCC and bacterial count in milk by the adding of 30 and 45 g of probiotic per day per cow. Moreover, blood parameters such as RBC, Hb, lymphocyte, and monocyte contents were improved in cows fed probiotics causing changes in local and systemic immunity for alleviating subclinical mastitis. The present study demonestrated that the probiotic (Bacillus coagulans MTCC 25250) supplementation to the diet can be a new alternative prophylaxis for control of subclinical mastitis in cow. Further studies with larger sample size and an establisment of mechanistic approach of probiotics will be important aspects in order to control subclinical mastitis precisely.
Acknowledgements
We are grateful to the Square Pharmaceuticals PLC., Bangladesh for providing the bacterial strain Bacillus coagulans MTCC 25250 (Trade Name: MastiAid Powder). Also, the authors are thankful to Hlamrasong Marma, Md. Nazmul Islam Bappy, Md. Mashfiq Hossain and Md. Khademul Islam for their technical assistance, data, and sample collection.
A special thanks to Md. Fokruddin Raji (farm owner) for his cooperation and support.
Novelty Statement
This is a novel study for evaluating the dietary supplement of probiotic Bacillus coagulans MTCC 25250 against subclinical mastitis in dairy cows. The research findings highlighted that application of Bacillus coagulans MTCC 25250 strain can be a new alternative to mitigate subclinical mastitis in dairy cows.
Author’s Contribution
Conceptualization: SS, JPR and HK. Methodology: SS, AKM, MIA. Software: SS, MD, DA. Validation: SS, JPR, JDG, HK. Formal analysis: HOTA, SS, JPR, SSUA. Investigation: JDG, HK. Resources: HK, SS. Data curation; SS, AKM. Writing original draft preparation: SS. Writing review and editing: SS, JPR, MIA, MD, SSUA, HK. Visualization: MD, AKM, DA. Supervision: HK, JDG, SS. Project administration: SS, HK. Funding acquisition: HK, JPR, SS. Scientific corrections and editing for English: ND. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the research grant (VetMed/003/2205) from the Square Pharmaceuticals PLC., Bangladesh to Sudeb Saha. This research was also supported by the JSPS Research Fellow (22F22080) to Haruki Kitazawa.
Data availability
Data may be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors have declared no conflict of interest.
References
Ali AKA, Shook GE (1980). An optimum transformation for somatic cell concentration in milk. J. Dairy Sci., 63: 487-490. https://doi.org/10.3168/jds.S0022-0302(80)82959-6
Barbano DM, Ma Y, Santos MVD (2006). Influence of raw milk quality on fluid milk shelf life. J. Dairy Sci., 89: E15-E19. https://doi.org/10.3168/jds.S0022-0302(06)72360-8
Bradley AJ, Green MJ (2000). A study of the incidence and significance of intramammary enterobacterial infections acquired during the dry period. J. Dairy Sci., 83: 1957-1965. https://doi.org/10.3168/jds.S0022-0302(00)75072-7
Cheng JB, Wang JQ, Bu DP, Liu GL, Zhang CG, Wei HY, Zhou LY, Wang, JZ (2008). Factors affecting the lactoferrin concentration in bovine milk. J. Dairy Sci., 91: 970-976. https://doi.org/10.3168/jds.2007-0689
Cheng WN, Han SG (2020). Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments. A review. Asian-Australas. J. Anim. Sci., 33: 1699-1713. https://doi.org/10.5713/ajas.20.0156
Choonkham W, Intanon M, Chewonarin T, Bernard JK, Suriyasathaporn W (2021). Effects of supplemental Bacillus subtilis, injectable vitamin E plus selenium, or both on health parameters during the transition period in dairy cows in a tropical environment. Trop. Anim. Health Prod., 53: 1-9. https://doi.org/10.1007/s11250-021-02741-z
Cobirka M, Tancin V, Slama P (2020). Epidemiology and classification of mastitis. Animals, 10: 2212. https://doi.org/10.3390/ani10122212
Dalanezi FM, Joaquim SF, Guimarães FF, Guerra ST, Lopes BC, Schmidt EMS, Cerri RLA, Langoni H (2020). Influence of pathogens causing clinical mastitis on reproductive variables of dairy cows. J. Dairy Sci., 103: 3648-3655. https://doi.org/10.3168/jds.2019-16841
Dalton JC (2006). Antibiotic residue prevention in milk and dairy beef. Western Dairy News, 6: W79-W80.
Dingwell RT, Leslie KE, Schukken YH, Sargeant JM, Timms LL (2003). Evaluation of the California mastitis test to detect an intramammary infection with a major pathogen in early lactation dairy cows. Can. Vet. J., 44: 413.
Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G (1989). A body condition scoring chart for Holstein dairy cows. J. Dairy Sci., 72: 68-78. https://doi.org/10.3168/jds.S0022-0302(89)79081-0
Fredebeul-Krein F, Schmenger A, Wente N, Zhang Y, Krömker V (2022). Factors associated with the severity of clinical mastitis. Pathogens, 11: 1089. https://doi.org/10.3390/pathogens11101089
Ghazanfar S, Anjum MI, Azim A, Ahmed I (2015). Effects of dietary supplementation of yeast (Saccharomyces cerevisiae) culture on growth performance, blood parameters, nutrient digestibility and fecal flora of dairy heifers. J. Anim. Plant Sci., 25.
Islam S, Howlader MMR, Saha S, Bhowmik N (2014). Hemato-biochemical changes of layer birds during egg production reared in Sylhet region of Bangladesh. Int. J. Agric. Innov. Res., 2: 491-494.
Jamali H, Barkema HW, Jacques M, Lavallée-Bourget EM, Malouin F, Saini V, Stryhn H, Dufour (2018). Invited review: Incidence, risk factors, and effects of clinical mastitis recurrence in dairy cows. J Dairy Sci., 101: 4729-4746. https://doi.org/10.3168/jds.2017-13730
Kabir ME, Alam MJ, Hossain MM, Ferdaushi Z (2022). Effect of feeding probiotic fermented rice straw-based total mixed ration on production, blood parameters and faecal microbiota of fattening cattle. J. Anim. Health Prod., 10: 190-197. https://doi.org/10.17582/journal.jahp/2022/10.2.190.197
Kander M (2004). Effect of Bifidobacterium sp. on the health state of piglets, determined on the basis of hematological and biochemical indices. Electron. J. Pol. Agric. Univ. Ser. Vet. Med., 7.
Kober AH, Saha S, Islam MA, Rajoka MSR, Fukuyama K, Aso H, Villena J, Kitazawa H (2022). Immunomodulatory effects of probiotics: A novel preventive approach for the control of bovine mastitis. Microorganisms, 10: 2255. https://doi.org/10.3390/microorganisms10112255
Lambo MT, Chang X, Liu D (2021). The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals, 11: 2805. https://doi.org/10.3390/ani11102805
Mahesh MS, Mohanta RK, Patra AK (2021). Probiotics in livestock and poultry nutrition and health. Adv. Probiot. Sustain. Food Med., pp. 149-179. https://doi.org/10.1007/978-981-15-6795-7_7
Merati Z, Towhidi A (2022). Effect of a Multispecies probiotics on productive and reproductive performance of Holstein cows. Iran. J. Appl. Anim. Sci., 12: 237-247.
Mousa S, Elsayed A, Marghani B, Ateya A (2019). Effects of supplementation of Bacillus spp. on blood metabolites, antioxidant status, and gene expression pattern of selective cytokines in growing Barki lambs. J. Adv. Vet. Anim. Res., 6: 333. https://doi.org/10.5455/javar.2019.f351
NRC (2001). Nutrient requirements of dairy cattle: National Academies Press, Washington DC, USA.
Özüsağlam MA (2010). Importance of Bacillus coagulans bacterium as probiotic in animal nutrition. Süleyman Demirel Üniversitesi Ziraat Fakültesi Dergisi, 5: 50-57.
Peng H, Wang JQ, Kang HY, Dong SH, Sun P, Bu DP, Zhou LY (2012). Effect of feeding Bacillus subtilis natto fermentation product on milk production and composition, blood metabolites and rumen fermentation in early lactation dairy cows. J. Anim. Physiol. Anim. Nutr., 96: 506-512. https://doi.org/10.1111/j.1439-0396.2011.01173.x
Qiao GH, Shan AS, Ma N, Ma QQ, Sun ZW (2010). Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J. Anim. Physiol. Anim. Nutr., 94: 429-436. https://doi.org/10.1111/j.1439-0396.2009.00926.x
R Core Team (2016). A Language and Environment for Statistical Computing. R Found Stat Comput 1: 409.
Rajala-Schultz PJ, Torres AH, DeGraves FJ (2011). Milk yield and somatic cell count during the following lactation after selective treatment of cows at dry-off. J Dairy Res., 78: 489-499. https://doi.org/10.1017/S0022029911000690
Saha S, Ara A (2012). Chemical and microbiological evaluation of pasteurized milk available in Sylhet city of Bangladesh. Agriculturists, 10: 104-108. https://doi.org/10.3329/agric.v10i2.13147
Saha S, Pory FS, Ahmed SSU, Khan MMH (2022). Physico-chemical and microbial properties of black Bengal goat milk. Int. J. Agric. Innov. Res., 11: 1-7.
Sarvesha K, Satyanarayana ML, Narayanaswamy HD, Rao S, Yathiraj S, Isloor S, Mukartal SY, Singh SV, Anuradha ME (2017). Haemato-biochemical profile and milk leukocyte count in subclinical and clinical mastitis affected crossbred cattle. J. Exp. Biol. Agric. Sci., 5: 1-6. https://doi.org/10.18006/2017.5(1).001.006
Shkromada O, Pikhtirova AV, Pecka-Kiełb, E, Skliar O, Musiienko Y (2022). Use of probiotic Bacillus megaterium NCH 55 for treatment of subclinical mastitis in cows–preliminary study. Mac. Vet. Rev., 45: 209-214. https://doi.org/10.2478/macvetrev-2022-0023
Souza VL, Lopes NM, Zacaroni OF, Silveira, VA, Pereira, RAN, Freitas JA, Almeida R, Salvati GGS, Pereira, MN (2017). Lactation performance and diet digestibility of dairy cows in response to the supplementation of Bacillus subtilis spores. Livest. Sci., 200: 35-39. https://doi.org/10.1016/j.livsci.2017.03.023
Spaniol JS, Oltramari CE, Locatelli M, Volpato A, Campigotto G, Stefani LM, Da Silva AS (2015). Influence of probiotic on somatic cell count in milk and immune system of dairy cows. Comp. Clin. Path., 24: 677-681. https://doi.org/10.1007/s00580-014-1966-y
Sun P, Wang JQ, Deng LF (2013). Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal, 7: 216-222. https://doi.org/10.1017/S1751731112001188
Tashakkori N, Khoramian B, Farhoodi Moghadam M, Heidarpour M, Mashayekhi K, Farzaneh N (2020). Evaluating the effectiveness of two bovine mastitis vaccines and their influences on oxidant and antioxidant capacities of milk. Trop. Anim. Health Prod., 52: 1493-1501. https://doi.org/10.1007/s11250-019-02156-x
Urakawa M, Zhuang T, Sato H, Takanashi S, Yoshimura K, Endo Y, Katsura T, Umino T, Tanaka K, Watanabe H, Kobayashi H, Takada N, Kozutsumi T, Kumagai H, Asano T, Sazawa K, Ashida N, Zhao G, Rose MT, Kitazawa H, Shirakawa H, Watanabe K, Nochi T, Nakamura, T, Aso H (2022). Prevention of mastitis in multiparous dairy cows with a previous history of mastitis by oral feeding with probiotic Bacillus subtilis. Anim. Sci. J., 93: e13764. https://doi.org/10.1111/asj.13764
Wente N, Grieger AS, Klocke D, Paduch JH, Zhang Y, Leimbach S, tho Seeth M, Mansion-De Vries EM, Mohr E, Krömker V (2020). Recurrent mastitis persistent or new infections? Vet. Microbiol., 244: 108682. https://doi.org/10.1016/j.vetmic.2020.108682
Zhang Y, Shi C, Wang C, Lu Z, Wang F, Feng J, Wang Y (2018). Effect of soybean meal fermented with Bacillus subtilis BS12 on growth performance and small intestinal immune status of piglets. Food Agric. Immunol., 29: 133-146. https://doi.org/10.1080/09540105.2017.1360258
Zhou Y, Zeng Z, Xu Y, Ying J, Wang B, Majeed M, Majeed S, Pande A, Li W (2020). Application of Bacillus coagulans in animal husbandry and its underlying mechanisms. Animals, 10: 454. https://doi.org/10.3390/ani10030454
To share on other social networks, click on any share button. What are these?





