Effect of Dietary Protein: Energy Ratios on Nutrient Digestibility in Labeo rohita Fingerlings
Effect of Dietary Protein: Energy Ratios on Nutrient Digestibility in Labeo rohita Fingerlings
Muhammad Javed Iqbal1,*, Muhammad Afzal1, Khalid Abbas1 and Muhammad Shahid2
1Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad 38040
2Department of Biochemistry, University of Agriculture, Faisalabad 38040
ABSTRACT
This study investigated the effects of dietary protein to energy (P/E) ratios on the nutrients and minerals digestibility of Labeo rohita fingerlings. Twelve experiment diets containing four protein levels (24, 26, 28, and 30 %) at three dietary energy levels (2400, 2700, and 3000 kcal/kg) with P/E ratios from 80.00 to 125.00 mg / kcal were evaluated. Each diet was randomly assigned to triplicate groups of seventeen fish averaging 6.5 ±0.6 g (mean ±SD) for 10 wk. The chromic oxide in the diets was added as an additional inert marker to determine the digestibility of nutrients. The results from present study were demonstrated that the dietary P/E ratio significantly affects digestibility of protein, lipid and gross energy. In addition, the interactions of protein and energy significantly (p < 0.05) affected the digestibility of crude proteins, lipids, gross energy however, no significant (p > 0.05) effect was observed in digestibility of dry matter and ash. The increasing dietary P / E ratio significantly increased the digestibility of Na, Ca, P, Cu, Mn, Zn, and Fe. The diet with P / E ratio of 86.67 mg / kcal showed higher digestibility of dry matter (69.38 ±1.06), crude protein (88.18 ±1.59), lipid (93.43 ±0.22) and gross energy (86.50 ±0.57) while lower digestibility was observed in D12 (dietary protein 30% and dietary energy 3000 kcal/kg) with P/E ratio 100.00 mg / kcal. In conclusion, P/E ratio of 86.67 mg digestible protein / kcal digestible energy shows the maximum digestibility of nutrients and minerals in Labeo rohita fingerlings.
Article Information
Received 04 May 2019
Revised 30 June 2020
Accepted 12 September 2020
Available online 06 January 2021
(early access)
Published 12 November 2021
Authors’ Contribution
MJI conducted the research, performed the experiments and wrote the manuscript. MA, KA and MS supervised the research.
Key words
P/E ratio, Labeo rohita, Nutrients, Rohu, Digestibility.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190504200510
* Corresponding author: [email protected]
0030-9923/2022/0001-0063 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
Introduction
Labeo rohita (Rohu) is the most important fish species commonly cultured in Asia, particularly in the Indian subcontinent (Khan et al., 2004) because of its high growth rate, graceful body, market value, quality meat and consumer preference. The estimated production of this fish species in subcontinent was 9, 45,233 metric tons (FAO, 2014). The advances in fish industry is mainly dependent on the availability of cheap feed ingredients in terms of quality and quantity. In fish feed, fish meal is a tremendous nutrients sources such as minerals, vitamins, essential amino acids, vital fatty acids (Zhou et al., 2004) and growth factors. However, its increasing demand has led to high marketing cost and supply constraints (Lim et al., 2011).
The protein of plants and animals are used for fish growth and development, reproductive activities, body repairing processes (Huo et al., 2014) but excessive levels of dietary protein increase nitrogen excretion, resulting in deterioration of the water quality of culture media which creates alarming situation for fish growth (Craig and Helfrich, 2009). The required protein levels in fish feed is a major factor for optimal growth of aquaculture species. Similarly, the proper energy level in the diet also play important role for sustaining fish life. The cheap sources of dietary energy such as carbohydrates and lipids may spare protein as energy consumption whereas inadequate energy levels in the diet may increase production cost of fish (Okarie et al., 2007). However, dietary energy with excessive amount can reduce feed utilization more lipid deposition in the body led to poor growth (NRC, 2011). Therefore, optimum ratio between dietary protein and non-protein energy sources is associated with higher growth (Ai et al., 2004). Thus, keep in mind, adequate P/E ratios in diet may enhance fish growth, its production lead to generating more revenue in the fish industry. The balanced protein energy ratio is also provided proper calories and amino acids for rapid growth, development and quality fish meat.
Optimization of P/E ratio may vary depending on fish age, different dietary protein sources, dietary formula, seasonal factors and experimental design (Okorie et al., 2007). In some fish species, P/E ratios have been reported 98.3 mg protein/kcal energy for Singhi, Heteropneustes fossilis (Khan et al., 2012); 92.25 mg protein/kcal energy for sturgeon, Acipenser persicus (Mohseni et al., 2013); 150.75 mg protein/kcal energy for parrot fish, Oplegnathus fasciatus (Kim et al., 2016); 97.35 mg protein/kcal energy for lemon fin barb hybrid Hypsibarbus wetmorei × Puntinus gonionotus larvae (Anizah et al., 2017).
The objective of this study is to: (i) investigate the combine effects of protein and energy on nutrients digestibility of Labeo rohita fingerlings (ii) determine optimal P/E ratio for nutrients digestibility in Labeo rohita fingerlings.
Materials and methods
Experimental design and diet
43 factorial arrangement was used to formulate twelve experimental diets having four protein levels (24, 26, 28, and 30 %) and three energy levels (2400, 2700 and 3000 kcal/kg) at each protein level with P/E ratios ranging from 80.00 to 125.00 mg /kcal in. Ingredient composition of experimental diets is given in Supplementary Table I.
All ingredients (dry) were ground (0.05 mm) using cereal grinding machine (FFC-45, JIMO, China). All weighed ingredients with 1% chromic oxide (inert marker) were mixed using electric mixer for 12-15 min. Fish and soybean oils were slowly added while mixing. A suitable dough of each diet was prepared by slowly adding 15% water. The dough was pelleted using lab Extruder (Model SYSLG 30-IV) for making floating pellets size (1-2 mm) and then dried in open air at room temperature for about 10-12 h to reduce the moisture contents (Lovell, 1989).
All diets were packed in plastic bag and stored (-20°C) until fed. The feces were carefully collected and dried in an oven before chemical analysis.
Fish and feeding conditions
L. rohita (Rohu) experimental fish were procured from Government Fish Seed Hatchery and allowed to acclimate for two weeks and fed with a commercial diet containing 30% protein. Fish averaging 6.5 ±0.06 g (mean ±SD) were randomly distributed into V shaped tanks each having 70 L water capacity. The fish were rearing for two weeks on basal diet. At the start of feeding experiment, 17 fish were stocked per tank of 36 tanks (12 treatment, 3 replicates). L. rohita fingerlings were fed once daily up to apparent satiation. After 2 h of feeding, uneaten diet was siphoned manually. The water temperature was kept 25.1-28.9°C, pH was 7.3-8.5 and dissolved oxygen 5.6-7.2 mg/L. The experiment was lasted for 13 weeks. The compressed air containing oxygen was supplied from air compressor to all the tanks. The fish were bathed with 5 g/L salt solution to kill pathogenic agent if any as described by Rowland and Ingram (1991).
Sampling and chemical analyses
Feed ingredients, diets, feces were homogenized using a mortar and pestle and analyzed following AOAC (1995). The proximate composition of experimental diet is given in Supplementary Table II. Dry matter was determined by convection heating at 105°C for 12-13 h. Micro Kjeldahl apparatus was used to determine crude protein (N×6.25). Lipid in the samples were extracted by Soxtec (HT2 1045) extraction method. Oxygen Bomb Calorimeter (Parr Instrument Company, Moline, United state America) was used to determine the gross energy. Ash was measured by help of muffle furnace and to ignite at 550-600°C for 4-5 h. The nutrient composition (%) of faces of treatment diets fed to L. rohita fingerlings is displayed in Supplementary Table III.
For the estimation of minerals, each sample from feed and feces was digested in a mixture of boiling nitric acid and per-chloric acid (2:1) following AOAC (1995). The minerals composition (%) of faces of practical diets to L. rohita fingerlings is given in Supplementary Table IV. After preparation of appropriate dilution, minerals contents such as calcium, magnesium, zinc, copper, manganese and iron were estimated by atomic absorption Spectrophotometer (Hitachi Polarized Atomic Absorption Spectrometer, Z-8200). The content of chromic oxide and phosphorus were coalorimetrically determined at 720 nm absorbance through Spectrophotometer (UV-VIS 2001) (Supplementary Table II). The sodium and potassium of feed and feces were estimated using Flame Photometer (Jenway PFP-7, UK). The phosphorus was analyzed by UV-VIS Spectrophotometer (U-2001, Hitachi) at 720 nm absorbance AOAC (1995).
Digestibility studies
The additional (1%) chromic oxide was added as an inert marker in the experimental diets to assume the amount of marker in feed and feces. After feeding session, fecal material was collected by adjusting the valves of each tank alternatively on daily basis. The fecal matter of each treatment was dried in the microwave oven and stored at -20°C until use for further chemical analysis. Total 5-6 (g) of fecal matter from each treatment group was collected. The contents of chromic oxide in the diets and feces were estimated using oxidation method with molybdate reagent (Divakaran et al., 2002) using UV-VIS 2001 spectrophotometer at 370 nm absorbance. The minerals digestibility i.e., sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), zinc (Zn), manganese (Mn), copper (Cu) of experimental diets was determined at the end of experiment using chromic oxide as inert marker.
Calculation of nutrient digestibility
The ADC (%) of nutrients and minerals of treatment diets was calculated according to following formula described in NRC (1993):
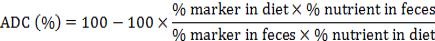
Table I.- ADC (%) of nutrients and minerals of practical diets fed to L. rohita fingerlings.
|
Protein (%) |
24 |
26 |
28 |
30 |
||||||||
|
Energy (kcal/kg) |
2400 |
2700 |
3000 |
2400 |
2700 |
3000 |
2400 |
2700 |
3000 |
2400 |
2700 |
3000 |
|
P/E (mg/kcal) |
100.00 |
88.89 |
80.00 |
108.33 |
96.30 |
86.67 |
113.67 |
103.70 |
93.33 |
125.00 |
111.1 |
100.00 |
|
Test Diets |
D1 |
D2 |
D3 |
D4 |
D5 |
D6 |
D7 |
D8 |
D9 |
D10 |
D11 |
D12 |
|
Dry matter |
66.74 ±2.13ab |
65.13 ±1.14abc |
64.34 ±2.44abc |
66.46 ±1.82abc |
66.57 ±0.35ab |
69.38 ±1.06 a |
59.72 ±0.63bc |
62.99 ±1.71abc |
63.01 ±2.13abc |
61.83 ±2.75abc |
61.77 ±2.06abc |
57.711 ±0.13c |
|
Crude protein |
85.58 ±0.89 ab |
81.79 ±0.69 b |
81.54 ±1.56 b |
85.09 ±0.77 ab |
86.03 ±0.64ab |
88.18 ±1.59a |
82.75 ±0.69ab |
84.32 ±1.59 b |
87.37 ±0.26ab |
86.99 ±1.68 ab |
85.84 ±0.36 ab |
83.21 ±0.26ab |
|
Lipid |
86.70 ±0.94 b |
89.43 ±1.86 ab |
90.86 ±0.46 ab |
87.31 ±0.74 b |
89.03 ±0.31ab |
93.43 ±0.22 a |
81.93 ±0.39c |
88.99 ±0.95 ab |
90.73 ±0.38ab |
80.52 ±1.21 c |
86.58 ±1.18 b |
87.82 ±0.38 b |
|
Gross energy |
79.60 ±1.33 bc |
74.48 ±0.97 cd |
72.02 ±1.96 d |
79.30 ±1.00 bc |
78.07 ±0.53bc |
86.50 ±0.57 a |
69.17 ±0.82d |
79.16 ±1.39 bc |
79.70 ±1.17bc |
81.10 ±1.47 ab |
81.08 ±1.01ab |
83.05 ±0.27ab |
|
Ash |
85.35 ±0.92abc |
85.60 ±0.40abc |
83.41 ±1.18bcd |
87.51 ±0.61 a |
83.34 ±0.47bcd |
81.82 ±0.66cd |
86.30 ±0.22ab |
84.75 ±0.66abcd |
81.95 ±0.91cd |
84.08 ±1.16abcd |
82.34 ±0.92cd |
80.87 ±0.13 d |
|
Sodium |
76.65 ±1.40ab |
79.84 ±0.74ab |
80.09 ±1.36ab |
72.85 ±1.85ab |
74.04 ±4.81ab |
87.38 ±2.07a |
66.03 ±1.20b |
70.04 ±7.58b |
71.25 ±4.24b |
80.19 ±1.64ab |
66.71 ±1.62b |
65.40 ±0.88b |
|
Potassium |
87.11 ±0.74ab |
88.01 ±1.85ab |
90.32 ±0.69ab |
85.98 ±3.14b |
88.48 ±0.42ab |
92.93 ±0.20a |
88.50 ±0.59ab |
89.17 ±1.07ab |
89.15 ±1.01ab |
90.26 ±0.76ab |
89.64 ±0.57ab |
89.56 ±0.09ab |
|
Phosphorus |
74.09 ±1.52c |
77.96 ±1.62bc |
81.52 ±1.06ab |
77.14 ±0.42bc |
78.31 ±1.62bc |
85.05 ±0.92a |
78.86 ±0.28bc |
78.58 ±1.03bc |
77.86 ±1.25bc |
82.33 ±1.22ab |
80.90 ±0.96ab |
79.04 ±0.42bc |
|
Calcium |
83.67 ±0.94ab |
80.17 ±0.82bc |
76.63 ±2.39c |
83.40 ±1.64ab |
85.67 ±1.75ab |
88.16 ±0.49a |
84.49 ±0.05ab |
85.51 ±0.56ab |
85.96 ±0.67ab |
80.84 ±1.15bc |
82.55 ±0.88abc |
84.21 ±0.24ab |
|
Magnesium |
91.06 ±0.59ab |
90.52 ±0.32ab |
90.30 ±0.67ab |
90.98 ±0.47ab |
91.11 ±0.12ab |
91.92 ±0.33a |
89.06 ±0.20bc |
90.02 ±0.47abc |
90.09 ±0.79abc |
89.79 ±0.72abc |
89.66 ±0.56abc |
87.52 ±0.46c |
|
Zinc |
74.39 ±1.41ab |
67.51 ±1.22bcd |
65.69 ±2.48cd |
69.83 ±1.27abcd |
71.33 ±0.06abcd |
76.61 ±0.60a |
63.30 ±0.95d |
72.15 ±1.54abc |
73.13 ±1.33abc |
65.32 ±3.38cd |
66.51 ±1.40bcd |
69.16 ±0.70abcd |
|
Copper |
80.48 ±2.28abc |
72.47 ±4.12bc |
83.24 ±1.77abc |
83.55 ±1.57abc |
91.15 ±3.79a |
87.21 ±1.25ab |
90.83 ±3.86a |
79.55 ±2.62abc |
67.4 9 ±0.52c |
80.83 ±0.45abc |
80.24 ±2.93abc |
73.57 ±8.76abc |
|
Manganese |
81.62 ±0.99a |
80.58 ±0.35a |
73.28 ±2.02b |
80.22 ±1.08a |
81.61 ±0.28a |
82.01 ±0.30a |
84.37 ±0.26a |
82.94 ±1.09a |
82.60 ±1.13a |
83.76 ±1.08a |
81.83 ±0.92a |
80.97 ±0.32a |
|
Iron |
81.94 ±1.17a |
84.87 ±0.40a |
85.20 ±0.71a |
78.94 ±1.21abc |
82.19 ±1.77a |
86.43 ±0.33a |
72.06 ±1.37bc |
71.13 ±2.23c |
70.77 ±3.75c |
77.64 ±2.55abc |
81.07 ±0.98ab |
82.83 ±1.61a |
Analysis of Variance (P value)
|
AN-OVA |
DM (%) |
CP (%) |
Lipid (%) |
Gross ener-gy (%) |
Ash (%) |
Ca (%) |
P (%) |
Na (%) |
K (%) |
Mg (%) |
Cu (%) |
Mn (%) |
Zn (%) |
Fe (%) |
|
Prot-ein |
< 0.000 |
< 0.008 |
< 0.000 |
< 0.000 |
< 0.005 |
< 0.000 |
< 0.011 |
< 0.001 |
> 0.608 |
< 0.000 |
< 0.013 |
< 0.00 |
< 0.00 |
< 0.00 |
|
Ene-rgy |
> 0.907 |
>0.706 |
< 0.000 |
< 0.003 |
< 0.000 |
> 0.739 |
< 0.006 |
> 0.326 |
< 0.023 |
> 0.584 |
> 0.075 |
< 0.00 |
< 0.04 |
< 0.02 |
|
Prot-ein × Ener-gy |
> 0.236 |
< 0.005 |
< 0.031 |
< 0.000 |
> 0.143 |
< 0.002 |
< 0.000 |
< 0.004 |
> 0.111 |
< 0.033 |
< 0.005 |
< 0.00 |
< 0.00 |
> 0.34 |
|
P/E |
> 0.124 |
< 0.004 |
< 0.000 |
< 0.000 |
< 0.004 |
< 0.000 |
< 0.001 |
< 0.004 |
> 0.083 |
> 0.172 |
< 0.002 |
< 0.00 |
< 0.00 |
< 0.00 |
|
P. Valu es |
||||||||||||||
|
24% prot-ein |
65.40ab |
82.97b |
88.99ab |
75.37b |
88.99ab |
80.15b |
77.85b |
78.85a |
88.47a |
90.62ab |
78.73b |
78.49b |
69.19ab |
84.0a |
|
26% prot-ein |
67.47a |
86.43a |
89.92a |
81.29 a |
89.92a |
85.74b |
80.16ab |
78.08a |
89.13a |
91.33a |
87.30a |
81.27a |
72.58a |
82.52a |
|
28% prot-ein |
61.90bc |
84.76ab |
87.21b |
76.01b |
87.21b |
85.32a |
78.43ab |
68.10b |
88.93a |
89.72bc |
79.28b |
83.30a |
69.52ab |
71.32b |
|
30% prot-ein |
60.43c |
85.34ab |
84.69c |
81.74a |
84.69c |
82.53b |
80.75a |
70.76b |
89.81a |
88.98c |
78.21b |
82.18a |
66.99b |
80.51a |
|
240 kcal DE |
63.68a |
85.10a |
84.11c |
77.29b |
84.11c |
83.10a |
78.10b |
73.93a |
87.96b |
90.22a |
83.91a |
82.49a |
68.20b |
77.64b |
|
270 kcal DE |
64.11a |
84.49a |
88.50b |
78.20b |
88.50b |
83.47a |
78.93ab |
72.65a |
88.82ab |
90.32a |
80.85a |
81.74a |
69.37ab |
79.81ab |
|
300 kcal DE |
63.61a |
85.04a |
90.50a |
80.32a |
90.50a |
83.74a |
80.86a |
76.02a |
90.48a |
89.95a |
77.87a |
79.71b |
71.14a |
81.31a |
Data are mean of triplicate. Means same column sharing a same superscript letter are not significantly different by Tukey s test (p > 0.05).
Data analyses
Statistical analysis of nutrient digestibility data for each variable was performed by using one-way and two-way analysis of variance (ANOVA). Significant difference between treatment groups was tested by the Tukey multiple range test (p < 0.05) (Steel et al., 1997). Minitab (8.1.1) statistical package (Minitab, College Park, PA) was used for statistical analysis.
Results
Dry matter digestibility
The digestibility coefficient of dry matter ranged from 57.71% to 69.38 %. Significant differences (p<0.05) were observed among the digestibility of protein in varying diets. But the digestibility of protein was not significantly different (p>0.05) between D1 and D4. Similarly the protein digestibility was not significantly different (p>0.05) between D10 and D11. However, D6 (69.38%) had the highest coefficient of digestibility, followed by D9 (87.37%), then D10 (86.99 %) (Table I).
Protein digestibility
The digestibility coefficient of protein ranged from 81.54% to 88.18%. Significant differences (p<0.05) were observed among the digestibility of protein in varying diets. But the digestibility of protein was not significantly different (p>0.05) between D2 and D3. Similarly the protein digestibility was not significantly different (p>0.05) among D1, D4 and D11. However, D6 (88.18%) had the highest coefficient of digestibility, followed by D1 (66.74%), then D4 (66.46 %) (Table I).
Lipid digestibility
The lipid digestibility coefficient ranged from 80.52% to 93.43%. A significant differences among the digestibility of lipid in various diets were found (p<0.05). But lipid digestibility between D2 and D5 was not significantly different (p>0.05). Similarly, lipid digestibility between D3 and D9 was not significantly different (p> 0.05). However, D6 (93.43%) had the maximum digestibility coefficient, followed by D3 (90.86%), then D9 (90.73%), lastly D2 (89.43%) (Table I).
Gross energy digestibility
The digestibility coefficient of gross energy ranged from 69.17% to 86.30%. The digestibility of the gross energy was significantly different (p<0.05) in various diets.
But gross energy digestibility was not significantly different (p>0.05) among D1, D4 and D8.Similarly, gross energy digestibility was not significantly different (p>0.05) between D10 and D11. However, the D6 showed highest digestibility coefficient of gross energy (86.30%), followed D12 (83.05%) (Table I).
Ash digestibility
The digestibility coefficient of ash ranged from 80.87% to 87.51%. The digestibility of the ash was significantly different in various diets (p<0.05). But ash digestibility was not significantly different (p>0.05) between D1 and D2. Similarly, ash digestibility was not significantly different (p>0.05) between diet D3 and D5, between D8 and D10, between D6 and D9. However, the D4 (87.51%) showed highest digestibility coefficient of ash, followed by D7 (86.30%) (Table I).
The diet with proper P/E ratio enhanced the ADC (%) of nutrients and minerals of treatment diets in L. rohita fingerlings (Table I). The higher digestibility of dry matter, crude protein, lipid and gross energy was observed in fish fed D6 with P/E ratio 86.67 mg/kcal which differed significantly (p<0.05) from all other treatment while higher digestibility of ash was observed in fish fed 108.33 P/E ratio diet.
Factorial plots of interactions of dry matter, crude protein, lipid, ash and gross energy digestibility with protein and energy levels are shown in Figure 1. The digestibility of minerals such as potassium and magnesium were not significantly (p> 0.05) affected by protein energy P/E ratios whereas the digestibility of sodium, phosphorus, calcium, zinc, copper, manganese and iron was significantly (p<0.05) affected by protein energy ratios. However, the digestibility of Na, Mg, P, Ca, K, Zn and Fe were found highest with P/E ratio 86.67 mg/kcal diet while the highest digestibility of Cu and Mn was found in fingerlings fed D5 and D7, respectively. Factorial plots of interactions of minerals such as Na, K, P, Ca, Mg, Zn, Cu, Mn and Fe digestibility with protein and energy levels are shown in Figure 2.
Discussion
The digestibility of dry matter, crude protein, lipid and gross energy was higher in fish fed 86.67 mg/kcal P/E ratio diet while digestibility of ash was higher in fish fed 108.33 mg/kcal P/E ratio diet. The lower digestibility values of dry matter and ash were observed in D12 with P/E ratio 100.00 mg/kcal while lower digestibility of crude protein, lipid and gross energy was noticed in D3 (80.00 mg/kcal), D10 (125.00 mg/kcal) and D7 (113.67 mg/kcal), respectively. In the current study, the digestibility values of dry matter are agreed with the results of Rahman et al. (2016). The mean values of digestibility of crude protein, lipid, gross energy was significantly (p < 0.05) improved by the (P/E) ratio in all diets while dry matter digestibility was non-significantly (p > 0.05) affected. The results of this study are agreed with the findings of Rivas-Vega et al. (2013). In the present study, protein digestibility is closer to the values reported by Hossain et al. (2000). The diet containing 80 mg/kcal is negatively affected the digestibility of protein. In the present study, lipids digestibility values are closer to the digestibility values (90.1 to 95.7%) in Japanese flounder reported by Sato (1999). The decreasing trend of digestibility of lipid at higher protein level might be due to more nitrogen excretion that negatively affected lipid digestibility.
The digestibility of ash was highest in fingerlings fed diet with P/E ratio 108.33 mg /kcal. The digestibility of ash was significantly lower at high dietary energy and protein levels. The higher dietary lipid level may cause more fecal excretion that reduce digestibility of ash. In addition, digestibility of energy was found highest in fish fed D6 (86.67 mg/kcal ratio). The digestibility of dietary energy values ranged from 83.05 to 72.02 (%) are similar with values reported by Windell et al. (1978) in rainbow trout, however, lower than values reported by Cho et al. (1982).The herbivorous fish species have lower digestibility of dietary energy with high lipid diet than carnivorous fish (Lovell, 1989). This study is consistent with the findings of Rivas-Vega et al. (2013).
The digestibility values of Na, K, P, Ca, Mg, Zn and Fe were highest in fish fed D6 while Cu and Mn digestibility was highest in group of fish fed D5 and in D7, respectively. The digestibility of Na, P, Ca, Zn, Cu, Mn, and Fe was significantly (p < 0.05) affected by P/E ratio in the diets while the digestibility of K and Mg not significantly (p > 0.05) affected. These finding are agreed with the results of Hardy et al. (2011). The digestibility values of Na, K, Ca, Mg, Zn and Fe were decreased by increasing protein levels at each dietary energy level while digestibility values of P, Cu and Mn were increased by increasing levels of dietary protein at each dietary energy level. The finding of this study is agreed with the result of Hardy et al. (2011).
As a result of the present study we concluded that P/E ratio of 86.67 mg / kcal can be successfully utilized in L. rohita fingerlings diet to maximize growth performance.
There is supplementary material associated with this article. Access the material online at: https://dx.doi.org/10.17582/journal.pjz/20190504200510
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Ai, Q.H., Mai, K.S., Li, H., Zhang, C., Zhang, L., Duan, Q., Tan, B., Xu, W., Ma, H., Zhang, W. and Liufu, Z., 2004. Effects of dietary protein to energy ratios on growth and body composition of juvenile Japanese sea bass, Lateolabrax japonicas. Aquaculture, 230: 507-516. https://doi.org/10.1016/j.aquaculture.2003.09.040
AOAC, 1995. Official methods of analysis of the AOAC International. AOAC International, Gaithersburg, MD, USA.
Anizah, M.R., Saad, C.R., Kamarudin, M.S. and Rahim, A.A., 2017. Effect of dietary protein and protein energy ratio on the growth performance of lemon fin barb hybrid Hypsibarbus wetmorei × Puntius gonionotus larvae. Iran. J. Fish. Sci. 16: 711-721.
Cho, C.Y., Slinger, S J. and Bayley, H.S., 1982. Bioenergetics of salmonid fishes: Energy intake, expenditure and productivity. Comp. Biochem. Physiol., 73: 25-41. https://doi.org/10.1016/0305-0491(82)90198-5
Cortes, J.E., Colmenares, H.V., Cerecedo, R.C. and Paramo, J.N., 2004. Effect of dietary protein level on the growth and survival of pre-adult freshwater crayfish Cherax quadricarinatus (von Martens) in mono sex culture. Aquacult. Res., 35: 71-79. https://doi.org/10.1111/j.1365-2109.2004.00988.x
Craig, S. and Helfrich, L.A., 2009. Understanding fish nutrition, feeds, and feeding. Virginia Cooperative Extension, pp. 420-256.
Divakaran, S., Leonard, G.O. and Ian, P.F., 2002. Methods for determination of chromic oxide in shrimp feeds. J. Agric. Fd. Chem., 50: 464-467.
FAO, 2014. The state of World fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Roma, pp. 223.
Hardy, R.W., Gatlin-III, D.M., Bureau, D., Abramo, L.R., Davis, D.A., Halver, J.E., Krogdahl, A., Medale, F.S., Shiau, Y. and Tocher, D.R., 2011. Nutrient requirements of fish and shrimp. The National Academies Press, Washington, D.C., pp. 376.
Hossain, M.A., Jahan, P. and Kikuchi, K., 2000. Nutrient digestibility coefficients of diets with varying energy to protein ratio for Japanese flounder, (Paralichthys olivaceus). Bangladesh J. Fish. Res., 4: 105-112.
Huo, Y.W., Jin, M., Zhou, P.P., Li, M., Mai, M.K.S. and Zhou, Q.C., 2014. Effect of dietary protein and lipid levels on growth, feed utilization and body composition of juvenile swimming crab, Portunus trituberculatus. Aquaculture, 434: 151-158. https://doi.org/10.1016/j.aquaculture.2014.08.011
Khan, M.A., Jafri, A.K. and Chadha, N.K., 2004. Growth and body composition of rohu, Labeo rohita fed compound diet: Winter feeding and rearing to marketable size. J. appl. Ichthyol., 20: 265-270. https://doi.org/10.1111/j.1439-0426.2004.00550.x
Khan, M.A. and Shabi, F.A., 2012. Effect of varying protein to energy ratios on growth, nutrient retention, somatic indices, and digestive enzyme activities of Singhi, Heteropneustes fossilis (Bloch). J. World Aquacult. Soc., 43: 490-501. https://doi.org/10.1111/j.1749-7345.2012.00587.x
Kim, K.W., Kim, K.D., Han, H.S., Moniruzzaman, H.S.M., Yun, H., Lee, S. and Bai, S.C., 2016. Optimum dietary protein level and protein to energy ratio for growth of juvenile Parrot fish Oplegnathus fasciatus. J. World Aquacult. Soc., 10: 111. https://doi.org/10.1111/jwas.12337
Lim, S.J., Kim, S.S., Ko, G.Y. and Lee, K.J., 2011. Fish meal replacement by soybean meal in diets for Tigger puffer Takifugu rubripes. Aquaculture, 313: 165-170. https://doi.org/10.1016/j.aquaculture.2011.01.007
Lovell, R.T., 1989. Nutrition and feeding of fish. Van Nostrand Reinhold, New York, USA. https://doi.org/10.1007/978-1-4757-1174-5
Mohseni, M., Pourkazemi, M., Hosseni, M.R., Hassani, M.H.S. and Bai, S.C., 2013. Optimization of protein level and protein to energy ratio in sub-yearling Persian sturgeon, Acipenser persicus. Aquacult. Res., 44: 378-387. https://doi.org/10.1111/j.1365-2109.2011.03041.x
NRC, 1993. Nutrient requirements of fish. National Research Council, National Academy Press, Washington, DC, USA.
NRC, 2011. Nutrient requirements of fish and shrimp. National Research Council, National Academy Press, Washington, DC, USA.
Okorie, E.O., Kim, Y.C., Lee, S., Bae, J.Y., Yoo, J.H., Han, K., Park, G.J., Choi, S.M. and Bai, S.C., 2007. Re-evaluation of the dietary protein requirements and optimum dietary protein to energy ratios in Japanese eel (Anguilla japonica). J. World Aquacult. Soc., 38: 418-426. https://doi.org/10.1111/j.1749-7345.2007.00113.x
Rivas-Vega, M.E., Romero, A.G., Baeza, A.M., Sandoval, M.A., Lopez-Elias, J.A. and Soto, M.N., 2013. Effect of protein to energy ratio on growth performance, body composition and enzymatic digestive activity of juvenile Tilapia Oreochromis niloticus x O. mossambicus reared in seawater. Curr. Res. J. biol. Sci., 5: 30-35. https://doi.org/10.19026/crjbs.5.5469
Rahman, M.M., Han, H.S., Kim, K.W., Kim, K.D., Lee, B.J. and Lee, S.M., 2016. Apparent digestibility coefficients of the extruded pellet diets containing various fish meals for olive flounder Paralichthys oblivaceus. Fish. aquat. Sci., 19: 27. https://doi.org/10.1186/s41240-016-0027-7
Rowland, S.J. and Ingram, B.A., 1991. Diseases of Australian native fishes. In: Fisheries bulletin 4. NSW Fisheries, Sydney, NSW, Australia.
Sato, T., 1999. Development of formulated feed for juvenile Japanese flounder. PhD thesis, Dept. of Aquatic Biosciences, Tokyo University of Fisheries, Tokyo, pp. 13.
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1997. Principles and procedures of statistics, 3rd edn. McGraw Hill International Book Co. Inc., New York.
Windell, J.T., Foltz, J.F. and Sarokon, J.A., 1978. Effect of fish size, temperature and amount fed on nutrient digestibility of a pelleted diet by rainbow trout, Salmo gairdneri. Trans. Am. Fish. Soc., 107: 613-616. https://doi.org/10.1577/1548-8659(1978)107<613:EOFSTA>2.0.CO;2
Zhou, Q.C., Tan, B.P, Mai, K.S. and Liu, Y.J., 2004. Apparent digestibility of selected feed ingredients for juvenile cobia (Rachycentron canadum). Aquaculture, 241: 441-451.
To share on other social networks, click on any share button. What are these?









