Digit and Sesamoid Bones of Adult Chinkara (Gazella bennettii) (Sykes, 1831) (Mammalia: Bovidae): Morphology and Osteometry
Digit and Sesamoid Bones of Adult Chinkara (Gazella bennettii) (Sykes, 1831) (Mammalia: Bovidae): Morphology and Osteometry
Salahud Din
Department of Anatomy and Histology, Faculty of Veterinary and Animals Sciences, The Islamia University of Bahawalpur, Pakistan.
ABSTRACT
This study was designed to study gross morphological and basic osteometric features of the digit bones of Chinkara. The osteometry were performed manually through a digital vernier calliper on twenty (20) adult Chinkara (10 each male and females). The digits of Chinkara in each limb comprised the lateral and medial proximal, middle and distal phalanx. The first two phalanges were long bones. The shaft of the phalanx proximalis (P1) was thick proximally than distally and slightly arched. The phalanx media (P2) was shorter in length than the proximal. Its proximal articular surface was divided into two glenoid cavities “axial and abaxial’ by a dorsopalmar ridge. The phalanx distalis (P3) was triangular, with four surfaces and six borders. The descriptive analysis indicates, no statistically significant (p>0.05) differences between the lateral and medial phalanges of all measured parameters. All the measured parameters for the digits of the forelimb were statistically significantly different (p>0.05) between male and female adult Chinkara except greatest Length (Lg) of the phalanx media of the forelimb. Similarly, significant differences (p>0.05) were present in all the studied parameters of the P1, P2 and P3 of the hindlimb except for maximum breadth of the shaft (Bs) of the P2.
Article Information
Received 04 September 2021
Revised 06 October 2021
Accepted 27 October 2021
Available online 10 May 2022
(early access)
Published 22 February 2023
Key words
Chinkara, Digit bones, Sesamoid bones
DOI: https://dx.doi.org/10.17582/journal.pjz/20210904190928
* Corresponding author: [email protected]
0030-9923/2023/0003-1021 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Societ of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Chinkara (Gazella bennettii) is a beautiful ungulate species, its natural habitat is light forests, dry scrub, deserts, hills, and dry plains (Mallon and Kingswood, 2001). The body weight of male and female chinkara is about 23 kg and 16 kg, respectively (Mallon, 2008). Morphometry is a research area, observing differences in the distance between certain points, width, angles and rates through various statistical methods (Mitteroecker and Gunz, 2009; Salvagno and Albarella, 2017; Gündemir et al., 2020). The gross and morphometric examinations of the skeletal elements are important for comparing the skeletal differences between ancient and recent animals (Paral et al., 2004). Morphometric investigations were used and still using in archaeological studies for identifications of species and specific bones, in osteological remains (Paral et al., 2004). All the ruminants have two fully developed digits and each digit has three phalanges. These are, phalanx proximalis, phalanx media, and phalanx distalis (König and Liebich, 2020). The phalanx proximalis is the longest among the three phalanx (Nourinezhad et al., 2012). Phalanx media is almost half of phalanx proximalis in length, and the phalanx distalis looks like an irregular prism (Choudhary and Singh, 2016). The digits in the hindlimb are anatomically analogous to the bones of the digits in the forelimb, however, morphometrically phalanx proximalis and phalanx media are shorter in the hindlimb than forelimb (Sisson, 1975; Nickel et al., 1986). In morphological studies, every bone is described completely, according to its position, function, length, breadth and shape. It has been found that the lateral and medial digit bones of cattle differ in length (Schwarzmann, 2007; Nacambo et al., 2007; Muggli et al., 2011). The abaxial digit is reported to be longer than the axial digits in cattle, also, this dissimilarity in the length of digit bones had been stated in other artiodactyls (Keller et al., 2009). In Bovines, the digit bones are symmetrical in oxen (Nourinezhad et al., 2012), however, it is asymmetrical in cows (Keller et al., 2009), that is why biomechanical lesions of the foot are less common than the cattle. It is hypothesized by (Keller et al., 2009) about the asymmetry of the lateral and medial digit, on soft ground, a longer lateral digit is advantageous and provides good stability during the walk and a faster speed. It is also advantageous during aggression with rivals or making turns to spurt predators because the grasp would be better (Keller et al., 2009).
It was hypothesized that the morphological and osteometric characteristics of the chinkara digits and sesamoids will be adopted to barren plains and maintenance of balance in fast running and jumping. This study was designed to thoroughly describe the morphological and osteometric details of the chinkara digits, and compare the morphological features with the related ungulates from the literature. The comprehensive gross anatomical and osteometric obtained data will be helpful in the assessment and documentation of fast running artiodactyl digital bones, archaeozoological studies, easier evaluation of digital anomalies of the feet and provide knowledge of the proper understanding of biomechanical possessions of these animals by using morphometric data.
MATERIALS AND METHODS
Experimental design
In this study, digits and associated sesamoid bones of both thoracic limb and pelvic limb from twenty (20) specimens of an adult male and female adult chinkara (Gazella bennettii) of homogeneous groups from the same locality were studied, to reduce differences due to sampling from dissimilar races and sources to a minimum (Guintard and Lallemand, 2003). Bones specimens for morphological and osteometric studies were obtained after natural mortality of chinkara kept in a semi-captive environment, unrelated to skeletal abnormities. Age and sex of all the studied chinkara were noted at the time of post mortem examination, and carcasses of male and female chinkara were buried separately in a designated location within the park premises to obtain digit and sesamoid bones. After elapse of few months, the bones of each male and female chinkara were excavated. Gross features of each digit and sesamoid bone were studied thoroughly and described using the terminology given in (Nomina Anatomica Veterinaria, 2017). The dimensions of each bone were measured through a digital vernier calliper by two independent observers, and the mean values of each measurement were recorded. The gross morphological readings were described according to (Driesch, 1976).
The dimensions of the first and phalanx media (Fig. 1A, B) and phalanx distalis parameters (Fig. 1C) were analyzed. Analysis of all morphometric values was conducted with SPSS version 20.0 and was expressed as mean±standard deviation (SD) and means of the male and female adult chinkara were compared with a student t-test and differences were considered significant at P < 0.05. Furthermore, all data were subjected to a one-way analysis of variance (ANOVA). When intergroup differences between the sexes were statistically significant, the Tukey test was used to determine from which group the differences originated. The percent value of some parameters was calculated using the formula:
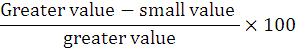
RESULTS
Morphological features of digits
In this study, two completely establish digits having three phalanges per digit were observed gross morphologically in the thoracic and pelvic limb of the adult chinkara i.e. proximal, middle and distal phalanx. The first two phalanxes were long bones, having shaft and extremities. Both extremities (proximal and distal) had articular surfaces and facets.
Phalanx proximalis
The proximal extremity (base) (Fig. 2) of the phalanx proximalis was alienated by a dorso-palmarly oriented deep sagittal groove into two concave articular surfaces (fovea articularis). The lateral articular surface was slightly extended proximal from the summit of the medial articular surface, also more concave and broader than the medial articular surface (Fig. 2). Both articular surfaces were smooth and elongated dorso-palmarly. The outlines of both articular surfaces were sharp. The palmar surface of the proximal end had two tuberosities separated by a deep and smooth depression (Fig. 3). Alienations between the articular surfaces were clearly visible from the palmer’s side; however, the alienations were not noticeable from the dorsal side because the deep sagittal groove was closed by a sharp and narrow ridge on the dorsal side, while it was opened on the palmar side. The proximal end was comparatively wider and laterally compressed than the distal (caput) one (Fig. 3). Two convex articular facets were notified on the palmer surface of the base (proximopalmar), axial and abaxial facet, these two facets were for the attachment of the proximal sesamoids, the axial articular facet was smaller and positioned lower than the abaxial articular facet.
The shaft or body was thick proximally than distally and having four surfaces (Fig. 3). The shaft was slightly arched towards the interdigital surface. The surfaces were axial, abaxial, dorsal and palmer. The dorsal and abaxial surfaces were continuous with each other. A smooth arched shaped muscular line was placed on the axial surface, which extended from the axial articular surface to the axial condyle on the distal end. The axial surface had a prominent nutrient foramen palmar to the muscular line and slightly distal to its middle. The palmar surface was flat and had two rough nodular elevations. These elevations were present laterally on either side on its distal aspect (Fig. 3) on both lateral and medial phalanx.
The distal extremity (Fig. 3) was alienated by a sagittal groove into two convex condyles. The abaxial condyle was prominently larger and wider than the axial condyle. The articular condyles had rounded shaped depressions on either side for ligamentous attachments (Fig. 3). The articular condyles were enlarged and encroached considerably into the shaft on the palmer surface making a clear line of demarcation between the body and the distal end.
Phalanx media
The phalanx media (Fig. 4) was shorter in length than the proximal one. The proximal surface was alienated into two glenoid cavities by a dorsopalmar ridge. The abaxial glenoid cavity was four-sided and distinctly larger than the axial, which was triangular (Fig. 5). The proximal end had a middle eminence on the dorsal surface and two tuberosities on the palmar surface. A rough depression was noticed between the tuberosities and the palmar limit of the two glenoid cavities. The overall shape of the proximal articular surface was trilateral (Fig. 5). The shaft of the phalanx media (Fig. 4) had three surfaces. These surfaces were axial, abaxial and palmar. The axial and abaxial surfaces of the phalanx media were flattened distally and had a nutrient foramen in the middle. The palmar surface was rough and concave. The distal end (Fig. 4) was smaller than the proximal one and is alienated by a sagittal groove into two condyles. Both condyles were placed obliquely and triangular-shaped from dorsopalmer direction. The width of both condyles was equal but the abaxial was longer than the axial.
Phalanx distalis
The phalanx distalis (Fig. 6) was completely enclosed by the claw. It was triangular, with four surfaces and six borders (Fig. 6). The dorsal border was sharp and had a prominent extensor process proximally for the attachment of the common digital extensor tendon. The extensor process was rough. The proximal articular surface was flattened sidewise, placed obliquely and divided by a dorsopalmar oblique ridge into two articular cavities. The overall articular surface was trilateral but the articular cavities were rectangular shaped. Both cavities were an equal size and placed at the same level. A transverse rough facet for the attachment of the distal sesamoid bone was placed on the palmer surface of the proximal articular end. A prominent rounded shaped nutrient foramen lying axially was observed on the proximal articular surface palmar to the extensor process (Fig. 6). The abaxial surface was nearly convex, rough and crossed by a faint dorsoventral ridge. The area palmar to the ridge was rough, elevated, and porous and had numerous small and large foramina and grooves; while, the area located dorsally to the ridge was smooth. The sole surface was almost centrally concave. The axial surface was smooth and had a groove in the middle and a prominent foramen, which was circular in outline and more towards the dorsal border. A rounded border was present between the axial and sole surface.
Sesamoid bones (Ossa sesamoidea)
In this research, two pairs of proximal sesamoids (two per digit) and one pair of distal sesamoid bone (one per digit) were observed.
Proximal sesamoid bones (Os sesamoidea proximalia)
The proximal sesamoid bones were located palmar to the metacarpophalangeal joint (fetlock joint). The proximal sesamoid bones were medial and lateral and were small, crescent-shaped bones. Both sesamoids were firmly attached. Of the two sesamoid bones, the lateral sesamoid bone one was wider and less elongated than the medial one (Fig. 7). The articular surface (fovea articularis) of each sesamoid was concave. The palmar surface (flexor surface) was rough and convex. The dorsal part had a facet for articulation with the first phalanx. The apex was pointed dorsally.
Distal sesamoid bones (Os sesamoideum distale)
The distal sesamoids were short bone and quadrilateral in shape. The distal sesamoid had two surfaces and two borders. The dorsal surface was divided by a sharp ridge into two concave areas, the axial of which was larger than the abaxial one. The palmar surface was rounded, smooth and convex (Fig. 7).
Descriptive studies of the digits and sesamoids
The osteological measurements of various digital and sesamoid bones of both adult male and female adult chinkara are tabulated in Tables I-VI. The analysis of the present study indicates that there were no statistically significant (p>0.05) differences between the left and right and lateral and medial phalanges of all measured parameters. Therefore, in this research, the mean values obtained make it possible to pool the data from the left and right sides and lateral and medial phalanges for duplication of the value number in the calculation.
Table I. Male and female morphometrics of the phalanges of the forelimb of the adult chinkara in cm (Mean±SD).
|
Male (n=10) |
Female (n=10) |
Mean±SD |
P value |
||
|
First phalanx |
Lg |
3.94 ±0.06 |
3.50±0.11 |
3.72±0.24 |
.023 |
|
Bp |
0.86±0.03 |
0.97±0.02 |
0.91±0.06 |
.006 |
|
|
Bs |
0.81±0.03 |
0.83±0.04 |
0.82±0.04 |
.12 |
|
|
Bd |
0.77±0.01 |
0.81±0.03 |
0.79±0.03 |
.001 |
|
|
Second phalanx |
Lg |
2.31 ±0. 07 |
2.00 ± 0.06 |
2.15±0.17 |
.96 |
|
Bp |
0.93±0.03 |
0.80± 0.02 |
0.86±0.07 |
.07 |
|
|
Bs |
0.72 ±0.01 |
0.81±0.04 |
0.77±0.24 |
.00 |
|
|
Bd |
0.77±0.03 |
0.67± 0.01 |
0.72±0.06 |
.00 |
|
|
Third phalanx |
Lg |
2.35 ±0.06 |
2.36 ± 0.01 |
2.35±0.04 |
.001 |
|
Ba |
0.69 ±0.03 |
0.68±0.01 |
0.69±0.02 |
.001 |
|
|
He |
1.53±0.03 |
1.50±0.01 |
1.51±0.03 |
.001 |
Differences are considered significant at P < 0.05. Lg, greatest length; Bp, maximum breadth of proximal extremity; Bs, maximum breadth of shaft; Bd, maximum breadth of distal extremity, Ba, maximum breadth of articular surface; He, maximum height including extensor process.
Table II. Male and female morphometrics of the phalanges of the hindlimb of the adult chinkara in cm.
|
Male (n=10) |
Female (n=10) |
Mean±SD |
P value |
||
|
First phalanx |
Lg |
3.96 ±0.16 |
3.57±0.05 |
3.76±0.23 |
.008 |
|
Bp |
0.96±0.08 |
0.95±0.03 |
0.96±0.06 |
.001 |
|
|
Bs |
0.92 ±0.07 |
0.80±0.04 |
0.86±0.08 |
.002 |
|
|
Bd |
0.85±0.04 |
0.77±0.11 |
0.81±0.09 |
.001 |
|
|
Second phalanx |
Lg |
2.17±0.14 |
2.05±0.03 |
2.11±0.11 |
.001 |
|
Bp |
0.86 ±0.05 |
0.81±0.01 |
0.83±0.04 |
.001 |
|
|
Bs |
0.72±0.06 |
0.67 ±0.06 |
0.70±0.06 |
.094 |
|
|
Bd |
0.72 ±0.02 |
0.66±0.02 |
0.69±0.03 |
.001 |
|
|
Third phalanx |
Lg |
2.34±0.04 |
2.36 ± 0.04 |
2.35±0.03 |
.013 |
|
Ba |
0.68 ±0.02 |
0.69 ±0.02 |
0.69±0.02 |
.001 |
|
|
He |
1.52 ±0.02 |
1.51 ±0.02 |
1.51±0.02 |
.06 |
Differences are considered significant at P < 0.05. For abbreviations, see Table I.
All the measured parameters for the digits of the forelimb were statistically significantly different (p>0.05) between male and female adult chinkara except Lg. Similarly, significant differences (p>0.05) were present in all the studied parameters of the P1, P2 and P3 of the hindlimb except for maximum breadth of shaft (Bs) of the P2. In the sesamoid bones of the forelimb, statistically significant (p>0.05) differences in the greatest length of the abaxial sesamoid, bm of the axial and distal sesamoid between male and female adult chinkara. In hindlimb sesamoid bone, bm in both abaxial and axial were statistically (p>0.05) significant. In most of the parameters, significant differences (p>0.05) were observed in the mean values of the phalanges of the forelimb and hindlimb in both male and female adult chinkara.
Table III. The mean values of the measurements (Mean±SD) for the digits bones of the forelimb and hindlimb bones of chinkara.
|
Forelimb |
Hindlimb |
||||
|
Male |
Female |
Male |
Female |
||
|
First phalanx |
Lg |
3.94±0.06a |
3.50±0.11b |
3.96±0.16abc |
3.57±0.05c |
|
Bp |
0.86±0.03a |
0.97±0.02b |
0.96±0.08b |
0.95±0.03b |
|
|
Bs |
0.81±0.03a |
0.83±0.04a |
0.92 ±0.07b |
0.80±0.04c |
|
|
Bd |
0.77±0.01a |
0.81±0.03a |
0.85±0.04b |
0.77±0.11c |
|
|
Second phalanx |
Lg |
2.31±0.07a |
2.00±0.06b |
2.17±0.14c |
2.05±0.03b |
|
Bp |
0.93±0.03a |
0.80±0.02b |
0.86 ±0.05c |
0.81±0.01b |
|
|
Bs |
0.72±0.01a |
0.81±0.04b |
0.72±0.06ab |
0.67±0.06ab |
|
|
Bd |
0.77±0.03a |
0.67±0.01b |
0.72 ±0.02c |
0.66±0.02b |
|
|
Third phalanx |
Lg |
2.35±0.06a |
2.36±0.01a |
2.34±0.04a |
2.36±0.04a |
|
Ba |
0.69±0.03a |
0.68±0.01a |
0.68 ±0.02a |
0.69±0.02a |
|
|
He |
1.53±0.03a |
1.50±0.01b |
1.52±0.02ac |
1.51±0.02c |
|
a, b, c: Means in a row with different superscript letters are statistically different (p < 0.05). For abbreviations, see Table I.
Table IV. Measurements (Mean± SD) of the sesamoid bones of forelimb of adult chinkara in cm.
|
Male |
Female |
Mean± SD |
P value |
||
|
Abaxial |
Lg |
0.95±0.02 |
0.94±0.04 |
0.95±0.03 |
.05 |
|
Bm |
0.45±0.04 |
0.41±0.03 |
0.43±0.05 |
.33 |
|
|
Axial |
Lg |
0.81±0.03 |
0.82±0.04 |
0.82±0.03 |
.16 |
|
Bm |
0.32±0.02 |
0.35±0.02 |
0.34±0.04 |
.03 |
|
|
Distal sesamoids |
Lg |
0.44±0.04 |
0.41±0.04 |
0.43±0.04 |
.93 |
|
Bm |
0.89±0.09 |
0.85±0.04 |
0.88±0.08 |
.05 |
Differences are considered significant at P < 0.05. For abbreviations, see Table I.
The proximal phalanx of the forelimb in male adult chinkara was 0.40% shorter and 11.69 % thinner than the proximal phalanx of the hind limb. However, the 2nd phalanx of the forelimb was 6.54 % taller and 1.29 % broader than the phalanx media of the hindlimb. The phalanx distalis of the hindlimb was 0.48% smaller than the phalanx distalis of the forelimb. In female chinkara, different values from the male chinkara were found, the proximal phalanx of the forelimb in adult female chinkara was 1.71% shorter, 3.67% broader than the proximal phalanx of the hind limb. The 2nd phalanx was 2.33 % shorter and 20.25% broader than the phalanx media of the hindlimb. The first phalanx of the forelimb was 41.40 % greater as compared to the phalanx media in male adult chinkara, while in it 42.84% greater in female chinkara. Similarly, the first phalanx of the hindlimb was 45. 28 % greater as compared to the phalanx media in male adult chinkara, while it was 42.53% greater in the female chinkara.
Table V. Measurements (Mean± SD) of the sesamoid bones of the hindimb of adult chinkara in cm.
|
Male |
Female |
Mean± SD |
P value |
||
|
Abaxial |
Lg |
1.28±0.04 |
1.29±0.03 |
1.29±0.04 |
.12 |
|
Bm |
0.46±0.04 |
0.47±0.03 |
0.47±0.05 |
.07 |
|
|
Axial |
Lg |
1.09±0.05 |
1.09±0.05 |
1.09±0.04 |
.50 |
|
Bm |
0.49±0.02 |
0.49±0.03 |
0.49±0.03 |
.01 |
|
|
Distal sesamoids |
Lg |
0.64±0.04 |
0.63±0.03 |
0.63±0.03 |
.76 |
|
Bm |
1.20±0.04 |
1.17±0.04 |
1.19±0.04 |
.26 |
Differences are considered significant at P < 0.05. For abbreviations, see Table I.
Table VI. The mean values (Mean±SD) of the measurements for the sesamoid bones of the forelimb and hindlimb bones of chinkara.
|
Forelimb |
Hindlimb |
||||
|
Male |
Female |
Male |
Female |
||
|
Abaxial |
Lg |
0.95±0.02a |
0.94±0.04a |
1.28±0.04b |
1.29±0.03b |
|
Bm |
0.45±0.04a |
0.41±0.03b |
0.46±0.04c |
0.47±0.03c |
|
|
Axial |
Lg |
0.81±0.03a |
0.82±0.04a |
1.09±0.05b |
1.09±0.05b |
|
Bm |
0.32±0.02a |
0.35±0.02b |
0.49±0.02c |
0.49±0.03c |
|
|
Distal sesamoids |
Lg |
0.44±0.04a |
0.41±0.04a |
0.64±0.04b |
0.63±0.03b |
|
Bm |
0.89±0.09a |
0.85±0.04a |
1.20±0.04b |
1.17±0.04b |
|
a, b, c: Means in a row with different superscript letters are statistically different (p < 0.05). For abbreviations, see Table I.
DISCUSSION
The gross morphological and osteometric features of the chinkara digits are still not explored. To test the hypothesis that the digits bone of chinkara will be adoption in these animals for fast running, therefore, the present study analyzed comprehensively and thoroughly the digits morphologically and morphometrically. Studying and researching the normal anatomic development of a specific area is not only important for preliminary basic studies but also required for a precise diagnosis of the lesions (Raes et al., 2010). These anatomical and metrical explorations and investigations of the skeletal parts are important for the documentation of species, forensic exploration and calculations of animals’ age. Specific components of the skeletal system are used to describe bodily proportions, normal growth and adaptation to seasonal and environmental variations (Zannèse et al., 2006). This study certifies two completely established digits in adult chinkara, each digit comprised the Phalanx proximalis, phalanx media and phalanx distalis. The first digit was absent, the second and fifth was remaining as a dewclaw, the same pattern of digits development has been reported in related wild and domestic animals (Siddiqui et al., 2008), in black Bengal goat (Capra hircus), in cattle (Getty, 1975) and in goats (Al- Sharoot et al., 2013).
In this study, it was observed that the shaft of the proximal phalanx was four-sided. This observation was parallel to the studies of Choudhary et al. (2014) in chital (Axis axis), Choudhary and Singh (2016) in Indian blackbuck (Antilope cervicapra), Al-Sharoot et al. (2013) in goats, while a contradiction to the current study was seen in reports of Sisson (1975) in Ox, in which this phalanx was reported to have three-sides. Anatomical features related to the shaft of the phalanx were described by the classical anatomist but the functions related to the morphology of the shaft are still not explored. The four surfaces of the shaft seen in this study and reported earlier by other researchers in wild ungulates i.e., Chital and Indian blackbuck and small ruminants (goat) may increase surface area for the attachment of muscle which may have an impact on fast running.
The medial articular facet was smaller and postioned distal than the lateral one in chinkara. Similar description has been narrated in black Bengal goat (Siddiqui et al., 2008), in Indian black buck (Choudhary and Singh, 2016), goat (Al-Sharoot et al., 2013) and in (Sisson, 1975). However, this finding showed dissimilarity with Choudhary et al. (2014) in chital, who reported that the medial articular facet was larger than the lateral one. It was also noted in the current study that the axial articular facet located on the palmer surface of the basis was comparatively smaller and positioned lower than the abaxial articular facet. In contrast to the current study, the axial articular facet was reported larger than the abaxial one in similar wild ungulates i.e., in Chital (Choudhary et al., 2014) and in Indian black buck (Choudhary and Singh, 2016). It can be inferred from the results of this study and previous literature that the position and size of articular facets are even different in very closely related animals. However, it was stated that the muscle attachment sites vary considerably in animals even between the same species (Zumwalt, 2006). In this context, further studies are required in these animals to find out the connection between the anatomic characteristics and function of the attached soft tissues and their significance in galloping, jumping and fast running.
In another interesting morphological feature on the axial surface of the shaft was the presence of an arched shaped and prominent muscular line extended from the axial articular facet to the axial condyle on the distal end. However, reports regarding this muscular line in small ruminants and also large ruminants are not available in the literature. The anatomical features of the distal extremity identified in chinkara were analogous to the reports of Choudhary et al. (2014) in Chital, Choudhary and Singh (2016) in Indian black buck, Siddiqui et al. (2008) in goat and (Sisson, 1975) in Ox.
The present study showed that the articular condyles on the distal end encroached considerably into the shaft on the palmer surface making a clear line of demarcation between the shaft and the distal end. However, in contrast to the present investigations, in camel Abid Al-Redah and Hussin (2016) reported dorsal extensions of the articular condyles into the shaft. These features of the articular condyles are not described in related wild ruminants and domestic small ruminants. However, it was reported by Ocal et al. (2004) that this extension is not found in ruminants. Looking to the movement capabilities in these animals, the encroachment may be adopted for an increasing area for joint mobility in these animals. However, further studies are needed in live chinkaras to observe the ranges of movement in the pastern joint.
The gross morphologic features of the phalanx media observed in chinkara were equivalent to the studies of Choudhary et al. (2014) in Chital, Choudhary and Singh (2016) in Indian black buck and goat (Siddiqui et al., 2008).
The major elements of the biomechanics of locomotion are feet which are evolved due to severe adaptations. The stress factors increase foot sole pressure or force area in mammals despite greater foot areas (Strickson et al., 2020). In wild animals, the feet adaptive response to stress are on a very narrow margin, therefore, captive animals kept on walking areas having pressures higher than normal create increased incidences of mechanically induced pathologies (Panagiotopoulou et al., 2019; Regnault et al., 2017). The distal phalanx of the adult chinkara was triangular, smaller than the other phalanxes. Its abaxial surface was rough, elevated, and porous and had numerous small and large foramina and grooves. Similar morphologic features of the distal phalanx were described in impala (Mostafa et al., 2006), and in goats (Siddiqui et al., 2008; Al-Sharoot et al., 2013). However, further in vivo radiological and computed tomographic studies of the distal phalanx and related soft tissues are required, to more elaborate morphological adaptations in these animals. The results of Ford (1990) in antelope, Frandson et al. (2009) in domestic animals and Choudhary et al. (2014) in chital regarding the number of sesamoid bones were similar to our study.
The second part of this study analyzed osteometrically the digits in both sexes of adult chinkara through digital vernier calliper. Different measurements were carried out on each digit. It is evident from many reports that morphology and physiology are closely interrelated, because suitable anatomical structures assist an animal to accomplish definite functions. In this study, the osteometric values of the left and right, lateral and medial in proximal, middle and distal phalanges in the forelimb and hind limb of the male and female were not statistically significant (P < 0.05). Similarly, previous researchers reported that there was no statistical difference between the right and left phalanxes (Ocal et al., 2004; Castelló, 2016; Nourinezhad et al., 2012) in native Khuzestan buffaloes, and (Ocal et al., 2004) in Holstein bulls. Results of the present study support these studies. Contrary to the current study, Muggli et al. (2011) reported significant differences in the lengths of lateral and medial individual digital bones, and total lengths of the three phalanges in cattle of different ages. The present study indicated that no asymmetry between the left, right, lateral and medial digits showed an equal distribution of weight on both phalanges. The symmetrical digit bones may contribute to allocate equal weight on feet during standing or walking. These animals are fast running and present in harsh, steep hilly areas that might be a possible reason for symmetrical digits. Symmetrical digital bones in the oxen result in equal weight loading to the feet during standing and walking, which is the reason why the biomechanical lesions of the feet are less common than in cattle (Nourinezhad et al., 2012). However, biomechanical lesions of the feet and their causes are still not investigated in the chinkara or other related wild ungulates.
It is reported in the literature that in antelope, goat and blue bulls (Boselaphus tragocamelus) the phalanx proximalis is the longest bone among all phalanges as present study as well (Choudhary and Singh, 2016; Bharti and Singh, 2018). Different osteometric parameters measured in this study for the P1, P2 and P3 of the forelimb were distinctively lower than blackbuck (Choudhary and Singh, 2016). However, the Lg of all the three phalanges was higher than goat (Siddiqui et al., 2008). The greatest length and breadth of phalanx media were indicated to be 1.88±0.03cm and 0.94 ± 0.05cm. respectively. The same parameters were found to be 2.15±0.17cm and 0.86±0.07cm, respectively in the present study.
The Lg and Bp values of phalanx media were reported to be statistically significant in the studies on camels by Nourinezhad et al. (2012) and on domestic cattle by Gündemir et al. (2020). It was found in the present study that only the Bp value was statistically significant(P<0.05).
Clinically it is proven that a higher occurrence of limb diseases, especially laminitis, was reported in the hindlimb in contrast to the front limb (Kofler, 1999). Classical anatomy books describe that P1 and P2 were shorter in the hind limb than in the forelimb. However, these reports were not supported by morphometric data. In the present study, it was found that the Lg, Bp, Bs and Bd of the P1 was significantly higher in the hind limb as compared to the forelimb in same-sex of the chinkara.Similar reports were also narrated in domestic cattle (Gündemir et al., 2019). However, the Lg, Bp and Bd of the PII were significantly higher in the forelimb as compared to the hindlimb in the same-sex of the chinkara.
The average greatest length and maximum breadth of axial proximal sesamoids and distal sesamoid bone were 0.82±0.03 cm, 0.34±0.04 cm, 0.43±0.04 cm and 0.88±0.08 cm, respectively. However, Choudhary et al. (2014) reported 1.58±0.07, 0.75±0.00, 0.56±0.00 and 0.87±0.00 cm, respectively in chital.
CONCLUSIONS
The phalanx bones (Ossa phalanges) of the chinkara have not been studied so far. The morphological and osteometric results of the present study are useful for understanding of biomechanical properties of antelope species. The overall gross morphological and osteometric data showed similarities with other related wild ungulates and small and large ruminants, but also several individualities in chinkara. This study was based on the bone samples of adult chinkara. Further studies are needed to be performed on bones of different age groups under different environmental conditions through a radiograph and computed tomography.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abid Al-Redah, S.A., and Hussin, A.M., 2016. Anatomical study of bone of camel foot. Proceeding of 5th International Scientific Conference, College of Veterinary Medicine University of Basrah, Iraq. Iraq. Bas. J. Vet. Res., 15: 95.
Al-Sharoot, H.A., Abid, E.A., and AL-Baghdady, F., 2013. Anatomical study of the digits of fore limbs in goat. Al-Qadisiya J. Vet. Med. Sci., 12: 2-28. https://doi.org/10.29079/vol12iss2art254
Bharti, S.K., 2016. Osteo-morphological studies on the skull and appendicular skeleton of blue bull (Boselaphustrago camelus). Phd thesis. GB Pant University of Agriculture and Technology, Pantnagar.
Bharti, S.K. and Singh, I., 2018. Morphological and morphometrical studies on the phalanges and sesamoid bones of blue bull (Boselephus tragocamelus). In: International Conference on Food Security and Sustainable Agriculture, Thailand. pp. 81-85.
Budras, K.D., and Robert, E., 2003. Bovine anatomy: An illustrated text. 1st ed. Schlutersche, pp. 2-3.
Castelló, J.R., 2016. Bovids of the world: antelopes, gazelles, cattle, goats, sheep and relatives, Vol. 104. Princeton University Press, New Jersey, USA.
Choudhary, O.P., and Singh, I., 2016. Gross and morphometrical studies on phalanges and sesamoid bones of Indian blackbuck (Antilope cervicapra). J. Wildl. Res., 4: 1-5. https://doi.org/10.5958/2277-940X.2016.00028.0
Choudhary, O.P., Mathur, R., Joshi, S., and Yadav, S., 2014. Gross and biometrical studies on sesamoid bones of chital Axis axis. Vet. Pract., 15: 286- 287.
Driesch von den, A., 1976. A guide to the measurement of animal bones from archeological sites. Peabody Museum Bulletin, Harvard University, Massachusetts.
Ford, P.J., 1990. Antelope, deer, Bighorn sheep and mountain goats: A guide to the carpals. J. Ethnol. Biol., 10: 169-181.
Frandson, R.D., Wilke, W.L. and Fails, A.D., 2009.Anatomy and physiology of farm animals. Willey Blackwell, pp. 71-74.
Getty, R., 1975. The Sisson and Grossman’s anatomy of the domestic animal 5th Ed., Philadelphia, USA, pp. 748-762.
Guintard, C., and Lallemand, M., 2003. Osteometric study of metapodial bones in sheep (Ovis aries L. 1758). Annls. Anat., 185: 573-583. https://doi.org/10.1016/S0940-9602(03)80131-0
Gündemir, O., Özkan, E., and Mutuş, R., 2020. Morphometric study on the digital bones in the domestic cattle. Kafkas Univ. Vet. Fak. Derg., 26: 75-82.
Habel, R.E., 1982. Ruminant introduction. In: Sisson and Grossman’s. Anatomy of the domestic animals. Saunders Philadelphia, pp. 821-822.
Keller, A., Clauss, M., Muggli, E., and Nuss, K., 2009. Even-toed but uneven in length: The digits of artiodactyls. Zoology, 112: 270-278. https://doi.org/10.1016/j.zool.2008.11.001
Kofler, J., 1999. Clinical study of toe ulcer and necrosis of the apex of the distal phalanx in 53 cattle. Vet. J., 157: 139-147. https://doi.org/10.1053/tvjl.1998.0290
König, H.E., and Liebich, H.G., 2020. Veterinary anatomy of domestic animals. Edition, pp. 7.
Mallon, D.P., 2008. Gazella bennettii. IUCN red list of threatened species. Version 2014.3. International Union for Conservation of Nature. Global survey and regional action plans IUCN Gland Switzerland.
Mallon, D.P., and Kingswood, S.C., 2001. Antelopes. Part 4: North Africa, the Middle East and Asia.
Mitteroecker, P., and Gunz, P., 2009. Advances in geometric morphometrics. Evol. Biol., 36: 235–247. https://doi.org/10.1007/s11692-009-9055-x
Mostafa, M., Koma, L.M., and Sengoba, O., 2006. Radiographic visualization of the metacarpus and phalanges in the impala (Aepyceros melampus). Vet. Arch., 76: 75-83.
Muggli, E., Sauter-Louis, C., Braun, U., and Nuss, K., 2011. Length asymmetry of the bovine digits. Vet. J., 188: 295-300. https://doi.org/10.1016/j.tvjl.2010.05.016
Nacambo, S., Hassig, M., Lischer, C., Nuss, K. 2007. Difference in the length of the medial and lateral metacarpal and metatarsal condyles in calves and cows – A post-mortem study. Anat. Histol. Embryol., 36: 408–412.
Nickel, R., Schummer, A., Seiferle, E., Wilkens, H., Wille, K.H., and Frewein, J., 1986. The anatomy of the domestic animals. Volume 1. The locomotor system of the domestic mammals. Parey Im Mvs, Berlin.
Nomina Anatomica Veterinaria, 2017. Nomina Anatomica Veterinaria. 6th ed. World Association of Veterinary Anatomist, New York. pp. 1-178.
Nourinezhad, J., Mazaheri, Y., Pourmahdi, B.M., and Daneshi, M., 2012. Morphometric study on digital bones in Native Khuzestan water buffaloes (Bubalus bubalis). Bulgarian J. Vet. Med., 15: 228-235.
Ocal, M.K., Sevil, F., and Parin, U., 2004. A quantitative study on the digital bones of cattle. Annls. Anat., 186: 165-168. https://doi.org/10.1016/S0940-9602(04)80034-7
Panagiotopoulou, O., Pataky, T.C., and Hutchinson, J.R., 2019. Foot pressure distribution in white rhinoceroses (Ceratotherium simum) during walking. Peer J., 7: 6881. https://doi.org/10.7717/peerj.6881
Páral, V., Tich. F. and Fabi, M., 2004. Functional structure of metapodial bones in cattle. Acta Vet. Brno., 73: 413-420.
Raes, E.V., Vanderperren, K., Pille, F., and Saunders, J.H., 2010. Ultrasonographic findings in 100 horses with tarsal region disorders. Vet. J., 186: 201-209. https://doi.org/10.1016/j.tvjl.2009.07.026
Regnault, S., Dixon, J.J., Warren-Smith, C., Hutchinson, J.R., and Weller, R., 2017. Skeletal pathology and variable anatomy in elephant feet assessed using computed tomography. Peer J., 5: 2877. https://doi.org/10.7717/peerj.2877
Salvagno, L., and Albarella, U., 2017. A morphometric system to distinguish sheep and goat postcranial bones. PLoS One, 12: 0178543. https://doi.org/10.1371/journal.pone.0178543
Schwarzmann, B., Köstlin, R., and Nuss, K., 2007. Größenunterschiede zwischen den lateralen und medialen Zehenknochen und Klauen von Kälbern. Tierarztl. Prax., 35: 341–349. https://doi.org/10.1055/s-0037-1621550
Siddiqui, M.S.I., Khan, M.Z.I., Moon, S., Islam, M.N., and Jahan, M.R., 2008. Macro-anatomy of the bones of the fore limb of black bengal goat (Capra hircus). Bangl. J. vet. Med., 6: 59–66. https://doi.org/10.3329/bjvm.v6i1.1340
Sisson, S., 1975. Ruminant, porcine and carnivore osteology. In: Sisson and Grossman’s the anatomy of the domestic animals, Getty R. 5th edn. W.B. Sounder’s Co., Philadelphia; pp. 149.
Strickson, E.C., Hutchinson, J.R., Wilkinson, D.M., and Falkingham, P.L., 2020. Can skeletal surface area predict in vivo foot surface area? J. Anat., 236: 72-84. https://doi.org/10.1111/joa.13090
Zannèse, A., Baïsse, A., and Gaillard, J.M., 2006. Hind foot length: An indicator for monitoring roe deer populations at a landscape scale. Wildl. Soc. Bull., 34: 351-358. https://doi.org/10.2193/0091-7648(2006)34[351:HFLAIF]2.0.CO;2
Zumwalt, A., 2006. The effect of endurance exercise on the morphology of muscle attachment sites. J. exp. Biol., 209: 444–454. https://doi.org/10.1242/jeb.02028
To share on other social networks, click on any share button. What are these?









