Effect of Ginger Derived Phyto-Protease on Production Performance, Serum Biochemistry, Nutrient Digestibility, Gut Morphometry and Immunity of Broilers Fed High Level of Poultry By Product Meal-Based Diet
Effect of Ginger Derived Phyto-Protease on Production Performance, Serum Biochemistry, Nutrient Digestibility, Gut Morphometry and Immunity of Broilers Fed High Level of Poultry By Product Meal-Based Diet
Umair Ahmad1*, Asad Sultan1, Sarzamin Khan1 and Muhammad Tahir2
1Department of Poultry Science, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, Pakistan
2Department of Animal Nutrition, The University of Agriculture, Peshawar, Pakistan
ABSTRACT
An experiment was conducted to assess the potential of ginger-derived phyto-protease on production performance, gut health, immunity, and nutrient digestibility of broilers fed a high level of poultry by-product meal-based (PBM) diet. A total of 320-day-old broiler birds were assigned to four dietary treatments CON, N-CON, GPP1, and GPP2. CON a commercial corn-soybean meal-based diet as per ROSS-308 nutritional specifications. N-CON was a 6.5% PBM-based diet and to this group, a ginger-derived phyto-protease was added at two levels in treatments GPP1 (50 mg kg-1 feed) and GPP2 (100 mg kg-1 feed), respectively. Birds in group N-CON performed poorly for the performance traits like feed intake, weight gain and FCR. Phyto-protease addition at 100mg kg-1 feed exhibited significant effect on body weight, FCR, and carcass traits compared to the control. The highest body weight gain was recorded in birds fed GPP2, followed by birds on GPP1 and the lowest in N-CON fed birds. Ginger-derived phyto-protease supplementation significantly lowered serum cholesterol (TG and LDL) while HDL was raised in GPP2 compared to the N-CON group showing a positive effect on lipid profile. Protein digestibility and AME were improved in the GPP2 group compared to N-CON. Gut health was improved in terms of better integrity, villus height, crypt depth, and villus surface area by birds in the GPP2 group. These findings demonstrated that adverse effects associated with using a high level of locally available animal protein concentrates could be ameliorated by supplementing birds with a ginger phyto-protease enzyme.
Article Information
Received 31 October 2022
Revised 18 November 2022
Accepted 25 December 2022
Available online 14 February 2023
(early access)
Published 12 April 2024
Authors’ Contribution
UA planned and executed experimental work, collected samples, and performed lab work. AS planned the experiment, helped in manuscript preparation, and formulated ration. SK helped in the article writing. MT analyzed the data and helped in lab work.
Key words
Phyto-protease, Digestibility, Histo-morphology, PBM, Broilers
DOI: https://dx.doi.org/10.17582/journal.pjz/20221031071042
* Corresponding author: umairvet@gmail.com
0030-9923/2024/0003-1233 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The feed efficiency of poultry needs to be improved significantly because feed is the commodity with the highest cost about 70% of the total production (Deek et al., 2020). Poultry production at the commercial level is facing a big challenge regarding quality feed availability at stable prices on a regular basis. The poultry industry relies on soybean meal as the primary source of protein which fulfills up to 77% of its protein requirement (Ogunji, 2004). Many countries including Pakistan that imports full-fat soybean or meal face high price fluctuations and supply problems most of the time. According to USDA (2021) report, Pakistan imports 2.4 MMT of soybean costing 842 million US$ annually. Therefore, alternative indigenously available cheap protein sources will not only add versatility to feed ingredients for poultry but will also minimize soybean dependency to a certain extet (Shuaib et al., 2022). A locally manufactured poultry by-product meal (PBM) can potentially replace the plant protein source. PBM meal is produced by processing the inedible parts like viscera, feet, feathers, and heads of poultry carcass (Senkoylu et al., 2005) and is used as a protein source in the mono-gastric animal diet in many countries. Approximately 37% of the live weight of a broiler is considered slaughter house waste (Meeker and Hamilton, 2006) while non-edible parts account for approximately 28% of the live weight percentage of a broiler. In Pakistan, since there were 1407 million broilers in 2021 (Pakistan Economic Survey, 2021) with an average weight of 2kg and 28% non-edible parts they would produce 787.92 million kg of waste product annually which needs to be managed efficiently to ensure environmental safety and reduce pollution. The use of this waste product as PBM in poultry ration will not only minimize this waste burden but will also minimize the cost of soybean meal and will act as a partial replacement for soybean. The CP value of PBM ranges from 45-65% (Karapanagiotidis et al., 2019) but aside from its benefits, there are some barriers to the incorporation of poultry by-product meal in broilers’ diets which are its poor digestibility, quality of raw materials, processing conditions, variation in nutritive composition and inappropriate inclusion levels in poultry feed (Jafari et al., 2022).
Several researchers suggest different levels of PBM replacement with soybean meal, some suggest a 10% inclusion level however, Hassanabadi et al. (2008) reported a 5% inclusion level without adverse effects on broilers’ performance while Jafari et al. (2022) reported reduced feed efficiency and growth rate beyond 5% inclusion level. To overcome these inherent problems of poultry by-product meal and make it suitable for use in broilers’ diets several processing methods like fermentation or bio-procession, rendering, and enzymatic valorization can be effectively applied (Barnes et al., 2015; Lewis et al., 2019).
Enzymatic valorization with exogenous protease extracted from ginger might improve the feeding value of PBM by complementing the endogenous enzyme system, improving the breakdown of certain proteins, and enhancing digestibility (Classen et al., 1991). These inconsistent results about PBM inclusion level in broiler diets urge further studies to figure out the correct inclusion level without compromising broilers’ health. Therefore, this study was conducted to investigate the effect of soybean meal replacement by 6.5% PBM on broilers’ performance, nutrient digestibility, serum biochemistry and gut morphology of broilers supplemented with ginger derived phyto-protease enzyme at different concentrations.
MATERIALS AND METHODS
Experimental layout and bird’s husbandry
This study was approved by the ethical committee of the Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, Pakistan and was conducted in the poultry farm of the university. A total of 320-day-old broilers (ROSS-308) were purchased from a local hatchery and randomly distributed in four treatment groups in a completely randomized design. Each group had 4-replicates with 20 birds per replicate. Birds in all the treatment groups were presented with starter (0-21 days) and finisher (22-35 days) diets Table I as per
Table I. Experimental diets composition.
|
Ingredients |
Starter phase (day1-21) |
Finisher phase (day 22-35) |
||
|
CON |
N-CON |
CON |
N-CON |
|
|
Corn |
57.2 |
63.73 |
64.98 |
71.45 |
|
Soybean meal 44% |
35.4 |
26.1 |
28.1 |
18.7 |
|
Poultry by-product meal |
-- |
6.50 |
-- |
6.50 |
|
Animal fat |
3.57 |
1.37 |
3.58 |
1.43 |
|
DCP |
1.51 |
0.34 |
1.26 |
0.09 |
|
Limestone |
1.10 |
0.63 |
1.01 |
0.55 |
|
DL-methionine |
0.25 |
0.25 |
0.19 |
0.18 |
|
L-lysine HCL |
0.07 |
0.25 |
0.53 |
0.22 |
|
L-threonine |
0.02 |
0.06 |
0.01 |
0.05 |
|
NaCl |
0.40 |
0.40 |
0.40 |
0.40 |
|
Vitamin premix1 |
0.25 |
0.25 |
0.25 |
0.25 |
|
Mineral premix2 |
0.09 |
0.09 |
0.09 |
0.09 |
|
Calculated nutrients % |
||||
|
DM |
87.4 |
87.4 |
87.4 |
87.2 |
|
M.E (Kcal/kg) |
3100 |
3100 |
3180 |
3180 |
|
Crude protein |
21.5 |
21.5 |
18.5 |
18.5 |
|
True protein |
19.0 |
19.15 |
16.74 |
16.44 |
|
E.E |
4.67 |
4.44 |
6.07 |
4.69 |
|
C.F |
2.17 |
2.19 |
2.08 |
2.12 |
|
Ca |
0.87 |
0.87 |
0.76 |
0.76 |
|
Total P |
0.62 |
0.66 |
0.62 |
0.58 |
|
Avail. P |
0.51 |
0.44 |
0.38 |
0.38 |
|
Na |
0.22 |
0.21 |
0.18 |
0.21 |
|
Cl |
0.27 |
0.31 |
0.27 |
0.31 |
|
Choline |
1.85 |
2.05 |
1.74 |
1.93 |
|
Folate |
0.86 |
0.87 |
0.82 |
0.83 |
|
D LYS |
1.15 |
1.15 |
0.95 |
0.95 |
|
D MET |
0.57 |
0.55 |
0.48 |
0.45 |
|
D TSAA |
0.87 |
0.87 |
0.74 |
0.74 |
|
D THR |
0.77 |
0.77 |
0.65 |
0.65 |
|
D TRP |
0.25 |
0.22 |
0.21 |
0.18 |
|
D ARG |
1.37 |
1.34 |
1.18 |
1.12 |
|
D VAL |
1.07 |
1.02 |
0.92 |
0.88 |
11kg of premix provided Vitamin E 80 mg, vitamin D3 3,000 IU, A 15,000 IU, vitamin B1 3 mg, vitamin K3 3 mg, vitamin B2 8 mg, vitamin B3 60 mg, vitamin B5 15 mg, vitamin B12 0.02 mg, Choline chloride (60%) 700 mg, vitamin B6 4 mg, vitamin B9 2 mg, vitamin H 0.2 mg/Kg.
2Zn (as ZnSO4.H2O) 80 mg, Mn (MnSO4.H2O) 80 mg, Cu (CuSO4.H2O) 10 mg, Se 0.2 mg, Fe (FeSO4.H2O) 60 mg, I (KI) 1 mg.
CON, control; N-CON, 6.5% PBM-based diet; DCP, di calcium phosphate; DM, dry matter; ME, mobilizable energy; EE, ether extract; CF, crude fiber; LYS, lysine; MET, methionine; TSAA, total sulphure amino acid; THR, Threonine; TRP, TRP, tryptophan; ARG, arginine; VAL, valine
(ROSS-308) requirements using Brill Formulation® software. At different stages of rearing optimum environmental conditions of temperature, humidity, ventilation, and light were maintained as required. Ginger was purchased from the local market, washed, and sliced into small pieces. 200mL potassium phosphate buffer 100mM (pH 7.0) and 100g ginger were homogenized, then filtered through cheesecloth and centrifuged at 10,500x for 30 min. The supernatant was collected and used as a ginger protease (GP) with some modifications as described by Nafi et al. (2013). There were four dietary treatments (CON group; N-CON group, GPP1 group and GPP2 group). CON group was a normal corn-soybean meal diet, N-CON was a diet in which soybean meal was partially replaced with 6.5% PBM, GPP1 and GPP2 were the same 6.5%PBM (N-CON) based diets but added with ginger derived Phyto-protease enzyme at 50mg/kg and 100mg/kg.
Performance parameters
Feed intake was calculated by the formula as feed offered minus feed rejected. The final bird weight was subtracted from the initial weight to determine body weight gain. For each experimental unit, the feed conversion ratio (FCR) was calculated on weekly basis by dividing the feed consumed by the body weight of the birds.
To obtain dressing percentage carcass weight was divided by live weight (Badar et al., 2021). The relative weight of lymphoid organs (spleen, bursa, thymus) was calculated as organs weight as percent of BW= weight of an organ in g/ body weight in g × 100.
Three birds per replicate were randomly selected for blood collection from wing veins in EDTA tubes. To separate serum, the samples were centrifuged at 3000 RPM for 15 min and then stored at -20oC till further analysis. Serum was analyzed by using commercial kits through standard procedures and protocols after thawing for determination of total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), and serum triglycerides (Asghar et al., 2021).
Hemagglutination inhibition (HI)
In this trial, birds were vaccinated through drinking water against New Castle Disease Virus (NDV) on days 7th and 17th. On day 7th birds were vaccinated against infectious bronchitis (IB). To determine antibody titer against NDV and IB three birds per replicate were randomly slaughtered for blood collection on day 35. A commercially available laboratory kit for HI was used for the determination of NDV antibody titer as described by Asghar et al. (2021). Antibody titer against IB was determined through ELISA.
Intestinal histomorphology
To evaluate intestinal histomorphology, 3cm duodenal tissue samples were taken from the duodenum center of three birds per replicate on day 35th. The digesta was gently removed and the tissue was flushed with normal saline and then preserved in buffer formalin (10%) for 48 h. In the histopathology lab, the tissue samples were washed with tap water and then treated with an alcohol solution. Using cassettes, a properly cut tissue section was embedded in paraffin wax. Microtome was used to cut tissue sections into 4µm, then mounting those tissue sections on slides and staining with hematoxylin and eosin (H and E). Under a computer-aided light microscope using image analysis (Leica Camera AG, Solms, Germany) histological indices were analyzed as described by Laudadio et al. (2012).
Nutrients digestibility
Five birds per replicate were shifted to metabolic cages on day 36 for total fecal collection for the last four days till day 42. A weighed amount of feed was offered daily and feces were collected early the next morning for four days and weighed. A representative sample (20%) of feces was placed in plastic bags and stored at -20oC till further analysis. For the determination of ileal nutrient digestibility 0.2% Cr2O3 was used as an indigestible marker. The fecal samples within the pen were pooled and dried in an oven at 65oC. The samples were ground to pass through a 0.5mm sieve. Similarly, digesta from the ileum portion of the small intestine of all the birds within the cage were collected and then frozen immediately. After drying, the samples were ground to pass through a 0.5mm sieve. The samples of diet, excreta and ileal digesta were analyzed for proximate analysis as described by AOAC (2005), and for calcium and phosphorus analysis by AOAC (2000). The concentration of chromium was determined with a UV absorption spectrophotometer (Hitachi Z-5000 Automatic Absorption Spectrophotometer, Tokyo, Japan) using the method of Pan et al. (2016). Adiabatic bomb calorimeter (Par instrument USA) was used to determine the gross energy of feed and fecal samples and then AME and nutrient digestibility were calculated using the formula of Stefanello et al. (2020) shown below.
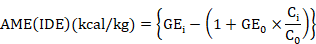
Where GEi is gross energy (kcal/kg) of diet; GEo is gross energy (kcal/kg) of excreta. Ci and Co are concentrations of marker in the diet and digesta or excreta.
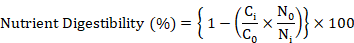
Where; Ci and Co are concentrations of marker in the diet and digesta or excreta (%), respectively; Ni and No are concentrations of nutrient in the diet and digesta or excreta (%), respectively.
Economics of the study (cost analysis)
Per chick cost was calculated for all the groups. The market rate of live birds per kg was used to calculate the mean gross return per chick.
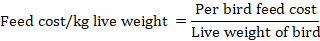
Statistical analysis
The collected data was analyzed by the standard procedure of (ANOVA) analysis of variance using a completely randomized design (CRD) as suggested by Steel and Torrie (1981) and the Tukey test was used for significance among means with a level of significance set at (P<0.05).
RESULTS
Growth performance
The growth performance results of the birds are shown in Table II. Feed intake was significantly (P>0.05) lower in the N-CON group in both starter (1-21 day) and finisher phases (21-35 day), while the feed consumption of the same N-CON feed supplemented with ginger-derived Phyto-protease enzyme in GPP1 and GPP2 groups was comparable with CON group. Ginger derived Phyto-protease enzyme supplementation significantly improved the body weight gain of broilers in both phases. Feed efficiency was improved (P<0.05) by ginger protease enzyme in GPP1 and GPP2 groups compared to CON and N-CON groups.
Carcass traits, lymphoid organs weight, and serum biochemistry
Results related to carcass traits, lymphoid organs weight, and serum biochemistry are shown in Table III. The carcass yield of broilers was significantly affected by ginger protease enzyme in GPP1 and GPP2 groups compared to N-CON. Carcass yield was highest in GPP2 and lowest in the N-CON group. Ginger derived phyto-protease enzyme significantly affected dressing percentage. The dressing percentage of N-CON was the lowest and GPP2 was the highest. The percent relative weight of lymphoid organs (spleen and bursa) was significantly affected by ginger protease. Maximum weight of the spleen and bursa was noticed in the GPP2 group and minimum in the N-CON group. No significant effect of ginger protease was observed on the thymus. Total cholesterol, LDL, and triglycerides were (P<0.05) lowered, and HDL levels were raised in ginger protease-supplemented groups compared to control and negative control groups, the maximum effect of ginger protease was observed in the GPP2 group. The lowest values of total cholesterol, LDL, and triglycerides were observed in ginger protease-supplemented groups and highest in the N-CON group. HDL level was improved and highest in the GPP2 group and lowest in the N-CON group.
Table II. Effect of different levels of a ginger derived phyto-protease enzyme on broiler bird’s performance fed on PBM based diet on day 21 and 35.
|
Parameters |
CON |
N-CON |
GPP1 |
GPP2 |
P value |
|
Starter phase |
|||||
|
Initial weight (g) |
39.7±0.54 |
39.2±0.55 |
39.0±0.49 |
40.6±0.58 |
0.192 |
|
BWG (g) |
704.2±0.62a |
679.7±1.05b |
713.2±0.62a |
719.7±1.75a |
0.001 |
|
Feed intake (g) |
1112±0.40a |
1076±1.24b |
1114±2.10a |
1121±2.25a |
0.003 |
|
FCR |
1.57±0.50a |
1.58±0.04a |
1.56±0.08ab |
1.55±0.75b |
0.001 |
|
Finisher phase |
|||||
|
BWG (g) |
1001±1.50ab |
955±2.30b |
1028±2.33a |
1042±1.87a |
0.009 |
|
Feed intake (g) |
2193±0.40a |
2116±2.74b |
2195±0.64a |
2204±0.85a |
0.002 |
|
FCR |
2.19±0.50a |
2.21±0.02a |
2.13±0.08b |
2.11±0.08b |
0.002 |
|
Overall period |
|||||
|
Final weight (g) |
1745±1.46b |
1674±1.93c |
1780±2.60ab |
1803±3.40a |
0.002 |
|
BWG (g) |
1705±1.46b |
1634±1.93c |
1741±2.60ab |
1762±3.40a |
0.001 |
|
Feed intake (g) |
3305±0.70a |
3192±3.61b |
3310±1.82a |
3326±1.82a |
0.008 |
|
FCR |
1.89±0.50a |
1.91±0.01a |
1.85±0.88b |
1.84±0.50b |
0.002 |
CON, Normal commercial ration; N-CON, 6.5% of PBM inclusion; GPP1, 6.5%PBM +50mgkg-1 phyto-protease; GPP2, 6.5% PBM+100mgkg-1 phyto-protease. Means within the same rows bearing different superscripts differ significantly (p ≤ 0.05), FI, Feed intake; BWG, body weight gain; FCR, Feed conversion ratio.
Table III. Effect of different levels of a ginger derived phyto-protease (GPP) enzyme on carcass traits and serum biochemistry of broiler birds fed on PBM based diets on day 35.
|
Parameters |
CON |
N-CON |
GPP1 |
GPP2 |
P-value |
|
Dressing percentage (%) |
62.3bc±0.20 |
61.2c±2.00 |
64.4ab±0.17 |
65.3a±0.09 |
0.049 |
|
Spleen (%) |
0.12b±0.88 |
0.10b±0.78 |
0.13ab±0.88 |
0.16a±0.50 |
0.001 |
|
Bursa (%) |
0.12ab±0.45 |
0.10b±0.01 |
0.12ab±0.45 |
0.15a±0.45 |
0.004 |
|
Thymus (%) |
0.36±0.08 |
0.36±0.01 |
0.35±0.78 |
0.35±0.64 |
0.297 |
|
Cholesterol (mg/dL) |
149.9ab±0.51 |
151.7a±0.71 |
137.8ab±0.61 |
134.7b±1.01 |
0.013 |
|
Triglycerides (mg/dL) |
46.5a±0.55 |
40.5ab±0.34 |
26.5c±0.34 |
31.0bc±0.25 |
0.001 |
|
HDL (mg/dL) |
63.8b±0.53 |
64.2b±0.72 |
71.6ab±0.29 |
78.5a±0.49 |
0.004 |
|
LDL (mg/dL) |
71.1a±0.23 |
70.9a±0.60 |
60.4ab±0.53 |
49.6b±0.23 |
0.008 |
For statistical details and details of treatments groups, see Table II. HDL, high density lipoprotein; LDL, low density lipoprotein.
Table IV. Effect of different levels of a ginger derived phyto-protease enzyme on immune response and intestine (duodenum) histomorphology of broilers fed on PBM based diets on day 35.
|
Parameters |
CON |
N-CON |
GPP1 |
GPP2 |
P-value |
|
ND (log2) |
4.22b±0.12 |
4.17b±0.14 |
5.15a±0.17 |
4.88a±0.08 |
0.005 |
|
IB (log2) |
4.95b±0.06 |
4.92b±0.13 |
4.97b±0.08 |
5.42a±0.08 |
0.008 |
|
Livability (%) |
90.0ab±0.00 |
85.0b±2.88 |
95.0ab±2.88 |
97.5a±2.50 |
0.014 |
|
Villi height (µm) |
730.9c±0.47 |
711.9d±0.60 |
897.1b±0.45 |
902.3a±0.68 |
0.002 |
|
Villi width (µm) |
50.8c±0.74 |
43.7d±0.59 |
55.4b±0.76 |
60.4a±1.22 |
0.001 |
|
Crypt depth (µm) |
82.5c±0.97 |
62.2d±0.79 |
90.2b±0.77 |
115.9a±0.87 |
0.002 |
|
VSA (mm) |
0.058c±0.71 |
0.048d±0.77 |
0.078b±0.74 |
0.085a±0.70 |
0.003 |
For statistical details and details of treatments groups, see Table II. ND, new castle disease; IB, infectious bronchitis; VSA, villus surface area.
Immunity and gut morphometry
The effect of soybean meal replacement with PBM at a 6.5% level added with or without ginger derived phyto-protease on broilers’ immune response, livability, and gut morphology is shown in Table IV. On day 35th the antibody titer against NDV was significant and highest in the GPP1 group while lowest in the N-CON group. The livability of broilers was significantly high in the GPP2 group compared to other treatment groups. The highest livability was observed in the GPP2 group and the lowest in the N-CON group. The antibody titer against infectious bronchitis was significantly affected by ginger protease supplementation. The highest titer was observed for the GPP2 group and the lowest in the N-CON group.
Gut morphometry of broilers was significantly affected and the GPP2 group observed significantly the highest villi length, width, and crypt depth compared to all other groups while the N-CON group showed the lowest villi length, width, and crypt depth. Villus surface area was significantly high in the GPP2 group compared to the N-CON group.
Nutrients digestibility
Results regarding nutrient digestibility and economics of broilers supplemented with ginger-protease enzyme are shown in Table V. Mean percent digestibility of dry matter, crude protein, crude fiber, calcium, and phosphorus improved significantly high in the GPP2 group and lowest in the N-CON group. The AME was significantly higher in the CON group followed by GPP2, and GPP1, and lowest in the N-CON group.
Economics
The results of economic parameters indicated that feed cost per chick was significantly low in the N-CON group but the gross return was significantly high in GPP2 and GPP1 groups compared to CON and N-CON groups. Despite the same feed cost GPP2 gross return and profit over feed cost were higher than the CON group.
Table V. Effect of different levels of a ginger derived phyto-protease enzyme on nutrients digestibility and economics of broiler birds fed on PBM based diets on day 42.
|
Parameters |
CON |
N-CON |
GPP1 |
GPP2 |
P-value |
|
DM (%) |
57.2b±0.12 |
57.5b±1.89 |
60.6ab±0.18 |
61.7a±0.01 |
0.012 |
|
CP (%) |
41.5c±0.16 |
41.5c±0.18 |
45.1b±0.07 |
46.1a±0.02 |
0.024 |
|
CF (%) |
18.6ab±0.22 |
18.4b±0.28 |
19.1ab±0.09 |
20.6a±0.19 |
0.045 |
|
Ca (%) |
40.0ab±0.64 |
39.4b±1.28 |
41.0ab±0.64 |
42.1a±0.11 |
0.049 |
|
P (%) |
38.7bc±0.26 |
37.5c±0.84 |
40.1ab±0.42 |
41.0a±0.39 |
0.002 |
|
AME (Kcal/Kg) |
2995a±0.64 |
2894b±4.20 |
2934ab±1.08 |
2943ab±0.85 |
0.035 |
|
Feed cost (PKR/Kg) |
70 |
60 |
66 |
70 |
N/A |
|
Feed cost/kg live weight |
132.5a±0.26 |
114.4c±0.47 |
122.7b±0.22 |
129.1ab±0.19 |
0.002 |
|
Gross return (PKR) |
349.ab±0.72 |
334.8b±0.99 |
356.0ab±0.68 |
360.6a±0.72 |
0.027 |
|
Profit over feed cost |
216.4±0.98 |
220.4±1.33 |
233.3±0.92 |
231.5±0.91 |
0.109 |
For statistical details and details of treatments groups, see Table II. DM, dry matter; CP, crude protein; CF, crude fiber; Ca, Calcium; P, phosphorus; AME, apparent metabolizable energy. Values are given in Pak-rupees (1US$=175).
DISCUSSION
This study was conducted to assess the effect of soybean meal replacement with poultry by-product meal at a 6.5% level of incorporation on broiler performance supplemented with or without ginger-protease enzyme. Feed intake, weight gain (WG), and FCR were improved with ginger protease supplementation in PBM-based diets. Depression in the growth performance of birds was obvious when fed PBM-based diets only, however with the supplementation of ginger-protease enzyme the growth performance improved significantly, this indicated that ginger-protease was responsible for complementing the endogenous secretion of proteolytic enzymes resulting in improved performance. The overall performance of birds was improved by enzyme supplementation which agrees with the previous findings of Cowieson et al. (2006), Mahmood et al. (2018), and Saaci et al. (2018) who noticed a positive correlation of aqueous ginger extract on body weight gain and feed intake of broilers. Mahmood et al. (2018) also reported improved feed intake with protease enzyme in PBM-based diets. Similarly, a positive effect of protease enzyme was noticed on carcass traits of broilers by Mahmood et al. (2018), while, Barazesh et al. (2013) reported that carcass characteristics were not affected by different concentrations of ginger opposing our observations. Similarly, broilers provided with protease in low-protein diets showed improvement in carcass yield (Ajayi, 2015). Enhanced utilization and deposition of protein might be responsible for improved carcass yield in phyto-protease-supplemented groups. Improvement in the percent weight of bursa and spleen was observed for ginger protease-supplemented groups (Raeesi et al., 2010) approving our observations. The per cent weight of the thymus was not affected by dietary ginger protease supplementation (Bondona et al., 2019) and these results agreed with our findings of a non-significant effect on thymus per cent weight. The non-significant effect of ginger protease on thymus weight was also confirmed by other researchers (Khushdil et al., 2012).
Serum cholesterol, triglycerides, and LDL level was significantly lowered while HDL level was improved in ginger protease-supplemented groups compared to CON and N-CON groups. Reduction in cholesterol and triglycerides were reported by feeding 0.2% ginger to broilers (Mohamed et al., 2012). The presence of HMG-COA (hydroxyl methyl glutaryl coenzyme A reductase) in ginger which is the rate-limiting step in cholesterol synthesis might be responsible for its hypolipidemic effect (Asghar et al., 2021). Cholesterol-lowering effects of the aqueous ginger extract were previously reported by Oleforuh et al. (2015). The antibody titer against Newcastle disease was significantly high among ginger derived phyto-protease supplemented groups on the 35th day. Deniz Azhir et al. (2012) also reported the immune-boosting effect of ginger on broilers’ immune systems. The increase in amino acid digestion by ginger protease might be responsible for the increase in antibody titer (Li et al., 2007) as dietary protein deficiency has long been known to impair immune functions and make humans and animals more susceptible to infectious diseases. The antioxidant effect of ginger and the presence of natural aromatic compounds like shogoals and gingerol might be another possible reason for the immune boosting effect of ginger protease (Khan et al., 2012).
Dry matter, crude protein, crude fiber, calcium, and phosphorus were significantly affected by ginger-protease enzyme in PBM-based diets. Our results were consistent with the observations of Kamel et al. (2015) and Duwa et al. (2020). However, Saaci et al. (2018) did not detect any significant effect of ginger extract on the DM and CP digestibility of broilers. This may be due to different types of feed, the duration of the experiment, and crude ginger extract. Digestibility of CP was improved by protease supplementation in broilers’ diet (Vieira et al., 2013). The AME was enhanced in ginger protease-supplemented groups which may be attributed to the extra-proteinaceous effect of protease (Cowieson and Roos, 2014) that aid in the digestibility of fats apart from proteins thus improving AME. The significant changes among treatment groups for nutrient digestibility may be attributed to ginger derived phyto-protease enzymes contributing positively to digestive functions by stimulating the endogenous enzyme system. Broiler health and performance are affected by the development of the small intestine which is an important digestive organ involved in nutrient absorption (Kawalilak et al., 2010). To evaluate the effects of nutrients on gastrointestinal physiology a common measurement tool is villus structure and anatomy. Moreover, crypt depth and villus height have been documented to show significant correlations regarding performance improvement. By increasing the crypt depth and absorptive surface area of the small intestine of broilers, ginger protease improved the digestive and absorptive capacity which resulted in faster growth rates (Prakash and Srinivasan, 2012). The reduction in villus length, width, crypt depth, and surface area may be due to poor absorption of nutrients in PBM-based diets, however, the gut morphometry was significantly affected and villi length, villi width, and crypt depth were significantly improved by ginger derived phyto-protease supplementation in a PBM-based diet. Our results agreed with the reports of Karangiya et al. (2016), Shewita and Taha (2018) and Asghar et al. (2021) that there were improvements in the villus height, width, and surface area of the birds’ duodenum. This increase in villi length and width causes an increase in the surface area of villi increasing the absorption of nutrients and resulting in improved growth and performance (Oladele et al., 2012). The electrolytes are produced through the excretory activity of crypt cells into the intestinal lumen ultimately boosting up digestive activities of the gut (Bowen, 2011).
CONCLUSION
The results of the current study clearly indicated that the negative effects of PBM on broilers’ growth performance can be successfully alleviated through the addition of ginger derived Phyto-protease. This significantly improved broilers’ performance, gut health, immunity, and nutrient digestibility in PBM-based diets. PBM may be used successfully as a partial replacement of soybean meal at 6.5% level of inclusion with the supplementation of ginger derived phyto-protease at (100 mg kg-1 feed) without any antagonistic effects on broilers health.
ACKNOWLEDGMENT
We acknowledge the staff of the Department of Poultry Science and Faculty of Animal Husbandry and Veterinary Sciences (FAHVS), The University of Agriculture Peshawar, Pakistan who provided technical and laboratory facilities. The research work is part of Ph.D degree program and as self-supported.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript and is self supported.
IRB approval
The study was approved by Board of Advanced Studies and Research (BASR), (NO.332PS/UAP).
Ethical statement
This study was approved by the animal welfare and care committee of the Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, Pakistan, and all the measures and tools were considered to minimize the pain and discomfort of birds during the conduction of this experiment.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Ajayi, H.I., 2015. Effect of protease supplementation on performance and carcass weights of broiler chickens fed low protein diets. Nigerian J. Agric. Fd. Environ., 11: 29-32.
AOAC, 2000. Official methods of analysis. Association of Official Analytical Chemists, Washington, DC. Methods 925.10, 65.17, 974.24, 992.16.
AOAC, 2005. Official methods of analysis of AOAC International. 18th ed. AOAC Int., Gaithersburg, MD. Method 935.14 and 992.24.
Asghar, M.U., Rahman, A., Hayata, Z., Rafique, M.K., Badard, I.H., Yar M.K. and Ijaz, M., 2021. Exploration of Zingiber officinale effects on growth performance, immunity and gut morphology in broilers. Braz. J. Biol., 83: 1678-4375. https://doi.org/10.1590/1519-6984.250296
Badar, I., Jaspal, M., Yar, M., Ijaz, M., Khalique, A., Zhang, L., Manzoor, A., Ali, S., Rahman, A. and Husnain, F., 2021. Effect of strain and slaughter age on production performance, meat quality and processing characteristics of broilers reared under tropical climatic conditions. Arch. Geflügelkd., 85: 1-17.
Barazesh, H., Pour, M.B., Salari, S. and Abadi, T.M., 2013. The effect of ginger powder on performance, carcass characteristics and blood parameters of broilers. Int. J. Adv. Biol. Biomed. Res., 1: 1645-1651.
Barnes, M.E., Brown, M.L. and Neiger, R., 2015. Comparative performance of two rainbow trout strains fed fermented soybean meal. Aquacult. Int., 23: 1227–1238. https://doi.org/10.1007/s10499-015-9879-6
Bondona, B., Joga, M., Deben, S., Bornalee. H. and Rafiqul, I., 2019. Effect of feeding garlic (Allium sativum) on haematological, serum biochemical profile and carcass characteristics in broiler chicken. Int. J. Curr. Microbiol. Appl. Sci., 8: 492-500. https://doi.org/10.20546/ijcmas.2019.810.054
Bowen, R., 2011. Villi, crypts and the life cycle of small intestinal enterocytes. Colorado State University, Fort Collins.
Classen, H.L., Graham, H., Inborr, J. and Bedford, M.R., 1991. Growing interest in feed enzymes to lead to new products. Feedstuffs, 4: 22–25.
Cowieson, A.J. and Roos, F.F., 2014. Bioefficacy of a mono-component protease in the diets of pigs and poultry: A meta-analysis of effect on ileal amino acid digestibility. J. appl. Anim. Nutr., 2: e13–e20. https://doi.org/10.1017/jan.2014.5
Cowieson, A.J., Singh, D.N. and Adeola, O., 2006. Prediction of ingredient quality and the effect of a combination of xylanase, amylase, protease and phytase in the diets of broiler chicks 2. Energy and nutrient utilisation. Br. Poult. Sci., 47: 490–500. https://doi.org/10.1080/00071660600830611
Deek, A.A.E., Wareth, A.A.A. and Osman, M.., 2020. Alternative feed ingredients in the finisher diets for sustainable broiler production. Sci. Rep., 10: 17743. https://doi.org/10.1038/s41598-020-74950-9
Deniz, A., Afshin, Z. and Ali, K., 2012. Effect of ginger powder rhizome on humoral immunity of broiler chickens. Eur. J. exp. Biol., 2: 2090-2092.
Duwa, H., Amaza, I.B., Dikko, M.I., Raymond, J.B. and Paullyne, U.O., 2020. Effect of ginger (Zingiber officinale) on the growth performance and nutrient digestibility of finisher broiler chickens in semi-arid zone of Nigeria. Niger. J. Anim. Sci., 22: 279-286.
Hassanabadi, A., Amanloo, H. and Zamanian, M., 2008. Effects of substitution of soybean meal with poultry by-product meal on broiler chicken’s performance. J. Anim. Vet. Adv., 7: 303–307.
Jafari, M., Ebrahimnezhad, Y., Janmohammadi, H., Nazeradl, K. and Nemati, M., 2022. Evaluation of protein and energy quality of poultry byproduct meal using poultry assays. Afr. J. agric. Res., 6: 1407–1412.
Kamel, N.F., Naela, M., Ragaa, El-Banna, R.A. and Mohameda, F.F., 2015. Effects of a monocomponent protease on performance parameters and protein digestibility in broiler chickens. Agric. Sci. Procedia, 6: 216–225. https://doi.org/10.1016/j.aaspro.2015.08.062
Karangiya, V.K., Savsani, H.H., Patil, S.S., Garg, D.D., Murthy, K.S., Ribadiya, N.K. and Vekariya, S.J., 2016. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World, 9: 245-250. https://doi.org/10.14202/vetworld.2016.245-250
Karapanagiotidis, I.T., Psofakis, P., Mente, E., Malandrakis, E. and Golomazou, E., 2019. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquacult. Nutr., 25: 3–14. https://doi.org/10.1111/anu.12824
Kawalilak, L., Franco, A.U. and Fasenko, G., 2010. Impaired intestinal villi growth in broiler chicks with unhealed navels. Poult. Sci., 89: 82-87. https://doi.org/10.3382/ps.2009-00284
Khan, R.U., Naz, S., Nikousefat, Z., Tufarelli, V., Javdani, M., Qureshi, M.S. and Laudadio, V., 2012. Potential applications of ginger (Zingiber officinale) in poultry diets. Worlds Poult. Sci. J., 68: 245-252. https://doi.org/10.1017/S004393391200030X
Khan, Z.U., 2021. The legal structure and role of livestock in Pakistan’s economy. Agriculture. 2: 17-44.
Khushdil, M., Chand, N., Khan, S., Qureshi, M.S. and Tanweer, A.J., 2012. Comparative effect of different schedules of administration of medicinal plants (Allium sativum, berberislycium, Eclipta alba and Mangiferaindica) infusion on the immunity and overall performance of broiler chicks. Sarhad J. Agric., 28: 319-326.
Laudadio, V., Passantino, L. and Perillo, A., 2012. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci., 91: 265-270. https://doi.org/10.3382/ps.2011-01675
Lewis, M.J., Francis, D.S., Blyth, D., Moyano, F.J., Smullen, R.P. and Turchini, G.M., 2019. A comparison of in-vivo and in-vitro methods for assessing the digestibility of poultry by-product meals using barramundi (Lates calcarifer); impacts of cooking temperature and raw material freshness. Aquaculture. 498: 187–200. https://doi.org/10.1016/j.aquaculture.2018.08.032
Li, P., Yin, Y.I., Li, D., Kim, S.W. and Wu, G., 2007. Amino acids and immune function. Br. J. Nutr., 98: 237-252. https://doi.org/10.1017/S000711450769936X
Mahmood, T., Mirza, M.A., Nawaz, H. and Shahid, M., 2018. Exogenous protease supplementation of poultry by-product meal-based diets for broilers: Effects on growth, carcass characteristics and nutrient digestibility. J. Anim. Physiol. Anim. Nutr., 102: 233-e241. https://doi.org/10.1111/jpn.12734
Meeker, D.L. and Hamilton, C.R., 2006. An overview of the rendering industry, national renderers association. In: Essential rendering: All about the animal by-products industry (ed. D.L. Meeker). National Renderers Association, Alexandria, VA, USA, pp. 1–16. https://doi.org/10.1002/047167849X.bio073.pub2
Mohamed, A.B., Al-rubaee, M.A.M. and Jalil, A.Q., 2012. Effect of ginger (Zingiber officinale) on performance and blood serum parameters of broiler. Int. J. Poult. Sci., 11: 143-146. https://doi.org/10.3923/ijps.2012.143.146
Nafi’, A., Foo, H.L., Jamilah, B. and Ghazali, H.M., 2013. Properties of proteolytic enzyme from ginger (Zingiber officinale Roscoe). Int. Fd. Res. J., 20: 363-368. https://www.researchgate.net/publication/286019913.
Ogunji, J.O., 2004. Alternative protein sources in diets for farmed tilapia. In: Nutrition abstracts and reviews: Series B: Livestock feeds and feeding. Wallingford, UK. No. 2004, pp. 10.
Oladele, O.A., Emikpe, B.O. and Bakare, H., 2012. Effects of dietary garlic (Allium sativum Linn.) supplementation on body weight and gut morphometry of commercial broilers. Int. J. Morphol., 30: 238-240. https://doi.org/10.4067/S0717-95022012000100042
Oleforuh-Okoleh, V.U., Ndofor-Foleng, H.M., Olorunleke, S.O. and Uguru, J.O., 2015. Evaluation of growth performance, hematological and serum biochemical response of broiler chickens to aqueous extract of ginger and garlic. J. agric. Sci., 7: 167–173. https://doi.org/10.5539/jas.v7n4p167
Pan, L., Zhao, P.F., Yang, Z.Y., Long, S.F., Wang, H.L., Tian, Q.Y., Xu, Y.T., X.u. X., Zhang, Z.H. and Piao, X.S., 2016. Effects of coated compound proteases on apparent total tract digestibility of nutrients and apparent ileal digestibility of amino acids for pigs. Asian Aust. J. Anim. Sci., 29: 1761-1767. https://doi.org/10.5713/ajas.16.0041
Prakash, U.N.S. and Srinivasan, K., 2012. Fat digestion and absorption in spice pretreated rats. J. Sci. Fd. Agric., 92: 503-510. https://doi.org/10.1002/jsfa.4597
Raeesi, M., Hoseini- Aliabad, S. A., Roofchaee, A., Zare-Shahneh, A. and Pirali, S., 2010. Effect of periodically use of garlic (Allium sativum) powder on performance and carcass characteristics in broiler chickens. World. Acad. Eng. Technol., 68: 1213- 1219.
Sa’aci, Z.A., Alabi, O.J., Brown, D. and Ng’ambi, J.W., 2018. Effect of aqueous ginger (Zingiber officinale) extract on growth performance nutrient digestibility and economy of feed conversion of broiler chickens. Anim. Nutr. Feed. Tech., 18: 225-231. https://doi.org/10.5958/0974-181X.2018.00021.5
Senkoylu, N., Samli, H.E., Akyurek, H., Agma, A. and Yasar, S., 2005. Performance and egg characteristics of laying hens fed diets incorporated with poultry by-product and feather meals. J. appl. Poult. Res., 14: 542–547. https://doi.org/10.1093/japr/14.3.542
Shewita, R.S. and Taha, A.E., 2018. Influence of dietary supplementation of ginger powder at different levels on growth performance, hematological profiles, slaughter traits and gut morphometry of broiler chickens. S. Afr. J. Anim. Sci., 48: 997-1008. https://doi.org/10.4314/sajas.v48i6.1
Shuaib, M., Hafeez, A., Chand, N. and Tahir, M., 2022. Effect of dietary inclusion of soybean hull on production performance and nutrient digestibility during peak egg production period with different phases in laying hens. Pakistan J. Zool., 55: 397-405. https://doi.org/10.17582/journal.pjz/20211105091115
Steel, R.G.D. and Torrie, J.H., 1981. Principles and procedures of statistics. McGraw-Hill Book Co. Inc, New York.
Stefanello, C., Rosa, D.P., Dalmoro, Y.K., Segatto, A.L. and Vieira, M.S., 2020. Protected blend of organic acids and essential oils improves growth performance, nutrient digestibility, and intestinal health of broiler chickens undergoing an intestinal challenge. Front. Vet. Sci., 6: 491. https://doi.org/10.3389/fvets.2019.00491
United States Department of Agriculture (USDA), 2021. Oilseeds and products annual. Report number., PK2021-0003.
Veldkamp, T. and Bosch, G., 2015. Insects: A protein rich feed ingredient in pig and poultry diets. Anim. Front., 5: 45-50.
Vieira, S.L., Angel, C.R., Miranda, D.J.A., Favero, A., Cruz, R.F.A. and Sorbara, J.O.B., 2013. Effects of a mono component protease on performance and protein utilization in 1-to 26 days of age Turkey poults. J. appl. Poult. Res., 22: 680–688. https://doi.org/10.3382/japr.2012-00558
To share on other social networks, click on any share button. What are these?










