Comparative Prevallance and Pathological Changes on Camel Brucelosis at the Selected Slaughterhouses in Garissa County, Kenya
Comparative Prevallance and Pathological Changes on Camel Brucelosis at the Selected Slaughterhouses in Garissa County, Kenya
Abdirahman Barre*, Karanja D Njuguna, Bebora Lilly Caroline and George Chege Gitao
Faculty of Veterinary Medicine, Department of Veterinary Pathology, Microbiology and Parasitology University of Nairobi, Kenya.
Abstract | The study aimed at determining the presence of the disease in camel slaughterhouses in Garissa County, through serological testing and pathological lesions that encountered at post mortem inspection of camel meat. Three sub-counties; Garissa Township, Dadaab and Balambale were purposefully recruited based on presence of camel slaughterhouses and accessibility. A hundred and sixty (160) camels were selected from 238 presented during the visits based on clinical manifestations suggestive of Brucellosis obtained upon ante-mortem examination and clinical history from owners. Sero-prevallance determination that involved the blood collection from the jugular and screening serum for attendance of Brucella antibodies using Rose Bengal Plate Test (RBPT), serum agglutination test, competitive- enzyme linked immune sorbent assay and double agar gel immunodiffusion test. The selected camels were followed into the slaughterhouse and pathological changes were identified grossly and microscopically based on alteration in organ and/tissue structure. The three main clinical signs that suggested brucellosis were lameness, swollen lymph nodes and abortion. Out of 160 samples tested, 15 (9.37%) were positive for Brucella antibodies and evenly distributed between counties; 8% (4/50) for Garissa Township; 10% (5/50) in Dadaab and 10% (6/60) in Balambale. Using chi-square (χ2), there was no statistically alteration in sensitivity among the four serological tests (p=0.999). Seventy-eight (48.7%) camels had one or more organs with lesions leading to condemnation at meat inspection. The common gross lesions encountered were fibrin depositions 3 (1.8%), enlargement of lung 2 (1.2%), pericarditis 38 (23.7%), and hepatomegaly with nodular liver lesions 79 (49.3%), enteritis 5 (3.1%), haemorrhages and congestion of visceral organs (lung and kidney) 6 (3.7%). Histopathology of sero-reactors revealed; cellular infiltration in lymph node 9 (5.6%), hypoplasia of lymphocytes 6 (3.7%), collapse of alveoli 5 (3.1%), oedema, congestion 4 (2.5%), fatty degeneration in liver 3 (1.8%) and haemorrhages in kidney1 (0.6%). In conclusion, brucellosis is prevalent in camel in Garissa County. Further extensive research should be done in the whole country. With respect to picking positive cases, RBPT is recommended as a screening test, since it is cheap, quick, and easy to carry-out. The other three can be used to establish respective antibody titres. The organs condemned at inspections are due to inflammatory processes that can be associated with brucellosis or other zoonotic diseases. Standard biosecurity measures at slaughterhouses and farms be enhanced the control and prevention of Brucella infection to animals and human.
Editor | Muhammad Abubakar, National Veterinary Laboratories, Park Road, Islamabad, Pakistan.
Received | November 05, 2022; Accepted | February 20, 2023; Published | March 08, 2023
*Correspondence | Abdirahman Barre, Faculty of Veterinary Medicine, Department of Veterinary Pathology, Microbiology and Parasitology University of Nairobi, Kenya; Email: [email protected], [email protected]
Citation | Barre, A., Karanja, D.N., Bebora, L.C., and Gitao, C.G., 2023. Comparative prevallance and pathological changes on camel brucelosis at the selected slaughterhouses in Garissa county, Kenya. Veterinary Sciences: Research and Reviews, 9(1): 1-17.
DOI | https://dx.doi.org/10.17582/journal.vsrr/2023/9.1.1.17
Keywords | Comparatives, Brucellosis, Sero-prevalence, Pathological lesions, Camel, slaughterhouse, Garissa county, Kenya
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Camels are an adaptable animal and has been domesticated by man. It’s been the quickest mode of transport in deserts due to that it’s also referred to as the ship of desert (Bahrawy et al., 2015). It is also used for economic and social aspects; they are used for milk, meat and conceal provide, as for different functions like transport, amusement, celebration and competition as in athletics and wonder show (Kaskous, 2016).
Camels (Camelus dromedarius) are foremost livestock in North-Eastern province wherever it offers nourishment to several individuals, particularly throughout the frequent droughts once different animals either die or are unthrifty (Wanjohi et al., 2012). This is as a result of the artiodactyl mammal is extremely fitted to hot desert, semi-desert, arid and semi-arid areas. The camels are prevailing domesticated animals in North-Eastern region where it gives sustenance to numerous individuals particularly amid the regular dry spells when different creatures either bite the dust or are unthrifty. Camel populace in Kenya is more than 1 million and about 54% of them are kept in Garissa and Wajir locale. Occupants of these bone-dry regions are for the most part of Somali root and are pastoralists.
Globally, camels are important for industrially and financially, in keeping with the continuing report there are about 600,000 camels in Kenya (According to Food and Agriculture Organization of the United Nations. Almost of all these are kept by migrant pastoralists in the arid lowlands of Northern Kenya. Camel brucellosis has been accounted for in all camel-raising nations. The contaminations are on the ascension in Old World camels (OWCs) because of the uncontrolled exchange of live creatures.
In Kenya, there are three sorts/types of a camel: Turkana (it is little in size; averaging 350 kg), Rendille/Gabbra (300 kg) and Somali (biggest in body estimate; 550 kg). The camels are utilized as multifunctional creatures in the peaceful generation framework. They are great drain makers: Delivering more drain contrasted and dairy cattle and little stock. They, accordingly, prove to be useful amid the dry season; the pastoralists incline toward camel drain to that of other domesticated animals’ creatures as a result of its delectable taste and its being nutritious (Kaindi et al., 2011).
About 60% of Garissa County population are pastoralists who keep around 300,000 camels that increase the animals’ economy of Carissa County. The indigenous camel breeds that found in North Eastern Kenya Are include: Somali breed, Turkana and Gabra. Camel Brucellosis is an infectious chronic bacterium, sickness of camel and other species, that mainly caused by individuals from class that influences for various individual species. Therefore, condemned organ (visceral organs) in camel slaughterhouses have been documented around the world. The disease spreads from herd to herd or from animal to animal or also from country to country (Garcell et al., 2016).
Brucellosis is furthermost Zoonosis infection in Animals and man. All most all the infections are transmitted from animals to man by drinking uncooked milk, meats as liver and kidneys from effected animal and close contacted of the animals through breathing, slaughtering and contaminated dusts. The illness is caused by bacteria genus called Brucella. There are two (2) common species in camel Brucella melitensis and Brucella abortus. In terms of camel production farms, the sero-prevalence is higher in intensive camel rearing farms while in extensive system farms the incidence is very low.
Organ condemnations have commercial and public health significance associated direct economic losses. Therefore, condition that leading to organ condemnation in camel slaughtered are bacterial and parasitic infections and non in factious organism.
The significant reasons for condemned organ in butcher stock are parasitic disease (Hydrated cyst and Fasciola spp. in camel) and bacterial diseases (brucellosis, leptospirosis and Tuberculosis).
Materials and Methods
Study area
This study was carried out in Garissa County (Figure 1). The county is one of the three countries in the North Eastern region in Kenya. It is located in Eastern Kenya bordering Somalia at the East, Wajir County and Isiolo County at the North, Tana River County at the West and Lamu County at the South. It lies latitude of 10 58’North and 20 1’ South and longitude of 380 34’ E and 410 32’ E. the county covers an area of 44,174.1 Km2. It is arranged at 0.46° South scope, 39.66° for East longitude and 152 meters height over the ocean level.
The agriculture and livestock are pilars of the county economy and they are the maine sources of occupation, livelyhood for farmers and other in the value chaine. The county is flate physiclly and topogaraphically and is low lying without hills, valleys and mountains. The county is principally a semi-arid area falling within ecological zone V-VI and receives an average rainfall of 275 mm per year. There are two rain seasons, the short rains from October to December and the long rains from March to May. The natural aired of the coounty temperatures are generally high throughout the year and range from 20oC to 39oC. The average temperature is however 36oC. The hottest months are September and January to March, while the months of April to August are relatively cooler (Wanjohi et al., 2012).
There are twenty (20) camel slaughter facilaties in the county. Six (6) are located in blambale sub-county.five (3) is in dadaab, eight (4) in township, two (2) is in Fafi sub-county, three (3) in lagdera sub-county and two (2) in Ijra sub-county. The others are uncategorized ones and don’t operate daily according to the sub-county veterinary officers (SCVO).
Study design
The study was carried out both prospective study (sero-prevalance) and cross-sectional survey (pathological lesions) in different slaughterhouses of Garissa County Kenya which were include Garissa central Sub-county (Garissa Township), Garissa East sub-County (Balambala) and Garissa west sub-county (Dadaab). For Garissa County Kenya, based on the The Avalability of animals (camels) and security. for the sero-prevalance study involved analysis of data from the screaning part of four (4) diferent serology tests. On the other parts of study, cross-sectional study entailed the poste-mortem inspection of the tested RBPT and slughterd carcases and exenterated organs, gross pathological lesions apprent brucellosis and collection of specimens for histopathology analysis.
Selection of slaughterhouses for collection of data
The sub-county Veterinary Officers (SCVOs) in the three study sites were visited to discuss sampling procedure, livestock movement and other necessary assistances. The animal handles at the slaughterhouses were interviewed before sampling. However, the disease under the study were introduced to the slaughterhouse owner and the Veterinary Professional doctor at the slaughter.
The three (3) slaughterhouses namely: Township, Dadaab and Balambale in Garissa-county were selected through convenient sampling methods. They were selected based on the higher number of camel availabilities for slaughter and security compering by the other slaughterhouses of the county and available resource for laboratory (data recording, sample collection, analysis materials and transportation lab material for sampling) and also availability of poste-mortem inspection instrument.
Study animals and sampling methods
All camels presented for slaughter during the times of visit were examined ante-mortem and records reviewed for signs suggestive of brucellosis. The study animals were apparently health and adult of both sexes. The animal details: tag number, species, sex, breed, age, and owner of the animals were noted and recorded in slaughterhouse interim data capture of sheet (Appendix 7.6). The three (3) slaughterhouses in the sub-counties were convenient selected for the study. This is because they slaughter a large numbered of camels, they are easy to reach and secure. Only slaughterhouses that handle camels were recruited and visited in a period of four (4) weeks.
Sample size determination
The sample size of various camel species and different slaughterhouses from the County of Garissa were bled for prevalence estimation of the brucellosis in camel and pathological lesions of the positive’s animals were determined for the following equation.
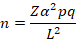
Where; n is required sample size; Zα= 1.96 the normal deviate at 5% level of significant; p A priori estimation of prevalence for the disease; q=1-p and Lis allowable error of estimation.
Slaughtered camel: using the highest prevalence estimation of 15% for brucellosis in camel and L is at 5%.
The required sample size ware calculated:
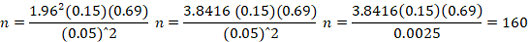
Therefore, the percentage (%) prevalence of the disease was calculated by dividing at ratio of the total number of positive from different slaughterhouses for 4:4:5 at Township: Dadaab: Balambale respectively, in order to get the required sample size. The study animals were sequentially selected for inspection and slaughtering on the slaughterhouses at a time to reach the total calculated sample size which is one hundred and sixty (160) camels.
Prospective study: Determination of Sero-prevalance in selected camel slaughterhouses in Garissa County Kenya
Sample size was redistributed to the three sub-counties propositioned based on previous in past slaughtered numbers. In the first two weeks Garissa-township sub-county slaughterhouses were visited and blood collected from fifty (50) brucellosis suspected camels. In the next two weeks, Dadaab and Balambale were also visited and Fifty (50) and sixty (60) brucellosis suspect camels, respectively. Ten millilitres (15ml) of blood was collected from jugular vein using gage 18 needle and 20 ml syringe for serology and the animals was followed to slaughter for collection of any condemned organs.
Serum preparation
The blood samples from camel slaughterhouses (160) prevalence were left to stand over-night in cool box with ice to allow the serum separation and clotting at Garissa veterinary office VIL (Veterinary Investigation Lab). The vials were labelled and stored in freezer (-20oC) at veterinary Investigation laboratory office in Garissa County. The serum was separated by using centrifuge and extracted the stander procedure.
Rose bengle plate test (RBPT)
The Rose Bengal test (RBT) was carried out using the method and OIE the antigen having been obtained from Spain (Rose Bengal- Antigen, rapid slide agglutination antigen, verw, blasé, Instituto de Salud de Navarra, RSA-RB:330-04:4000; in diagnostics ID vet 149. Spain). The temperature of the serum samples was raised to room temperature (21oC) before testing. Using micro-titre pipette a drop (25µl) of serum was placed on the glossy side of the tile: it was then mixed with the drop (25µl) of antigen. The tile was then rocked up-and-down for up to 4 minutes. Positive result appeared as pink agglutination, while no agglutination was taken as negative reaction. Positive and negative control were also set-up.
The results were readied by standard period of RBPT Test. After following the protocol, the agglutination denoted a positive test (+ve) while lack of it means a negative (-ve) results negative. The positive and the negative control were used to monitor the performance of procedure and to compere the interpretations.
This test was carried out using the method of Rose Bengal stained Brucella antigen having been from Spain (Rose Bengal-Antigen, rapid slide agglutination antigen, verw, blasé, Instituto de Salud de Navarra, RSA-RB: 330-04:4000; in diagnostics ID vet 149. Spain). Test serum was double diluted in micro0titre wells; first placing 90 µl of PBS (Phosphate Buffer Solution) in the first well 50 µl of PBS in the other wells. This was then followed by placing 10 µl of the test serum to the first well; mixed thoroughly, then 50 µl transferred to the next well and mixed thoroughly. The procedure was then repeated, transferring 50 µl of serum-PBS mixture from the second well to the 3rd one; continuing with the transference of 50 µl of thoroughly-mixed serum-PBS mixture to the next well until the last well. A volume of 50 µl was then removed from the last well and discarded. Then to each well, 50 µl of antigen was added, mixed thoroughly and the plate incubated Overnight. The positive result appeared as pinkish matt across the well, while negative reactions (no agglutination) appeared as a button at the button of the well. Positive and negative controls were also set up.
Competitive enzyme linked immuno-sorbent assay (c-ELISA) tests
The competitive enzyme linked immunosorbent assay (c-ELISA) ID screen: Copmelisa 400 A competitions ELISA kit for diagnosis of brucellosis. The reagents were prepared as per instructions of the kit manufacture (Veterinary laboratory of United Kingdom (UK). and test were prepared and carried out department of VPMP faculty of Veterinary Medicine University of Nairobi.
The serum was run in duplicate by using the comparison of the Optical Density (OD) for each sample. The cut-off point of the positive and negative control were calculated by different variable means of Optical Density. For the analysis results the lack of colour were indicated that the sample tested was positive. Appositive/negative cut-off were calculated as 60%of the mean of the optical density (OD) for the four (4) conjugate control wells. Any test sample that giving OD equal to or below the value was regarded as being positive. However, the sero-prevalence of the binding ratio was calculated by:
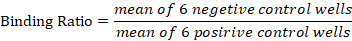
Table 1: The interpretation of brucellosis ELISA test results.
|
Serum |
|
|
Test result |
Rank |
|
P/R≤40% |
Negative |
|
40%<P/R <50% |
Doubtful |
|
50%<P/R≤70% |
Positive |
|
P.R<70% |
Strongly positive |
Ager Gel Immuno-diffusion test (AGID)
Slide ager gel double immunodifusion test (AGID) for anti Brucella antibody evaluated in this study is based on a single Brucella antigen, referred to the other antigens the test has been done in Bacteriology laboratory at University of Nairobi Department of Veterinary Pathology, Microbiology and Parasitology (VPMP). The test was used; following the method using 1.2 mm diameter puncture. Wells were dug into the solidified ager on the slide (6 in periphery and one at the centre); the central well was filled with the test serum while the outer wells were filled with the antigen. The slide was then incubated at room temperature in humid chambers/petri-dish and reading was done after 24 to 48 hours. Therefore, the presence of curved precipitation lines showed positive reaction. Positive and negative control ware also set-up.
Cross-sectional study: Establishing of Pathological lesions apparent from Brucellosis and organ condemnation
All camels that were suspected as brucellosis at ante-mortem examination were followed into the slaughterhouse. At the post-mortem inspection, organs condemned were grossly examined and sampled for histopathology.
Post-mortem examination
Poste-mortem inspection of organs that were positive in RBPT test was carried out as described by visual, observation, palpation and opening of the effected for each organ. From each Slaughterhouse visited, after anti-mortem examination of the animals were followed for further Pathological and histo-pathological examination. The lesions recorded (Appendix 7.7) with a special attention to the various changes in the organ condemnation based on size of the organ, colour, and transactional appearance. The lung, lymph nodes, heart, liver and kidney were grossly examined and processed for histopathology to rule out other diseases rather than Brucellosis. The observed lesions were described, for location, distribution, colour, size and recorded for diagnosis. The morphological lesions and other suspected abnormalities were also recorded.
The carcase was disposed of in slaughterhouse departmental disposal container after proper disinfectant of all surfaces and materials during post-mortem examination by using (Benzyl, dimethyl, Ammonium-chloride and Cooper manufactured, Kenya). Photographs of the lesions were taken using by a digital camera (sonny CSD–W920 and Mobile Camera A50 having three optical camera Magnification X40, X10, X100 and X400) and transferred into a computer and labelled appropriately.
Sample collection for histopathology
The Sampled tissue from different Slaughtered camel were fixed 10% Formalin for 48 hours as the stander protocol. The fresh tissue was removed as quickly as possible from different slaughterhouses of camel at Garissa, labelled the container and then transported to The University of Nairobi Department of Veterinary Pathology, Microbiology and Parasitology (VPMP). The tissues were trimmed with the thickened of 5 mm and dehydrated for the concentration of Alcohol for 70% to 95% at the intervals of one and half hour (½) by utilizing 80% of ethanol alcohol for 4 hours. They were cleared, infiltration with the liquid paraffin wax (paraplast) at 60 oC in two changed for the three hours per each and embedded in paper with wax, fixed into the wooden block by using hot searing spatula. The tissue was cut in to the 5µm by blocking and microtoming to the specimen. They were dewaxed in each spaceman for 5 minutes. The tissue was rehydrated and putted distilled water for 5 minutes in each section of the specimen.
The section was stained by using haematoxylin and eosin (H and E). The cover slip was applied by DPX (Dibutylphthalate xylene). The sectioned tissue was inspected under light microscope lens utilizing; x4, x10, and x40 amplification then the pathological lesions were recorded according to the affected organs.
Data analysis and presentation
The information (data) was gathered through descriptive examination from the investigation zones, revised composed and organized. So that the obtained data from, serological tests and pathological lesions were recorded in research notebook and entered the spread sheet of (Ms-Excel) and analysed by stata for windows (version 14.0). And also used Chi Square test (X2) for compering positivity of the disease from selected slaughtered camel through the pathological lesions of the infection to the other suspected diseases.
Results and Discussion
Prospective study: Sero-prevalence study results
In this case the study was based on four (4) different standard serological test so as to compare their results namely: Rose Bangle Plate (RBPT) serum Agglutination Test (SAT), Competitive Enzyme Immuno-Sorbent Test (c-ELISA) and Ager Gel Immuno-difussion Test (AGID). Therefore, using these serum techniques the results were looked at histological analysis to ensure that same sample has lesion for pathology.
Rose Bengal plate test (RBPT)
A one hundred (160) camel serum samples from Garissa sub-counties in selected slaughterhouses were tested using the Rose Bengal Plate Test (RBPT). Fifteen (15) samples (9.3%) tested positive (Table 2). From Garissa-township (n=50) four (4) samples (8.0%) were tested positive, fifty (n=50) samples from dadaab slaughterhouses six (6) (12.0%) were tested positive while sixty (n=60) samples from Balambale slaughterhouses five (5) (8.3%) were tested positive.
Table 2: Rose Bengal Plate Test (RBPT) results overall and with respect to the three-study area of Garissa County Kenya.
|
Slaughterhouse |
No. tested |
No. positive |
% Positive |
|
Overall |
160 |
15 |
9.3 |
|
Garissa township |
50 |
4 |
8 |
|
Dhadhaab |
50 |
6 |
12 |
|
Balambale |
60 |
5 |
8.3 |
Serum agglutination test (SAT)
A one hundred and sixty (160) samples of the camel serum from selected slaughterhouses in Garissa sub-counties were tested using the Serum Agglutination Test (SAT). Sixteen (16) samples (10.0%) tested positive. Garissa-township (n= 50), 4 samples (8.0%), were positive while 46 samples (28.75%) tested negative. from Dhadhab District (n= 50) 6 samples (12.0%) which were higher positive and Balambale also had 6 samples (10.0%) were tested positive. Therefore, after the result in SAT compared at percentage level, Dadaab had a highest percentage reactor of the samples (12.0%) than others.
Competitive enzyme linked immunosorbent assay test (cELISA)
The competitive enzyme-linked immunosorbent assay (cELISA) format has proven to be an accurate, reliable, easily standardized, and high-throughput method for detecting the brucella antigen in large and small animals. A one hundred sixty (160) serum samples from camel in three sub counties of Garissa County were tested used by the competitive enzyme-linked immunosorbent assay (cELISA).
The test results were as recorded in prospective. Table denotes that the fivty (50) samples were taken to Garissa-township forty-six (46) samples tested were negative (-Ve) while four (4) samples for (8.0%), were positive(+Ve) fifty (50) samples from Dhadhaab were a tested forty-five (45) tested negative (-Ve) while five (5), samples for (12.0%) tested positive(+Ve). and sixty (60) samples from Balambale were also tested fifty-four (54) samples tested negative (-Ve), while six (6) samples of (8.3%) tested positive (+Ve) dhadhaab was a higher percentage of reactors (12.0%) than garissa-township and balambale (8.0-8.3%). Therefore, representing the Results for the competitive enzyme-linked immunosorbent assay (cELISA) of three Sub-Counties in Garissa Kenya. The different of the results were shown below Table 4 written by the positive and the negative number in sub-counties as dhadhaab is much prevalence then the other.
Table 3: Serum Agglutination Test (SAT) results overall and with respect to the three study areas of Garissa County, Kenya.
|
Slaughterhouse |
No. tested |
No. positive |
% Positive |
|
Overall |
160 |
16 |
10 |
|
Garissa township |
50 |
4 |
8 |
|
Dhadhaab |
50 |
6 |
12 |
|
Balambale |
60 |
6 |
10 |
Table 4: Competitive enzyme-linked immunosorbent assay (c-ELISA) test results overall and with respect to the three study areas of Garissa County, Kenya.
|
Slaughterhouse |
No. tested |
No. positive |
% Positive |
|
Overall |
160 |
15 |
9.3 |
|
Garissa township |
50 |
4 |
8 |
|
Dhadhaab |
50 |
6 |
12 |
|
Balambale |
60 |
5 |
8.3 |
Therefore, Garissa-township (n = 50), 4 samples (8.0%), were positive while 46 samples (92.0%) were tested negative. from Dhadhab District (n= 50) five (5) samples for (10.0%) tested positive forty-five (45) samples for (90.0%) were negative tested which were higher positive and balambale also had six (6) samples (10.0%) were tested positive. Meanwhile, (54) fifty-four other samples of (90.0%) were tested negative by using competitive enzyme-linked immunosorbent assay (c-ELISA) were considered the strongly positive.
The unit value of competitive enzyme-linked immunosorbent assay (c-ELISA) obtained also indicated the level of antigen from different samples tested according to (Wanjohi et al., 2012).
Double gel diffusion test (DGD-T)
The agar gel immunodiffusion test (AGIDT) test has been used mainly by its high several authors that have reported its special ability to differentiate between S-19 vaccinated and naturally infected animals, when using soluble antigens. The test was performed following previous recommendations. Briefly, the gel was prepared in a 10% NaCl, 0.1 of HCl pH 7.2 buffer with 0.7% agarose. Polystyrene 100 mm diameter Petri dishes were filled with 9 ml of the agarose preparation and 3 mm wells punched in a circular pattern were filled with test and control sera, while in a central well the antigen was placed. Readings to detect precipitation lines were done at 24, 48 and 72 hours. A total of one hundred sixty (160) samples of the camel serum from selected slaughterhouses in Garissa sub-counties were tested using the Double Gel Diffusion Test (DGD-T). Eleven (11) samples were tested positive for (6.80%), while one forty-nine 149 negative tested. From Garissa-township District (n = 50), 2 samples for (4.0%), were positive while 48 samples for (28.75%) tested negative. from Dadab District (n = 50) 3 samples for (6.0%) which were positive and Balambale was higher positive with the 6 samples (10.0%) were tested positive and 54 tested negatives. therefore, the bellow figure transforming the different test that used by double gel immuno-diffusion test results from selected slaughtered camel in Garissa County of Kenya.
When sensitivities of the 4 serological tests were compared using by Chi square goodness of fit test, there was no significant difference between them, with respect to picking of positive cases (p was = 0.0999). Then gives the comparative results (percent) for the 4 serological tests, with respect to the study areas. However, the comparison of the four (4) tests, there was no statistically significant difference between the tests.
Table 5: Statistical significant of a Comparative results (percent) for the 4 serological tests, with respect to the study areas.
|
Tests |
Township (n=50) |
Dadaab (n=50) |
Balambale (n=60) |
Total No (n=160) |
|
RBPT |
4(8%) |
6(%) |
5(8.3%) |
15(9.3%) |
|
SAT |
4(8%) |
6(12%) |
6(10%) |
16(10%) |
|
c-ELISA |
4(8%) |
5(10%) |
6(10%) |
15(9.3%) |
|
AGID |
2(4%) |
3(6%) |
6(10%) |
11(6.8%) |
|
Average |
4(8%) |
5(10%) |
6(10%) |
14(8.75%) |
Cross-sectional study results
In total of one hundred and sixty (160) camels were inspected to examine for gross pathological lesions and histological lesions. Fifty (31%) were slaughtered at Garissa-township, fifty (31%) at dadaab and sixty (37%) at Balambale slaughterhouses.
Numbers of organ condemned
Of the160 camels that were inspected and examined, 78 (48.75%) of them had at least one pathological condition, 55 (70.5%) had one type of condition and 19(24.4%) had more than pathological lesions, while 38(48.7%) had no organ condemnation. At Garissa-township slaughterhouse, of 50 camels slaughtered 35(70%) had one (1) pathological lesion, (14%) had more than one (1) pathological conditions, while the other 8(16%) had no organ condemnation. At dadaab 50 camel were slaughtered, 30(60%) had one (1) pathological lesions, 10(20%) had same pathological conditions while other 10(20%) had no organ condemnation. At Balambale slaughterhouses, of 60 camels were slaughtered 40(66.6%) had no organ condemnation, 15 (25%) had more than one pathological lesions while others 5 (8.3%) had one same pathological lesions at the slaughter indicated Table 6.
Table 6: Number of pathological lesions of condemned organs, non-condemned and their %percentage proportion at the three study slaughterhouses.
|
Numbers of organs |
Slaughterhouses |
Total (%) |
||
|
Garissa township |
Dadaab |
Balambale |
||
|
Number of 1 pathology |
35 |
18 |
25 |
78(48) |
|
Numbers with 1 condition |
20 |
13 |
22 |
55(70) |
|
Condemnation numbers |
7 |
6 |
7 |
19(24) |
|
No. condemnation numbers |
16 |
8 |
14 |
38(48) |
|
Total inspected |
50 |
50 |
60 |
160 |
Organs of condemnation
Among the One hundred and sixty (160) slaughtered camels, 78 (48.7%) were lymph nodes. For Garissa-township 48(61.5%), at dadaab 18(23%) had condemned as whole, and Balambale 12(15.3%) were partially condemned 28(17.5%) of livers were condemned. For Garissa-township slaughterhouses 12(42.8%) had condemned as whole, at dadaab slaughterhouses 9(32.2%) were condemned as partially while Balambale slaughterhouses 7(25%) were condemned as whole 18(11.2%) of lung had also condemned at Garissa-township, 5(27.7%) has condemned as whole, at dadaab slaughterhouses 7(38.8%) had condemned as whole, while At Balambale slaughterhouses 3(16.6%) were condemned partially 20 (12.5%) kidney Garissa-township 9(45%), 6(30%) at dadaab and 5(25%) at Balambale slaughterhouses were condemned as whole 16(10%) heart muscle, 4(25%) at Township, 5(31.2%) at dadaab slaughterhouses and 7(43.7%) at Balambale slaughterhouses were condemned as partially Table 7.
Table 7: Types of condemned organs and their % percentage proportion at the three different study site slaughterhouses.
|
Organs |
Township SL |
Dadaab SL |
Balambale SL |
Total |
|
Lymph node |
48(61.5%) |
18(23%) |
12(15.3%) |
78 |
|
Liver |
12(42.8%) |
9(32.2%) |
7(25%) |
28 |
|
Lung |
6(33.3%) |
7(38.8%) |
5(27.7%) |
18 |
|
Heart muscle |
4(25%) |
5(31.2%) |
7(43.7%) |
16 |
|
Kidney |
9(45%) |
6(30%) |
5(25%) |
20 |
|
Total of inspected |
79 |
45 |
36 |
160 |
Clinical, gross and histopathology study results
A total of 160 samples were collected and examined during the period of study for laboratory analysis both gross and histopathology. According to the ante-mortem record, clinical manifestation was lameness 48(30.0%), swollen of lymph node 39(24.0%) (Figure 1), Orchitis 6(3.70%), infertility 7(4.3%), Abortion 8(5.0%), Abdominal pain 7(4.3%), decreased milk yield 7(4.3%), inflammation of testicles 6(3.7%), epididymitis 6(3.7%), Anorexia 7(4.3%), in appetence 7(4.3%), infection of urogenital 7(4.3%), and placental infection 6(3.7%). For more clinical manifestation that based slaughtered camel and examined animals.
For the gross condemned lesions encountered had include: fibrin depositions 7(4.3%), enlargement of lung 6(3.7%), pericarditis 38(23.7%), and hepatomegaly with nodular liver lesions 79(49.3%), enteritis 5(3.1%), haemorrhages 6(3.7%), congestion 8(5.0%), of visceral organs (lung and kidney) and abscess of lymph nodes 3(1.8%). the other gross pathological lesions based on slaughterhouses in Garissa County
The histopathology in counted were included cellular infiltrations 15 (6.2%), hypoplasia 3 (1.8%), collapse of alveoli 7 (4.3%), oedema 4 (2.5%), congestions 6 (3.7%), fatty degeneration 5 (3.1%), haemorrhages 9 (5.6%), immunoblastic infiltrations 8 (5.0%), increase in number of lymphocytes 9 (5.6%), pneumonia 10 (6.2%), lymphoblastic infiltrations 8 (5.0%),
Table 8: Pathological changes of condemned organs with the respect to Brucella sero-reactants in slaughtered camels in Garissa County.
|
Camel No. |
Cond.organs |
Clini. Signs |
Gross Lesions |
Histopathology |
|
SC-GT-12 |
Lymph node |
Swollen |
Enlargement and abscess |
Cellular infiltration |
|
Stomach |
loss of appetite |
Discoloration |
Haemorrhages |
|
|
SC-GT-14 |
Lung |
Lameness |
Change in colure , white spots |
collapse of alveoli, pinkish fluid materials with the alveoli (Oedema) |
|
SC-GT-24 |
Liver |
Lameness |
Distended |
Fatty degeneration |
|
Liver |
Placental infection |
Thickened of bile duct |
Diffuse of fatty infiltrations |
|
|
SC-GT-29 |
Lymph node |
Swollen of lymph nodes |
Swollen |
immunoblastic infiltration |
|
SC-GT-30 |
Heart |
Anorexia |
fibrins and haemorrhages |
destructions of fibrins |
|
SC-DA-64 |
Lung |
In appetence |
Discoloration |
Pneumonia |
|
SC-DA-69 |
Heart |
Placental infection |
Congested |
Lymphoblastic infiltrations |
|
SC-DA-70 |
Kidney |
Epididymitis |
Congested |
Congestion and haemorrhages |
|
SC-BA-120 |
Heart |
Infertility |
Fibrins |
Slightly destruction of fibrins |
|
SC-BA-132 |
Lung |
Abdominal pain |
Congested |
Polymorph-nuclei in Alveoli |
|
SC-BA-143 |
Kidney |
Abortion |
||
|
SC-BA-144 |
Lymph node |
Swollen of lymph nodes |
Enlarged in some areas |
Increase number of lymphocytes |
|
SC-BA-150 |
Heart |
Infection of urogenital |
Haemorrhages |
Macrophages and neutrophil infiltrations |
|
SC-BA-155 |
Liver |
Anoxia |
Hepatomegaly |
Oedematous mononuclear cells |
|
SC-BA-158 |
Kidney |
Abortion |
Congested |
Congested and haemorrhages |
fibrosis 7 (4.3%), macrophages and neutrophils 10 (6.2%), inflammatory cells 9 (5.6%), cellular injuries 8 (5.0%), accumulated of blood cells 8 (5.0%), compensatory Emphysema 9 (5.6%), increase in number of hepatocyte cells 10 (6.2%), inflammatory lesions in skin 8 (5.0%), and also recorded necrosis in myocardium 7 (4.3%). Therefore, the pathological changes of sero-reactants in condemned organs Table 8, with the respect of Brucellosis in slaughtered camel in Garissa County. For more pathological changes.
Gross morphology and histopathology appearances for the positive condemned organs lymph node
The examination of lymph node tissue from slaughtered camel in Garissa-Township whose sera was tested positive to the brucellosis had immunoblastic infiltrations, hypoplasia (meaning that underdevelopment or incomplete development of a tissue or organ), and few mature lymphocytes and increase number of lymphocytic cells were observed in lymph nodes for grossly and histopathology features. The lymph node of sampled from negative camel ten (10) and twelve (12) had similar lesions.
Lung tissue
The lung tissue from positive camel with brucellosis had verified collapse of alveoli, pinkish fluid materials with the alveoli (Oedema), Pneumonia, infiltrations of polymorphonuclear cells (is a type of immune cell that has granules (small particles) with enzymes that are released during infections, allergic reactions, and also asthma) and neutrophils in bronchioles for grossly and histopathology photomicrography However, the lung had thickened alveolar walls with interstitial mononuclear infiltrate, congestion in some areas and haemorrhages. There was negative sampled lung tissue of camel five and nine showed similar lesions.
Heart muscle
The heart tissue from seropositive camel of brucellosis had heavy fatty degeneration, lymphoblastic infiltrations in cardiac muscles, slightly destructions of fibrins in cardiac, in some areas there was macrophages and neutrophilic infiltrations for grossly and histopathology photomicrography and also
inflammatory cells are more in heart section. However, in a sampled sero-negatives camels eight (8) and Nine (9) had similar pathological lesions.
Liver
The liver tissue from seropositive camel of brucellosis was viewed fatty degeneration, Neutrophils, liver injuries, diffuse fatty infiltration (meaning that accumulation of excess fat in the liver) mononuclear cells, lymphoblastic cellular of infiltration and congestions in some areas for gross morphology of condemnation and features of histopathology photomicrography slight blockage of vessels and macrophages in live and enlargement of hepatocytes. Sampled liver with sero-negatives camel six and seven had similar pathological lesions.
Kidney
The kidney tissue from seroposive camel brucellosis were shown diifernt pathological lesions lymphoblastic cells of infiltration,congestions and hemorhages in some arreas for gross and histopathology photomicrography and also there was inflamatory cells with the perodominantly hetrophils in kidneys. The other kidney sampled of seronegetive from number thirteen and seven had simillar pathological leisions.
Results and Discussion
This study in Garissa camel slaughterhouses providing that a valuable occasion for producing with the camels’ prevalence data and pathological lesions that could be related with human health awareness. To determine the sero-prevalence of camel brucellosis, Four (4) different serological survey was conducted in camel slaughterhouses including the examination of blood specimens from different camel slaughterhouses in Garissa County and used, Rose Bengal Plate Test (RBPT), Serum Agglutination Test (SAT), Competitive Enzyme Linked Immune Sorbent Assay (c-ELISA) and Double Ager Gel Immunodiffusion Test (DAGID-T).
Sero-prevalence estimated that around 10% of 160/15 tested samples similar to (Alhaji et al., 2016). Understanding to the pathology and occurrences of Brucellosis in camel are important in veterinary problems that relating to the production losses and abortion. Therefore, the zoonotic diseases means that are also significant for medical professions to know about the in livestock. To establish the pathological lesions of apparent-Brucellosis in slaughtered camel, followed by the examination of sero-positive and serf-negative tested in serology for grossly and histologically.
Prospective study: Sero-prevalence
A total of one hundred and sixty (160) camel serum samples from Garissa slaughterhouses were confirmed by using Rose Bangle Plate Test (RBPT). Fifteen (15) samples (10%) tested positive. From Garissa-township (n=50) four (4) samples (8.0%) were tested positive, fifty (n=50) samples from dadaab slaughterhouses six (6) (12.0%) were tested positive while sixty (n=60) samples from Balambale slaughterhouses five (5) (8.3%) were tested positive. The one hundred and sixty (160) camel serum samples from Garissa County slaughterhouse were also tested by using serum agglutination test (SAT). Sixteen (16) samples (10.50%) were tested positive. From Garissa-township four (4) samples (8.0%) were tested positive. From dadaab six (6) samples (12.0%) tested positive. From Balambale six (6) samples (10.0%) were tested positive. For the 16 positive samples 10 samples had a titre of 1/10, 3 samples a titre of 1/20, 2 samples attire of 1/40, and 2 sample had a titre of 1/80 and 3 samples had a titre of 1/160. Similarly reported prevalence (Wanjohi et al., 2012).
The one hundred and sixty (160) had also tested using by Competitive Enzyme Linked Immune Sorbent Assay (c-ELISA). Fifteen (15) samples (9.3%) were tested positive. From Garissa-Township central of Garissa County (n=50), four (4) samples were tested positive. From dadaab (n=50), five (5) samples had tested positive. For Balambale (n=60), six (6) samples were tested positive. As reported similarly (Njeru, et al. 2016). The one hundred and sixty (160) camel serum samples from Garissa slaughterhouses were also tested by using AGID Test. eleven (11) samples (6.8%) were tested positive. From Garissa-Township (n=50), two (2) sera samples (4.0%) were tested positive. From dadaab (n=50), three (3) sera samples (6.0%), were tested positive. And from Balambale (n=60), six (6) sera samples had tested positive by using chi-square (χ2), there was no statistical difference in sensitivity between the four serological tests (p=0.999).
The present study for sero-prevalence result findings (10%) is similar to the previous reports from the different countries (Wanjohi et al., 2012). However, there was lower than some studies in Somalia, Tanzania, Ethiopia, Nigeria, Saudi Arabia and Yemen. Ser-prevalence was differed from other findings in neighbouring countries of Kenya (in the Afar region of Northeast Ethiopia. Individually, in the lower sero-prevalence in this study is not consistence with the other prevalence findings which showed that the infection is more prevalence among nomadic slaughterhouses in Garissa County Kenya. The sero-prevalence of Brucellosis in camel was lower in extensively kept pastoralists of camel in Garissa-township and dadaab slaughterhouses, while on the other hand had been reported in intensively kept pastoralists of camel was higher in Balambale slaughterhouses. Thus, several factors may affect increasing result of serological outcomes such as production system, overcrowding of restricted area, contacts between the animals, immune suppressive effective of trypanosomiasis that often prevalence in camel and cross-reacting bacteria of E-coli, Salmonella and Yersinia and uses of lower specificity tests. These factors have potential effects for serological findings. The sample sections and sampling for different animals may also be affect higher prevalence for the serology study. The higher prevalence of brucellosis represents the major challenges of both economics and public health problems. It is prospective that there is higher frequency of abortion/reproductive failures that may lead to the potential higher level of exposures of livestock owners and their families. It was very important to know that the RBPT is good diagnostic sensitivity compared to the other there (3) serological testes that have been done to the survey. So that, the RBPT is satisfactory screening test the test procedure for diagnosis of bovine brucellosis to be applied for camel brucellosis. Though camels are not known to be the host of Brucella organism, but it is well known to be susceptible for Brucella abortus and Brucella melitensis. Therefore, the disease is still remaining wildlife and domestic animals from the sources of human infection through, direct contacts and contamination of environment during parturition and abortion. Although, the infection in camel has been reported in Saudi Arabia, Sudan, Kenya, and Tanzania, Ethiopia and Somalia and other countries in the world. Generally, to control of the disease both animals and manes we need to keep the following: (1) improvising the hygiene (to reduce the direct contacts between infected and non-infected animals), (2) public awareness (to control and prevent the infection) and (3) proper disposal (to be disposed the effected foetus, tissues, discharges and poste-mortem equipment and to infect the contemned utensils).
Therefore, this study has been confirmed the presence of brucellosis in Garissa slaughterhouses of Kenya showing that the significant of sero-prevalence of (10% tested with RBPT, SAT c-ELISA and AGID). Further studies are more needed to improve the production of camel and diminish the risk transmissions of the infection to the human especially benchers. There is also needed control program for brucellosis in camel slaughterhouses and other animals. Standard biosecurity measures at slaughterhouses and farms be enhanced to control and prevent of Brucella infection to animals and human.
Cross-sectional study: Pathological lesions
The cross-sectional study 48% of slaughtered camel had one or more contaminations of organ at Garissa-township, dadab and Balambale slaughterhouses. Up on the histological features the main cause of contamination apart from Brucellosis were: Circulatory disturbance, inflammatory conditions.
The clinical manifestation of the slaughtered camel embraced swollen of lymph nodes (24%), sever lameness (30%) and abortion (5%). In the swollen of lymph nodes of the effected and non-effected of Brucellosis in slaughtered camels were enlarged and abscess that attributed to obstruction and discolorations of fluid. Microscopically, due to the miss stained of the slide some area appeared disorganized, cellular infiltrations, mononuclear inflammatory cells, immunoblastic infiltrations, increase number of lymphocytes and hypoplasia meaning that (underdevelopment or incomplete development of a tissue or organ). These lesions there was similar study in camel lymph nodes that come across in Sudan (Aljameel et al., 2013) and in Yemen (Hamza et al., 2017).
There is also liver of three (1.8%) obtained from the positive tested Brucellosis which had clinical manifestation of lameness at the anti-mortem record. Histopathologically, Fatty degeneration, Diffuse of fatty infiltrations, fibrosis, hepatocyte denegation and in some area necrosis. Similarly, to in Iran (Khaniki et al., 2013) and in Saudi Arabia (Mohamed, 2013). The adjusted area of the liver there was injury, congestion convoyed to inflammatory infiltrated cells and hepatocyte degenerations in some area. These grossly and histopathology findings generally decides with the study of Khaniki et al. (2013).
The lung of two (1.2%) from positive slaughtered camel was rejected at the slaughterhouses in dadab due to the enlargement, discoloration, white and red spots and fluid filed with cyst from the surface. Microscopically, collapse of alveoli, pinkish fluid materials with the alveoli (Oedema), mononuclear infiltrations of cells, slight blockage of vessels and macrophages, enlargement of hepatocyte cells. The adjacent area of bronchioles were congested a accompanied by slightly inflammatory cells. Similarly, and also, there a study in Saudi Arabia (Gameel and Yassein, 2010).
A heart from 4(2.5%) of tested positive Brucellosis that rejected to supply the slaughterhouses were condemned fibrins and haemorrhages. These gross conditions were also met at the slaughterhouse. Histopathological examinations, fibrins and haemorrhages, destructions of fibrins, lymphoblastic infiltrations, Macrophages and neutrophil infiltrations, fatty degenerations and inflammatory cells were found. Similar study has been done in Tanzania (Tembo and Neonga, 2015) and in Bangladesh (Mazumder et al., 2012).
Kidney of 2 (1.2%) from camel slaughtered were also condemned during the poste-mortem inspection as they were discoloured and congested and haemorrhages with white-dark-red under the renal cortex microscopically, conformed that the presence of inflammation in cells, infiltration, macrophages, haemorrhages and congestion. Previous studies had also done from lung tissue of slaughtered camel in Athi River, Kenya (Mutua et al., 2017).
The lung from 2(1.2%) slaughtered camel obtained from sero-negative sample that were also condemned during the poste-mortem inspection with the red-dark coloured under pleural cavity. These conditions were also come across at the slaughter. Histological examination, confirmed that presence of erythrocytes and pinkish materials in bronchi and bronchioles and there were symptoms indicating inflammation in the slaughtered camel lung. Similar study has done in Ethiopia with the absence of inflammatory cells in lung (Mamo et al., 2011).
Finally, there is correlation between the gross pathology and microscopically examination.
Conclusions and Recommendations
The sero-positivty demonstrated by the camel brucellosis brought-in for slaughter was about 10% this indicates that the disease is enzootic in the area, though the figure is lower than what has been reported in other areas of the county. The infection has both economic and public health importance; it is zoonotic.
- Camels from Balambale slaughterhouses showed higher sero-positivity, using by the four (4) different serological test.
- Camels that were sero-positive also had clinical and pathological lesions similar to the those brucellosis.
- The despite of the prevalence of the disease been below referred to other results to the county, but the disease is still posing a public health problem economic loss to the slaughterhouses in the county because of its zoonotic nature and its clinical manifestations that being similar to other disease.
- There was correlation of the positive tested animal, clinical and pathological lesion that observed in the different test of the study.
- There were some health canters in the county that carry out limits test of brucellosis in camel slaughterhouses but faces the shortage of test reagents and to record is inconsistence and unpredictable to this work.
- There is lack of awareness on risk factors of the infection into the slaughterhouse’s owners because of the methods of prevention and control strategies. Therefore, the risk factors are significant in the spread of the disease.
- There was a number of condemned organs due to the infectious and non-infectious that contributed by the sanitation levels of poor slaughtering of the animals such liver, lung and other visceral organs which is good for human consumption. And it was also taking parts to the economic losses of slaughterhouses in the county.
Acknowledgments
I would like to acknowledge university of Nairobi Faculty of Veterinary Medicine (FVM) Department of Veterinary Pathology, Microbiology and parasitology (VPMP) and My lovely Mom Aisha Abdurrahman Mohamed May Allah give her health, my father Dahir Barre that has pasted 2006, I wish Allah give Jana, My Mom Marian Sheikh Doon and my Lovely Mom Ruqiya Issa Ahmed Ulusow with her family, and also My second Father Moalim Ahmed Moalim Abdulla.
Author’s Contribution
Manually they have contribute all authors and some of them we have used Google scholar
Conflict of interest
The authors have declared no conflict of interest.
References
Abebe, G., Worku, Y., Mamo, G., and Nazir, S., 2017. Sero-prevalence and associated risk factors of brucellosis in camel at Akaki Abattoir, Central Ethiopia. J. Anim. Res., 7(4): 617-622. https://doi.org/10.5958/2277-940X.2017.00094.8
Abo-Elnaga, T.R., and Osman, W.A., 2012. Detection of pathogens of condemned organs of one humped camels (Camelus dromedarius) slaughtered in Matrouh abattoirs, Egypt. Glob. Vet., 9(3): 290-296.
Aichouni, A., Jeblawi, R., Dellal, A., Hammou, H., and Aggad, H., 2010. Breed variation in blood constituents of the one-humped camel (Camelus dromedarius) in Algeria. J. Camelid Sci., 3: 19-25.
Al-Garadi, M.A., Al-Hothi, A., and Al-Sharma, A., 2015. Bacteriological and serological study on brucellosis infection in camel (Camelus dromedaries), Al-Hodeida governorate, Yemen. Int. J. Adv. Res., 3(1): 786-791.
Alhaji, N.B., Wungak, Y.S., and Bertu, W.J., 2016. Serological survey of Camel brucellosis in Fulani nomadic breeds (Bos indicus) of North-central Nigeria: Potential risk factors and zoonotic implications. Acta Trop., 153: 28-35. https://doi.org/10.1016/j.actatropica.2015.10.003
Ali, S., Ali, Q., Abatih, E. N., Ullah, N., Muhammad, A., Khan, I., and Akhter, S., 2013. Sero-prevalence of Brucella abortus and mellitus among camel in Pothohar Plateau, Pakistan. Pak. J. Zool., 45(4): 1041-1046.
Aljameel, M.A., Halima, M.O., El-Eragi, A.M.S., El-Tigani-Asil, A.E., and Hamaad, H., 2013. Studies in abscesses lymphoid tissue of camels (Camels dromedaries) slaughtered in Nyala abattoir, Sudan. U. K. J. Vet. Med. Anim. Prod., 4(2): 39–52.
Aljameel, M.A., Halima, M.O., ElTigani-Asil, A.E., Abdalla, A.S., and Abdellatif, M.M., 2014. Liver abscesses in dromedary camels: Pathological characteristics and aerobic bacterial aetiology. Open Vet. J., 4(2): 118-123. https://doi.org/10.5455/OVJ.2014.v4.i2.p118
Assenga, J.A., Matemba, L.E., Muller, S.K., Malakalinga, J.J., and Kazwala, R.R., 2015. Epidemiology of Brucella infection in the human, livestock and wildlife interface in the Katavi-Rukwa ecosystem, Tanzania. BMC Vet. Res., 11(1): 189. https://doi.org/10.1186/s12917-015-0504-8
Babeker, E.A., Elmansoury, Y.H.A., and Suleem, A.E., 2013. The influence of season on blood constituents of dromedary camel (Camelus dromedaries). Online J. Anim. Feed Res., 3(1): 1-8.
Bankole, A.A., Berkvens, D., Geerts, S., Ieven, M., and Saegerman, C., 2011. Risk assessment of human brucellosis infection from consumption of raw camel milk sold by vendors in The Gambia. Anani Adeniran Bamkole, 10(50): 165.
Calderón, A., Tique, V., Ensuncho, C.F., and Rodriguez, V., 2010. Seroprevalence of Brucella abortus in water buffaloes (Bubalus bubalis) in Cordoba. Revista UDCA Actualidad and Divulgación Científica, 13(2): 125-132. https://doi.org/10.31910/rudca.v13.n2.2010.740
Chakiso, B., Menkir, S., and Desta, M., 2014. On farm study of camel fasciolosis in lemo district and its economic loss due to liver condemnation at Hossana Municipal abattoir, Southern Ethiopia. Int. J. Curr. Microbiol. Appl. Sci., 3(4): 1122-1132.
Chauhan, H.C., Patel, K.B., Patel, S.I., Patel, B.K., Chandel, B.S., Bhagat, A.G., and Shome, R., 2017. Serological survey of brucellosis in camel of Gujarat. Int. J. Curr. Microbiol. Appl. Sci., 6(4): 1815-1821. https://doi.org/10.20546/ijcmas.2017.604.217
Devrajani, K., Abubakar, M., Fazlani, A.S., Shahid, F., Ourban, A.S., and Imran, R., 2010. Occurrence and prevalence of bacterial species as identified from camel wound. Inter. J. Agro Vet. Med. Sci., 4(4): 96-104.
Ducrotoy, M.J., Bertu, W.J., Ocholi, R.A., Gusi, A.M., Bryssinckx, W., Welburn, S., and Moriyon, I., 2014. Brucellosis as an emerging threat in developing economies: lessons from Nigeria. PLoS Negl. Trop. Dis., 8(7): 30-08. https://doi.org/10.1371/journal.pntd.0003008
Ducrotoy, M.J., Conde-Alvarez, R., Blasco, J.M., and Moriyon, I., 2016. A review of the basis of the immunological diagnosis of ruminant brucellosis. Vet. Immunol. Immunopathol., 1(171): 81-102. https://doi.org/10.1016/j.vetimm.2016.02.002
Ducrotoy, M.J., Muñoz, P.M., Conde-Álvarez, R., Blasco, J.M., and Moriyón, I., 2018. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prevent. Vet. Med., 2(151): 57-72. https://doi.org/10.1016/j.prevetmed.2018.01.005
Dunlop, J.A., Bird, T.L., Brookhart, J.O., and Bechly, G., 2015. A camel spider from Cretaceous Burmese amber. Cretaceous Res., 1(56): 265-273. https://doi.org/10.1016/j.cretres.2015.05.003
El-Bahrawy, K.A., Khalifa, M.A., and Rateb, S.A., 2015. Recent advances in dromedary camel reproduction: An Egyptian field experience. Emir. J. Food Agric., 1(2): 350-354. https://doi.org/10.9755/ejfa.v27i4.19907
Ernest, E., Nonga, H.E., Kynsieri, N., and Cleaveland, S., 2010. A retrospective survey of human hydatidosis based on hospital records during the period 1990–2003 in Ngorongoro, Tanzania. Zoon. Publ. Health, 57(7-8): e124-e129. https://doi.org/10.1111/j.1863-2378.2009.01297.x
Faye, B., Chaibou, M., and Vias, G., 2012. Integrated impact of climate change and socioeconomic development on the evolution of camel farming systems. Br. J. Environ. Clim. Change, 2(3): 227-244. https://doi.org/10.9734/BJECC/2012/1548
Fromsa, A., and Jobre, Y., 2011. Infection prevalence of hydatidosis (Echinococcus granulosus, Batsch, 1786) in domestic animals in Ethiopia: A synthesis report of previous surveys. Ethiop. Vet. J., 15(2). https://doi.org/10.4314/evj.v15i2.67691
Gameel, A.A., and Yassein, N., 2010. Primary bronchiole-alveolar adenocarcinoma of lung condemned in a dromedary camel (Camelus dromedarius). Vet. Rec., 142: 252-252. https://doi.org/10.1136/vr.142.10.252
Garcell, H.G., Garcia, E.G., Pueyo, P.V., Martín, I.R., Arias, A.V., and Serrano, R.N.A., 2016. Outbreaks of brucellosis related to the consumption of unpasteurized camel milk. J. infect. Publ. Health, 9(4): 523-527. https://doi.org/10.1016/j.jiph.2015.12.006
Gyuranecz, M., Wernery, U., Kreizinger, Z., Juhász, J., Felde, O., and Nagy, P., 2016. Genotyping of Brucella melitensis strains from dromedary camels (Camelus dromedarius) from the United Arab Emirates with multiple-locus variable-number tandem repeat analysis. Vet. Microbiol., 1(186): 8-12. https://doi.org/10.1016/j.vetmic.2016.02.009
Hadush, A., and Pal, M., 2013. Brucellosis-An infectious re-emerging bacterial zoonosis of global importance. Int. J. Livest. Res., 3(1): 28-34. https://doi.org/10.5455/ijlr.20130305064802
Hamza, I.I., Shuaib, Y.A., Suliman, S.E., and Abdalla, M.A., 2017. Aerobic bacteria isolated from internal lesions of camels at Tambool slaughterhouse. J. Adv. Vet. Anim. Res., 4(1): 22-31. https://doi.org/10.5455/javar.2017.d185
Hassan-Kadle, A.A., 2015. A review on ruminant and human brucellosis in Somalia. Open J. Vet. Med., 5(06): 133. https://doi.org/10.4236/ojvm.2015.56018
Henning, L.N., Miller, S.M., Pak, D.H., Lindsay, A., Fisher, D.A., Barnewall, R.E., and Warren, R.L., 2012. Pathophysiology of the rhesus macaque model for inhalational brucellosis. Infect. Immune., 80(1): 298-310. https://doi.org/10.1128/IAI.05878-11
Hewitson, T.D., and Darby, I.A., 2010. Histology protocols (p. 229). New York, USA: Humana Press. 8(1): 20-29
Hughes, K., Archer, J., Constantino-Casas, F., Wozniakowski, G.J., and Baigent, S., 2016. Diagnostic investigation of Marek’s disease in a turkey. Vet. Rec. Case Rep., 4(1): e000291. https://doi.org/10.1136/vetreccr-2016-000291
Jad, Y.E., Khattab, S.N., Beatriz, G., Govender, T., Kruger, H.G., El-Faham, A., and Albericio, F., 2014. Oxyma-B, an excellent racemization suppressor for peptide synthesis. Organ. Biomol. Chem., 12(42): 8379-8385. https://doi.org/10.1039/C4OB01612B
Kagunyu, A.W., and Wanjohi, J., 2014. Camel rearing replacing cattle production among the Borana community in Isiolo County of Northern Kenya, as climate variability bites. Pastoralism, 4(1): 13. https://doi.org/10.1186/s13570-014-0013-6
Kaindi, D.W.M., Schelling, E., Wangoh, J., Imungi, J.K., Farah, Z., and Meile, L., 2011. Microbiological quality of raw camel milk across the Kenyan market chain. Book, 5(2): 79-83.
Kant, N., Kulshreshtha, P., Singh, R., Mal, A., Dwivedi, A., Ahuja, R., and Kumar, S., 2018. A study to identify the practices of the buffalo keepers which inadvertently lead to the spread of brucellosis in Delhi. BMC Vet. Res., 14(1): 329. https://doi.org/10.1186/s12917-018-1670-2
Kaskous, S., 2016. Importance of camel milk for human health. Emir. J. Food Agric., 2(3): 158-163. https://doi.org/10.9755/ejfa.2015-05-296
Keskes, S., Mechemeria, M., Tessema, T.S., Regassa, F., Adugna, W., and Dawo, F., 2013. Reproductive performance of Camelus dromedarius kept under Afar pastoral management system using progeny history testing. J. Camelid Sci., 4(6): 100-115.
Khamesipour, F., Rahimi, E., Shakerian, A., Doosti, A., and Momtaz, H., 2014. Molecular study of the prevalence of Brucella abortus and Brucella melitensis in the blood and lymph node samples of slaughtered camels by polymerase chain reaction (PCR) in Iran. Acta Vet., 64(2): 245-256. https://doi.org/10.2478/acve-2014-0023
Khaniki, G.R.J., Kia, E.B., and Raei, M., 2013. Liver condemnation and economic losses due to parasitic infections in slaughtered animals in Iran. J. Parasit. Dis., 37(2): 240-244. https://doi.org/10.1007/s12639-012-0172-6
Kumar, P., 2013. A clinical study on surgical affections of head and neck region of camels (Camelus dromedarius) (Doctoral dissertation, Rajasthan University of Veterinary and Animal Sciences, Bikaner-334001).
Liu, L., Benyeda, Z., Zohari, S., Yacoub, A., Isaksson, M., Leijon, M. and Belak, S., 2016. Assessment of preparation of samples under the field conditions and a portable real-time RBT- and ELISA assay for the rapid on-site detection of Brucellosis. 63(2): e245-e250. https://doi.org/10.1111/tbed.12261
Madboly, A.A., Ghazi, Y.A., Sokkar, S.M., Desouky, H.M., and Ahmed, Y.F., 2014. Serological, bacteriological, molecular and immunohistochemical techniques for diagnosis of brucellosis in buffaloes. Middle East J. Appl. Sci., 4(3): 762-770.
Madu, G.A., Adama, O.R., James, B.W., Hassan, M., Lubabatu, I., Esther, M., and Gulak, W.H., 2016. Sero-prevalence of camel brucellosis in three abbatoirs of Nothern Nigeria. J. Vet. Med. Anim. Health, 8(3): 15-20. https://doi.org/10.5897/JVMAH2015.0380
Mamo, G., Bayleyegn, G., Tessema, T.S., Legesse, M., Medhin, G., Bjune, G., and Ameni, G., 2011. Pathology of camel tuberculosis and molecular characterization of its causative agents in pastoral regions of Ethiopia. PLoS One, 6(1): e15862. https://doi.org/10.1371/journal.pone.0015862
Manish, K., Chand, P., Rajesh, C., Teena, R., and Sunil, K., 2013. Brucellosis: An updated review of the disease. Indian J. Anim. Sci., 83(1): 3-16.
Mazumder, A.C., Khatun, S., Nooruzzaman, M., Chowdhury, E.H., Das, P.M., and Islam, M.R., 2012. Histological analysis from field outbreaks in camel and other large animals. Bangladesh Vet., 29(2): 41-48. https://doi.org/10.3329/bvet.v29i2.14341
Mirzaei, M., Rezaei, H., Nematollahi, A., and Ashrafihelan, J., 2016. Survey of hydatidosis infection in slaughtered camel (Camelus dromedarius) in Tabriz area, Northwest Iran. J. Parasit. Dis., 40(2): 444-447. https://doi.org/10.1007/s12639-014-0523-6
Mohamed, E.G.S., 2013. Epidemiological study of brucellosis in camels (Camelus dromedarius) in Khartoum State, Sudan. Doc. Dissertation, Sudan Univ. Sci. Technol., 60(1): 33-41.
Muendo, E.N., Mbatha, P.M., Macharia, J., Abdoel, T.H., Janszen, P.V., Pastoor, R., and Smits, H.L., 2012. Infection of cattle in Kenya with Brucella abortus biovar 3 and Brucella melitensis biovar 1 genotypes. Trop. Anim. Health Prod., 44(1): 17-20. https://doi.org/10.1007/s11250-011-9899-9
Mutua, J.M., Gitao, C.G., Bebora, L.C., and Mutua, F.K., 2017. Antimicrobial resistance profiles of bacteria isolated from the nasal cavity of camels in Samburu, Nakuru, and Isiolo Counties of Kenya. J. Vet. Med., 6(4): 103. https://doi.org/10.1155/2017/1216283
Njeru, J., Wareth, G., Melzer, F., Henning, K., Pletz, M.W., Heller, R., and Neubauer, H., 2016. Systematic review of brucellosis in Kenya: Disease frequency in humans and animals and risk factors for human infection. BMC Publ. Health, 16(1): 853. https://doi.org/10.1186/s12889-016-3532-9
Nourani, H., and Salimi, M., 2013. Pathological study on liver of dromedary camels. J. Camel Pract. Res., 20(1): 97-100.
Onono, J.O., Ogara, W.O., Okuthe, S.O., Nduhiu, J.G., Mainga, A.O., and Nduati, D., 2010. Challenges of camel production in Samburu District, Kenya. J. Camelid Sci., 2(8): 1-5.
Patel, A.S., Patel, S.J., Patel, N.R., and Chaudhary, G.V., 2016. Importance of camel milk-An alternative dairy food. J. Livest. Sci., 4(7): 19-25.
Racloz, V., Schelling, E., Chitnis, N., Roth, F., and Zinsstag, J., 2013. Persistence of brucellosis in pastoral systems. OIE Rev. Sci. Tech., 32(1): 61-70. https://doi.org/10.20506/rst.32.1.2186
Salih, M.E.A.S., 2015. Epidemiological of brucellosis camels (Camelus dormedarius) in Alzulfi governorate, Saudi Arabia. Doctoral Dissertation, Sudan Univ. Sci. Technol., 12(2): 126-131.
Scolamacchia, F., Handel, I.G., Fèvre, E.M., Morgan, K.L., Tanya, V.N., and Bronsvoort, B.M.D.C., 2010. Serological patterns of brucellosis, leptospirosis and Q fever in Bos indicus cattle in Cameroon. PLoS One, 5(1): e8623. https://doi.org/10.1371/journal.pone.0008623
Seleem, M.N., Boyle, S.M., and Sriranganathan, N., 2010. Brucellosis: A re-emerging zoonosis. Vet. Microbiol., 140(3-4): 392-398. https://doi.org/10.1016/j.vetmic.2009.06.021
Shome, R., Gupta, V.K., Bhardwaj, B., Shome, B.R., Nagalingam, M., and Rahman, H., 2013. A report of seroprevalence of camel brucellosis in India. J. Camel Pract. Res., 20: 183-186.
Suvarna, S.K., and Layton, C., 2013. Bancroft’s theory and practice of histological techniques. Churchill livingstone. Elsevier. Aughey E, Frye FL. Comparative veterinary histology. Manson Publ., 21(2010): 173-186.
Swai, E.S., Schoonman, L., and Daborn, C., 2010. Knowledge and attitude towards zoonoses among animal health workers and livestock keepers in Arusha and Tanga, Tanzania. Tanzania J. Health Res., 12(4): 272-277. https://doi.org/10.4314/thrb.v12i4.54709
Tembo, W., and Nonga, H.E., 2015. A survey of the causes of camel organs and/or carcass condemnation, financial losses and magnitude of foetal wastage at an abattoir in Dodoma, Tanzania. Onderstepoort J. Vet. Res., 82(1): 1-7. https://doi.org/10.4102/ojvr.v82i1.855
Wanjohi, M., Gitao, C.G., and Bebora, L., 2012. The prevalence of Brucella spp. in camel milk marketed from north Eastern Province, Kenya. Res. Opin. Anim. Vet. Sci., 2(7): 51-56.
Wareth, G., Murugaiyan, J., Khater, D.F., and Moustafa, S.A., 2014. Subclinical pathogenic infection in camels slaughtered in Cairo, Egypt. J. Infect. Dev. Countries, 8(7): 909-913. https://doi.org/10.3855/jidc.4810
Warsame, R., and Grothey, A., 2012. Treatment options for advanced pancreatic cancer: A review. Exp. Rev. Anticancer Ther., 12(10): 1327-1336. https://doi.org/10.1586/era.12.115
Wattam, A.R., Inzana, T.J., Williams, K.P., Mane, S.P., Shukla, M., Almeida, N.F., and Whatmore, A.M., 2012. Comparative genomics of early-diverging Brucella strains reveals a novel lipopolysaccharide biosynthesis pathway. MBio, 3(5): e00246-12. https://doi.org/10.1128/mBio.00246-12
Wernery, U., 2014. Camel brucellosis: A review. Rev. Sci. Tech. (Int. Off. Epizoot.), 33: 839-857. https://doi.org/10.20506/rst.33.3.2322
Yadav, A.K., Kumar, R., Priyadarshini, L., and Singh, J., 2015. Composition and medicinal properties of camel milk: A review. Asian J. Dairy Food Res., 34(2): 83-91. https://doi.org/10.5958/0976-0563.2015.00018.4
To share on other social networks, click on any share button. What are these?





