Cross Versus Purebred Thai Chickens Concerning Carcass Characteristics, Performance, and Frozen Meat Quality
Research Article
Cross Versus Purebred Thai Chickens Concerning Carcass Characteristics, Performance, and Frozen Meat Quality
Khanitta Pengmeesri1,2* Thassawan Somchan1 , Doungnapa Promket1,3
1Branch of Animal Science, Department of Agricultural Technology, Faculty of Technology, Mahasarakham University, Mahasarakham, 44150, Thailand; 2Small Ruminant Research Unit, Mahasarakham University, Maha Sarakham, 44150, Thailand; 3Applied Animal and Aquatic Sciences Research Unit, Department of Agricultural Technology, Faculty of Technology, Mahasarakham University, Maha Sarakham, 44150, Thailand.
Abstract | Native chickens hold promise in niche markets, but purebred chickens lag due to slow growth, crossbreeding can solve it. Crossbred natives reach market weight by the 10th week, in contrast to purebreds taking 12 weeks. This study aims to examine carcass composition, meat quality, and muscle fiber in crossbred and purebred native chickens after a 4-week freeze at market weight. The research involves 100 purebred Thai native chickens (Yellow-whited tail, YWN) and 100 three-breed crosses (25% egg line, 25% meat line, 50% Yellow-whited tail native chicken, CBN), raised under identical open-house conditions for 10 and 12 weeks. CBN hits market weight (around 1.2 kg) in the tenth week, while YWN takes 12 weeks. At both weeks, ten chickens from each breed were randomly selected per 5 cages and slaughtered. Post-carcass dressing, breast and thigh sections were hermetically sealed and stored at -30°C for four weeks, followed by thawing at 4°C for 24 hours. The assessment covers thaw, cooking, and drip losses, along with shear force measurements. Muscle fibers in the breast and thigh were studied via Scanning Electron Microscopy. Using a Completely Randomized Design, meat quality records underwent analysis with four treatments: 10 weeks age of CBN (10CBN), 12 weeks age of CBN (12CBN), 10 weeks age of YWN (10YWT), and 12 weeks age of YWN (12YWT). Findings highlight distinctive shear force in breast meat across breeds, with YWN displaying higher levels. Younger chickens show more cooking loss in the breast. While thigh breed effects are absent, shear force fluctuates with age, higher in older birds. Meat quality between purebred and crossbred chickens displays disparities after freezing, favoring purebreds for quality. Crossbred natives reached market weight faster than purebreds, but after freezing, purebreds exhibited a quality advantage.
Keywords | Crossbred native chicken, Freezing meat, SEM muscle fiber, Indigenous chicken
Received | August 24, 2023; Accepted | September 20, 2023; Published | December 01, 2023
*Correspondence | Khanitta Pengmeesri, Branch of Animal Science, Department of Agricultural Technology, Faculty of Technology, Mahasarakham University, Mahasarakham, 44150, Thailand; Email: khanitta.c@msu.ac.th
Citation | Pengmeesri K, Somchan T, Promket D (2023). Cross versus purebred thai chickens concerning carcass characteristics, performance, and frozen meat quality. Adv. Anim. Vet. Sci. 11(12): 2030-2037.
DOI | http://dx.doi.org/10.17582/journal.aavs/2023/11.12.2030.2037
ISSN (Online) | 2307-8316

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The native Thai chicken (Gallus gallus domesticus) stands as a distinctive breed recognized for its unique attributes, including adaptability to local environments and disease resistance. The popularity of its meat for consumption is burgeoning due to its distinctive characteristics, such as firmness and elevated nutritional value (Charoensin et al., 2021), as well as its lower abdominal fat content compared to commercial broiler meat (Promket and Ruangwittayanusorn, 2021; Kaewkot et al., 2019).
Nevertheless, a notable drawback of the native Thai chicken is its slow growth rate, which demands significant time and feed investment to achieve market weight. In response, crossbreeding initiatives have been introduced to enhance growth performance while retaining desirable traits of the native breed (Chomchuen et al., 2022; Oleforuh-Okoleh and Wagoha, 2020; Jinyan et al., 2019). The marketable weight for domestic chickens typically ranges from 1.2 to 1.5 kg. Hybrid chickens, as documented by Rizzi et al. (2013), attain this weight within 10 weeks (Maliwan et al., 2017), in contrast to the 12 weeks required for purebred chickens. Consequently, disparities in age significantly influence chicken muscle development, a phenomenon confirmed by Nualhnupong and Wattanachan (2020). In recent years, the frozen food industry has witnessed substantial growth, accompanied by an increasing global demand for frozen meat products. Freezing, a prevalent preservation technique, offers several advantages such as extended shelf life, convenience, and inhibition of bacterial growth responsible for meat spoilage (Hussein et al., 2022). However, freezing also impacts the physicochemical attributes of chicken meat (Sabikun et al., 2019).
This study pursues to address the meat quality discrepancies between frozen purebred and crossbred native chickens at market weight, explaining the effects of freezing on native chicken meat and hybrid native counterparts.
MATERIAL AND METHODS
Animal Ethical Consideration
Institution Animal Care and Use Committee (IACUC) of Mahasarakham University, Mahasarakham, Thailand, approved the use of animals under this study (IACUC-MSU-21/2022).
Animal And Sample Preparation
The crossbred chicken comprises a composition of 25% egg line, 25% meat line, and 50% yellow-whited tail native chicken (CBN), while the purebred chicken is yellow-whited tail (YWN). Initially, one hundred 1-day-old chicks from each breed strain were randomly distributed among 5 cages. Each cage housed 20 chicks, occupying an area of 1.5 x 2.0 square meters (equivalent to 6 chicks per square meters). At the age of 7 days old, all chicks were vaccinated against Newcastle disease and bronchitis using eye-drop vaccination. These birds were raised in an environment with natural light conditions for 9 hours each day in an open-sided house with a zinc roof and 1.5-inch chain-link mesh installed from the roof to the floor. The mesh was also covered with 0.5-inch nylon netting. Smooth galvanized sheets, each 50 cm high, were installed around the house to d rats, external birds and snakes. Temperatures during the three-month rearing period averaged 27.92±1.70°C, 26.71±1.63°C, and 24.13±1.67°C. Humidity levels were 81.52±5.25%, 72.23±4.81%, and 65.10±3.63%, respectively. These chicks had unrestricted access to both feed and water, which were provided through a feed hopper for feed and a water container for water supply. Feed containing 21% protein provided during weeks 0 to 4, and 19% protein from weeks 4 to 12. Upon reaching 10 weeks of age (10CBN and 10YWN) and 12 weeks of age (12CBN and 12YWN), two chickens per cage (amounting to 10 birds per treatment per age) were selected for sampling and subsequent slaughter.
The slaughtering process involved a calm approach, swiftly incising across the neck just below the jawline to sever the major blood vessels and trachea, followed the method of Sabow and Majeed, (2020) and Wattanachant et al. (2005). A precise cut was made to ensure efficient bleeding. Subsequently, the chicken was hung upside down to facilitate thorough bleeding and enable blood drainage into a collection container. Post-bleeding, a brief scalding in hot water (60-65°C) aided feather removal. Hand-plucking of feathers followed this step. Upon feather removal, evisceration was executed by carefully extracting internal organs through a vent area incision, with careful avoidance of intestine rupture or carcass contamination. Cold water rinsing was performed to cleanse the carcass of debris, preparing it for further processing.
The eviscerated carcass, inclusive of head and feet, was weighed to establish the traditional carcass weight. Removal of the neck and shanks was carried out, and the resultant hot carcass was weighed. Subsequent steps encompassed carcass dressing and weighing of drum, thigh, wing, breast, fillet, offal, abdominal fat, heart, liver, and gizzard. Breast and thigh sections from each carcass of the two breed lines were individually weighed and placed within plastic zip-lock bags, prepare for drip loss assessment. Remaining components were stored in a -30°C refrigerator. After a 4-week period, six frozen breast and six frozen thigh portions per age and breed line were thawed in a refrigerator set at 4°C for 24 hours, followed by subsequent measurements of thawing loss, cooking loss, and texture analysis. Post-thawing loss process, meat was underwent cutting for examined and photograph in a scanning electron microscopy (SEM). Chicken part left over from meat quality testing are classified as biological waste, separated into plastic bags, and disposed of according to their type in the laboratory.
Cook Loss
For cook loss determination, the chicken breasts and thighs were meticulously trimmed, with the skin and bones being removed. Subsequently, each sample was weighed at ambient room temperature (28°C) and then carefully placed within an airtight zip-lock plastic bag. These samples were subjected to heating within a water bath (Memmert Water bath WNB 10, Memmert GmbH+Co. KG, German) set to 80°C for a duration of 10 minutes. Following the heating process, the samples were allowed to cool down to room temperature, and any residual moisture on the meat was absorbed using tissue paper. The cooked samples were subsequently re-weighed to facilitate the calculation of cook loss.
Shear Force
For shear force analysis, denoted as WB Shear force, the breast and thigh samples that had been utilized for cook loss testing were prepared by trimming them into dimensions of 1x1x4 cm³, aligning them parallel to the muscle fiber. These trimmed samples were subjected to shearing using a Warner-Bratzler U Slot Blade (Texture analyzer, TA-XT plus Stable Micro System Texture Analyzer, UK), with a constant speed of 10 mm/s, and shearing was executed perpendicular to the muscle fiber orientation. The peak force values (expressed in newtons) resulting from this shearing process were meticulously recorded for subsequent analysis.
SEM
Specimen from 10CBN and 12YWN breast were cut into small cubes (3*3*3 mm3) and immerse them in the fixative solution. In this study used 2.5% glutaraldehyde in phosphate buffer (pH 7.4). Leaved the samples in the fixative solution overnight then removed the meat sample to wash it with phosphate buffer solution (PBS) several times. After washing process, samples were dehydrated by gradually dehydrate the chicken meat sample by immersing it in a series of ethanol solutions with increasing concentrations by 25%, 50%, 70%, 85%, 90%, 96% and 100%). Each immersion should last about 10-15 minutes. Let them dry for 2 hours, the chicken meat sample needs to be mounted onto a sample stub. Apply a small amount of conductive adhesive carbon tape to the sample stub. Place the dried chicken meat sample onto the adhesive, making sure it sticks securely to the stub. Subsequently, the sample stub was introduced into the SEM chamber (Scanning electron microscopes JEOL model JSM-6460LV, JEOL, Ltd., Japan), and the relevant parameters were duly adjusted for optimal imaging and analysis.
Statistical Method
The data about carcass composition and meat quality underwent analysis using Analysis of Variance (ANOVA) within a Completely Randomized Design (CRD). The statistical model employed was defined as follows:
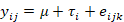
where yij = represents the observations of carcass composition and meat quality; μ = overall mean; τi= denotes the effect of treatment (where i= 10CBN, 12CBN, 10YWN and 12YWN); eij = random error associated with observation yij. To establish significant distinctions between means, Duncan’s Multiple Range Test was applied, considering a p-value threshold of less than 0.05 (SAS, 1998) to indicate statistical significance.
RESULTs
The study showed significant weight disparities between CBN chickens, caused for accelerated growth, and YWN chickens, evident as early as the first day of age, consequently delineating distinct growth paths. Notably, CBN chickens attained the market’s target weight of 1.2 kg within a span of approximately 10 weeks, whereas YWN necessitated 12 weeks to attain a comparable weight threshold. Importantly, findings from the experiment revealed that 12CBN displayed a superior body weight compared to the other groups (p<0.01). This underscores 12CBN’s potential suitability for consumers seeking specific size requirements for particular purposes. The results underscore that, within uniform rearing conditions, CBN chickens exhibited superior weight gain performance in comparison to YWN counterparts.
The observed growth patterns in this investigation substantiate the potential of hybridization in augmenting growth rates and alleviating the inherent growth limitations intrinsic to native chicken breeds. Consequently, this approach appears as a practical means to overcome the challenges posed by slow growth in native breeds (see Figure 1).
Carcass Composition
In traditional carcass dressing in native chicken, which includes the head and feet, the results highlight significant insights. Notably, at the 12th week, 12CBN achieved the highest traditional carcass weight (1249.91 g), while at the 10th week, 10CBN exhibited a greater weight than 10YWN (1050.85 vs. 866.64 g) (p < 0.01). Intriguingly, when focusing on market weight, 10CBN and 12YWN,
Table 1: Carcass composition of crossbred and purebred native chicken at 10th and 12th week
| Item | 10CBN | 12CBN | 10YWN | 12YWN | SEM | p-value |
| Body weigh |
1334.00bc |
1542.0a |
1052.50c |
1206.0bc |
41.260 | 0.001 |
|
Traditional carcass1 |
1050.85b |
1249.91a |
866.64c |
1003.57bc |
34.092 | 0.001 |
| Traditional carcass % |
78.475c |
80.975b |
82.378ab |
83.194a |
0.455 | 0.001 |
| Hot carcass |
897.59b |
1080.11a |
734.72c |
872.78cb |
29.958 | 0.001 |
| Hot carcass % |
67.05b |
69.97a |
69.83a |
70.68a |
0.403 | 0.004 |
| Drum% |
12.896b |
13.760a |
13.857a |
14.044a |
0.158 | 0.044 |
| Thigh% | 15.000 | 14.692 | 14.108 | 14.262 | 0.214 | 0.452 |
| Wing% | 12.299 | 11.506 | 12.425 | 12.328 | 0.155 | 0.125 |
| Breast% |
14.790ab |
15.174a |
13.444b |
13.902ab |
0.244 | 0.040 |
| Fillet% | 4.659 | 4.845 | 4.245 | 4.571 | 0.082 | 0.676 |
| Offal% |
17.421a |
14.095b |
12.860bc |
12.728c |
0.370 | 0.001 |
| Abdominal fat% |
0.576a |
0.542a |
0.179b |
0.092b |
0.062 | 0.004 |
| Head% | 26.107 | 26.100 | 26.812 | 26.903 | 0.378 | 0.813 |
| Hart% | 0.634 | 0.487 | 0.616 | 0.577 | 0.027 | 0.228 |
| Liver% |
0.236bc |
0.214c |
0.276a |
0.249b |
0.006 | 0.001 |
| Gizzard% | 3.083 | 2.950 | 2.994 | 2.922 | 0.074 |
0.886 |
Note: 10CBN: crossbred native chicken slaughter at 10th week; 12CBN: crossbred native chicken slaughter at 12th week; 10YWN: purebred native chicken slaughter at 10th week; 12YWN: purebred native chicken slaughter at 12th week
abcMeans within the row with different superscripts differ significantly (P<0.05)
1 The eviscerated carcass, inclusive of head and feet
Table 2: Drip loss percent comparison (Drip24 and Drip48) for breast and thigh of four treatment groups
| Item | 10CBN | 12CBN | 10YWT | 12YWT | SEM | P value | |
| Breast | |||||||
| Drip24 |
6.228a |
5.196b |
6.200a |
5.472b |
0.133 | 0.005 | |
| Drip48 |
7.570ab |
6.724c |
7.795a |
6.887bc |
0.140 | 0.010 | |
| Thigh | |||||||
| Drip24 | 3.796 | 4.266 | 4.500 | 4.428 | 0.113 | 0.114 | |
| Drip48 |
6.123ba |
5.558b |
7.008a |
5.875b |
0.178 |
0.020 |
Note: 10CBN: crossbred native chicken slaughter at 10th week; 12CBN: crossbred native chicken slaughter at 12th week;10YWN: purebred native chicken slaughter at 10th week; 12YWN: purebred native chicken slaughter at 12th week; Drip24 and Drip48: Drip loss percent at 24-hour period and 48-hour period
abc Means within the row with different superscripts differ significantly (P<0.05)
Table 3: Comparison of meat quality after freezing for breast and thigh of four treatment Groups
| Item | 10CBN | 12CBN | 10YWT | 12YWT | SEM | P value | |
| Breast | |||||||
| Thaw loss% | 4.114 | 3.426 | 3.123 | 3.110 | 0.182 | 0.168 | |
| Cook loss% |
24.655a |
23.762ab |
20.562c |
21.598bc |
0.494 | 0.004 | |
| Shear force (N) |
17.867b |
18.617ab |
19.733ab |
20.568a |
0.370 | 0.037 | |
| Thigh | |||||||
| Thaw loss% | 3.741 | 3.305 | 3.227 | 2.981 | 0.159 | 0.419 | |
| Cook loss% | 24.157 | 22.785 | 22.632 | 21.781 | 0.364 | 0.135 | |
| Shear force (N) | 18.577 | 19.57 | 18.850 | 19.833 | 0.231 |
0.178 |
Note: 10CBN: crossbred native chicken slaughter at 10th week; 12CBN: crossbred native chicken slaughter at 12th week;10YWN: purebred native chicken slaughter at 10th week; 12YWN: purebred native chicken slaughter at 12th week
abcMeans within the row with different superscripts differ significantly (P<0.05)
both having attained this criterion, displayed no significant difference (1050.85 vs. 1003.57 g). Thus far, concerning traditional carcass percentage, 12YWN exhibited a higher carcass percentage than both 10CBN and 12CBN (83.194 vs. 78.475 and 80.975%, respectively). (Table 1)
Hot carcass dressing paralleled the patterns observed in traditional carcass dressing. However, it is worth noting that the hot carcass percentage was markedly lower in 10CBN (67.056%), likely attributable to a comparatively higher presence of offal and abdominal fat (p < 0.01). Additionally, abdominal fat content was lower (p<0.01) in both age groups of purebred chickens in comparison to their crossbred counterparts.
Regarding specific components of carcass composition, the analysis revealed similarities between 10CBN and 12YWN in terms of traditional carcass weight and percentage, as well as proportions of thigh, wing, breast, fillet, heart, liver, and gizzard. Notably, within this context, 10CBN exhibited a lower drumstick percentage compared to 12YWN.
Meat Quality
Table 2 presents the results pertaining to meat quality, about the analysis of drip loss percentages across the four treatment groups. Slaughtering chickens at 10 weeks of age (10CBN and 10YWN) resulted in higher drip loss percentages (p<0.01) compared to the older age groups (12CBN and 12YWN). Remarkably, a particularly substantial Drip24 was observed in 10CBN as compared to 12YWN. Notably, within the context of the thigh part, 10YWN exhibited a Drip48 of 7.008%, surpassing both 12YWN and 12CBN (p < 0.05).
After a 4-week freezing period, the carcass segments underwent a thawing process. This involved removing them from the freezer and subsequently storing them at a temperature of 4 degrees Celsius for 24 hours.
The outcomes pertaining to meat quality, encompassing thaw loss%, cook loss%, and shear force of the breast and thigh of chickens, have been presented in Table 3. Within the scope of this study, it was determined that there existed no significant difference in thaw loss% between treatments, for both breast and thigh portions. However, it is noteworthy that the cook loss% in 10CBN breast was markedly higher, specifically by 24.655%, compared to both 10YWT and 12YWN (p<0.01). In terms of shear force, the breast meat of 10CBN displayed the lowest force at 17.867 N (p<0.05). In contrast, the breast meat of native chickens at 12 weeks exhibited a shear force of 20.568 N.
SEM
The Scanning Electron Microscopy (SEM) images of the breast meat fibers from the 10CBN and 12YWN treatment groups are depicted in the photographs (Figure 2). These images offer a visual insight into the size variations of the muscle fibers, indicated by arrows. Notably, two specific images were selected for description, as the analysis of the thigh meat fibers did not yield significant differences. The SEM images provide a comparative view of the structural characteristics of muscle fibers between the 10CBN and 12YWN groups, shedding light on potential distinctions in their microarchitecture.
DISCUSSION
Growth Performance
The observed variations in growth performance between CBN and YWN can be attributed to their distinct genetic compositions. As highlighted in previous studies (Singh et al., 2021; Deng et at., 2022) crossbreeding involving the incorporation of broiler meat-type genes into the genetic makeup of chickens, as seen in the case of CBN, has been proven to confer an advantageous growth ability.
This genetic modification facilitates a faster growth rate in crossbred chickens compared to their purebred counterparts, addressing a fundamental limitation associated with purebred strains. This trend aligns with the larger objective of enhancing the overall performance and productivity of poultry breeds in the face of evolving market demands and sustainability goals.
Carcass Composition
At a comparable live weight during slaughter, crossbred chickens were at 10 weeks of age (10CBN), while purebred chickens were at 12 weeks of age (12YWN). The carcass weights did not exhibit significant differences, yet the percentages of traditional and hot carcasses were notably higher in 12YWN. Comparisons between native and crossbred chickens in terms of carcass percentage have been explored in several studies, yielding mixed results. Some studies have demonstrated that crossbred chickens resulting from crosses between native and layer breeds exhibit higher carcass percentages than native chickens (Kaewkot et al., 2019). Conversely, other research has reported no significant disparity in carcass percentages between native chickens and native x broiler hybrids (Cruz et al., 2018) Additionally, in the report of Promket et al. (2016), no significant differences were observed between 3-breed or 4-breed crosses from Chee native chickens. This divergence underscores that carcass percentages in native and crossbred chickens can vary depending on the specific breeds and genetic backgrounds involved. In the present study, as crossbreds have 25% genes from meaty and 25% from egg types, it is possible that this genetic makeup might not significantly influence carcass percentages.
Abdominal fat percentages are subject to variation due to various factors, including feed and genetic composition. In our study, all chickens were raised under uniform feed and conditions. Notably, the abdominal fat percentage from 12YWN was 0.092%, which was lower than that of both 10CBN and 12CBN. Instances of low abdominal fat in native chickens have been documented in literature, as highlighted by Chee (Promket and Ruangwittayanusorn, 2021), Pradu Hang Dum (Kaewkot et al., 2019), and in slow-growing breeds as reported by N’Dri et al. (2006).
Meat Quality
Freezing can apply a detrimental influence on the quality and structural integrity of chicken breast cells, subsequently affecting attributes like texture and color (Rinwi et al., 2023). Following a freezing duration of 4 months, the thawed breast and thigh meats at market weight exhibited no significant differences in terms of thaw loss%.
The freezing process can lead to the formation of crystalline ice, which can induce damage to cell structures. Cook loss percentages ranging from 20.562% to 24.655% were notably higher in the 10CBN group. Essentially, this implies that when CBN chicken meat is subjected to cooking, a substantial amount of moisture is released, resulting in reduced tenderness, as compared to native chicken meat. Notably, cooking loss% was found to be lower in native chicken meat compared to broiler chicken meat (Jaturasitha et al., 2002). However, when comparing layer chicken and their crossbred counterparts, no significant differences were observed (Jaturasitha et al., 2008). It is important to mention that cooking loss% was found to be elevated in frozen meat as compared to thawed meat (Zhuang and Savage, 2013), as well as exceeding that of fresh and chilled chicken meat (Mothershaw et al., 2009). Shear force serves as a determinant of meat tenderness, with an increase in shear force corresponding to an increase in the age of slaughter (Li et al., 2019; Nualhnuplong and Wattanachan, 2020). Firmness is a preferred characteristic of native chicken meat, and this attribute is influenced by the thickness of muscle fibers (Weng et al., 2022). In the context of our study, breast meat from 12YWN exhibited higher shear force compared to 10CBN, with the muscle fibers of the pectoralis major in 12YWN being slightly larger than those in 10CBN.
CONCLUSIONS AND RECOMMENDATIONS
In this study, crossbred (10CBN) and purebred (12YWN) native chickens were evaluated for carcass composition, meat quality, and muscle fiber characteristics after a 4-week freeze at market weight. 10CBN achieved market weight more rapidly, whereas 12YWN exhibited favorable meat quality parameters.
Carcass composition analysis indicated similar weights between the breeds, with delicate differences in traditional and hot carcass percentages. Freeze-induced changes were observed in meat quality, including thaw loss, cook loss, and shear force. 10CBN exhibited higher cook loss, potentially affecting tenderness and juiciness.
Shear force measurements demonstrated that 12YWN had higher values, influencing meat tenderness. Muscle fiber observations aligned with this trend.
Further research should delve into genetic factors influencing meat quality disparities and explore strategies for combining growth advantages of 10CBN with desirable meat quality traits of 12YWN. Sensory evaluations and post-freezing treatment investigations are recommended for informed breeding and production decisions.
ACKNOWLEDGMENTS
This research project was financially supported by Mahasarakham University
NOVELTY STATEMENT
This study presents a novel examination of carcass composition, meat quality, and muscle fiber characteristics in crossbred native chicken, specifically a three-breed cross between egg-line, growth-line, and native, in comparison to purebred native chickens. Unlike previous research that often focused on isolated aspects of meat quality or growth performance, our study uniquely integrate these factors, revealing a comprehensive understanding of the interplay between genetic composition and meat quality after a 4-week freeze at market weight.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR’S CONTRIBUTION
KP: Initiated the study’s objectives, designed the experiments, and verified the data before analysis.
TS: Designed the data collection process and prepared samples for testing based on meat science concepts.
DP: Analyzed and interpreted the results.
REFERENCES
Charoensin S, Laopaiboon B, Boonkum W, Phetcharaburanin J, Villareal MO, Isoda H, Duangjinda M (2021). Thai Native Chicken as a Potential Functional Meat Source Rich in Anserine, Anserine/Carnosine, and Antioxidant Substances. Animals.; 11(3):902. https://doi.org/10.3390/ani11030902
Chomchuen K, Tuntiyasawasdikul V, Chankitisakul V, Boonkum W (2022). Comparative study of phenotypes and genetics related to the growth performance of crossbred Thai indigenous (KKU1 vs. KKU2) chickens under hot and humid conditions. Vet. Sci. 9(6):263. https://doi.org/10.3390/vetsci9060263c
Cruz FL, Katheryne L, Saraiva V, Silva GE, Nogueira TM, Silva AP d, Faria PB (2018). Growth and carcass characteristics of different crosses of broiler chickens reared under an alternative system. Semina-ciencias Agrarias, 39(1):317-328. https://doi.org/10.5433/1679-0359.2018V39N1P317
Deng S, Xing T, Li C, Xu X, Zhou G (2022). The Effect of Breed and Age on the Growth Performance, Carcass Traits and Metabolic Profile in Breast Muscle of Chinese Indigenous Chickens. Foods. 11(3):483. https://doi.org/10.3390/foods11030483
Jaturasitha S, Kayan A, Michael W (2008). Carcass and meat characteristics of male chickensbetween Thai indigenous compared with improved layer breeds and their crossbred. Arch Tierz Dummerstorf. 51: 283-294.
Jaturasitha S, Leangwunta V, Leotaragul A, Phongphaew A, Apichartstrungkoon A, Simasathitkul N, Vearasilp T, Worachai L, terMeulen U (2002). A comparative study of Thai native chicken and broiler on productive performance, carcass and meat quality. Proceeding of conference on International.
Jinyan T, Ning G, Haibin Z, Xiujin L, Jiaqi L, Hao Z, Xiquan Z, Zhe Z (2019). Performance of whole genome prediction for growth traits in a crossbred chicken population. Poult. Sci., https://doi.org/10.3382/PS/PEY604
Kaewkot C, Ruangsuriya J, Kreuzer M, Jaturasitha S (2019). Carcass and meat quality of crossbreds of Thai indigenous chickens and Rhode Island Red layer chickens as compared with the purebreds and with broilers. Anim. Prod. Sci. https://doi.org/10.1071/an18759
Li J, Yang C, Peng H, Yin H, Wang Y, Hu Y, Chunlin Y, Jiang X, Du H, Qingyun L, Liu Y (2019). Effects of slaughter age on muscle characteristics and meat quality traits of Da-Heng meat type birds. Animal. 10(1):69. https://doi.org/10.3390/ANI10010069
Maliwan P, Khempaka S, Molee W (2017). Evaluation of various feeding programmes on growth performance, carcass and meat qualities of Thai indigenous crossbred chickens. 47(1). https://doi.org/10.4314/SAJAS.V47I1.4
Mothershaw AS, Gaffer T, Kadim I, Guizani N, Al-Amri I, Mahgoub O, Al-Bahry S (2009). Quality Characteristics of Broiler Chicken Meat on Salt at Different Temperatures. Int. J. Food Prop., 12(3), 681–690. https://doi.org/10.1080/10942910801993858
N’Dri AL, Mignon-Grasteau S, Sellier N, Tixier-Boichard M, Beaumont C (2006). Genetic relationships between feed conversion ratio, growth curve and body composition in slow-growing chickens. Brit. Poult. Sci., https://doi.org/10.1080/00071660600753664
Oleforuh-Okoleh VU, Wagoha R (2020). Variations in growth performance traits and economic analysis of two Nigerian indigenous chicken strains and their crossbred. Nigerian J. Anim. Prod., https://doi.org/ 10.51791/NJAP.V44I4.625
Promket D, Ruangwittayanusorn K, Somchan T (2016). The Study of Carcass Yields and Meat Quality in Crossbred Native Chicken (Chee). Agricult. Agricult. Sci. Proced., https://doi.org/10.1016/J.AASPRO.2016.12.014
Rinwi SG, Sun Da-W, Ma J, Wang Q-J (2023). Effects of isochoric freezing on freezing process and quality attributes of chicken breast meat, Food Chem., 405 part B. https://doi.org/10.1016/j.foodchem.2022.134732.
Rizzi C, Contiero B, Cassandro M (2013). Growth patterns of Italian local chicken populations. 92(8). https://doi.org/10.3382/PS.2012-02825
Sabikun N, Allah, B, Ishamri I, Young H, Hwang M, Shafiur R, Seon TJ (2019). Changes in physicochemical characteristics and oxidative stability of pre- and post-rigor frozen chicken muscles during cold storage. J. Food Sci. Technol.-mysore, https://doi.org/10.1007/S13197-019-03941-0
Sabow A, Majeed S (2020). Bleeding efficiency and keeping quality in broiler chicken meat subjected to two slaughtering methods. 22(2):152-158. https://doi.org/10.26682/CAJUOD.2020.22.2.17
Singh M, Lim AJ, Muir WI, Groves PJ (2021). Comparison of performance and carcass composition of a novel slow-growing crossbred broiler with fast-growing broiler for chicken meat in Australia, Poult. Sci. 100(3). https://doi.org/10.1016/j.psj.2020.12.063.
Weng K, Huo W, Li Y, Zhang Y, Zhang Y, Chen G, Xu Q (2022). Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci.. 101(1): 101537. https://doi.org/10.1016/j.psj.2021.101537
Zhuang H, Savage EM (2013). Comparison of cook loss, shear force, and sensory descriptive profiles of boneless skinless white meat cooked from a frozen or thawed state1, Poult. Sci., 92(11): 3003- 3009. https://doi.org/10.3382/ps.2012-02801.
To share on other social networks, click on any share button. What are these?






