Clinicopathological Studies on Urine Retention in Barki Rams
Research Article
Clinicopathological Studies on Urine Retention in Barki Rams
Asmaa A Darwish*
Department of Animal Health and Poultry, Animal and Poultry Production Division, Desert Research Center (DRC), Cairo, Egypt.
Abstract | Urine retention is a significant concern in sheep farming due to its economic implications. It may result in fluid and solute imbalance, renal failure, bladder rupture, azotemia, and death. This study aimed to examine the immunological and clinicopathological alterations associated with urine retention in sheep. Twenty Barki rams were apparently-healthy and considered the control group (CG) and twenty Barki rams suffered from urine retention considered the diseased group (DG). Blood samples were collected from both groups and immunological and clinicopathological parameters were estimated and statistically analyzed. Diseased rams displayed a significant (P<0.05) increase in the pro-inflammatory cytokines, acute phase proteins, free radicals, globulin, triglycerides, kidney function tests, and liver enzymes concentrations and a significant (P<0.05) decrease in IL-10, antioxidants, total protein, albumin, glucose, total lipids, cholesterol, minerals, electrolytes, and trace elements concentrations. The DG hemogram clarified a significant (P<0.05) microcytic hypochromic anemia accompanied by neutrophilic leukocytosis and lymphocytopenia, and their iron profile characterized by a significant (P<0.05) hypoferremia, hypotransferrinemia, and hyperferritinemia. After the urethral process amputation, ten rams showed marked improvement and returned healthy (survive group (SG)) and the other ten rams showed a noticeable deterioration and eventually died (dead group (DeG)). The estimated pro-inflammatory cytokines, APPs, and TAC yielded sensitivity and NPV as 100 % in DG (compared to CG) and DeG (compared to SG), but LR suggests TAC and IL-1β as the best markers for urine retention diagnosis and IL-1α, TNF-α, Fb, and ferritin as the best predictors for the urethral process amputation results. The study concluded that urine retention in rams resulted in a strong immune response with subsequent hemato-biochemical changes. TAC and IL-1β are reliable biomarkers for urine retention in sheep while, IL-1α, TNF-α, Fb, and ferritin are better for urethral process resection outcomes prediction.
Keywords | Urine retention, Immunological alterations, Clinicopathological alterations, Urethral process resection, Biomarkers.
Received | June 22, 2023; Accepted | July 20, 2023; Published | September 15, 2023
*Correspondence | Asmaa A Darwish, Department of Animal Health and Poultry, Animal and Poultry Production Division, Desert Research Center (DRC), Cairo, Egypt; Email: [email protected]
Citation | Darwish AA (2023). Clinicopathological studies on urine retention in barki rams. Adv. Anim. Vet. Sci. 11(10): 1673-1680.
DOI | http://dx.doi.org/10.17582/journal.aavs/2023/11.10.1673.1680
ISSN (Online) | 2307-8316

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Urine retention is a serious condition that often occurs due to the presence of stones and sediments in the urinary tract (urolithiasis). It can lead to kidney dysfunction, disturbances in fluid and solute balance, and renal failure. It can also result in bladder rupture and the occurrence of azotemia. Causes of urolithiasis include several factors such as inadequate nutrition, vitamin A deficiency, urinary tract anatomy, excessive estrogen administration, geographic and seasonal influences, hormonal factors, and urinary system infections (Ewoldt et al., 2008, Gazi et al., 2014, Sharun et al., 2021).
Diet plays a significant role in stone formation, with a high concentrate and low fiber diet inducing an imbalance in the calcium and phosphorus ratio. This imbalance, particularly low calcium and high phosphorus levels contributes to the formation of uroliths. Ideally, the calcium-to-phosphorus ratio should be 1:1 to 2:1, but it often ranges from 1:4 to 1:6, leading to increased phosphorus excretion in the urine. Clinical signs of urolithiasis vary depending on the location and duration of the obstruction, including abdominal pain, anorexia, muscle weakness, apathy, anuria, and dysuria (Makhdoomi and Gazi, 2013, Santarosa et al., 2021).
Obstructions are more common in males due to the narrower and longer penile urethra, which contains structures like the sigmoid flexure and vermiform appendix that increase the risk of obstructions. Early castration of young animals reduces the urethral diameter, thus increasing the risk of obstruction (Gazi et al., 2014, Sharun et al., 2021).
Understanding the systemic effects of urine retention is crucial to prevent the worsening of the condition and determining the stone’s location for surgical intervention. Once clinical signs appear, reversal becomes difficult, and surgical treatment may render animals unfit for reproduction. Therefore, assessing the hemato-biochemical profile of animals with urine retention is important. Recent research suggests the use of immunological biomarkers such as pro-inflammatory cytokines, acute phase proteins (APPs), and total antioxidant capacity (TAC) as sensitive diagnostic and prognostic tools for various animal diseases. However, limited studies have investigated these markers in urine retention in sheep (El-Deeb and Elmoslemany, 2016; Maciel et al., 2019).
Hence, this study aims to investigate the immunological and clinicopathological changes associated with urine retention in Barki rams, with a specific focus on the diagnostic and predictive value of pro-inflammatory cytokines, APPs, and TAC for urine retention and urethral process amputation outcomes respectively.
Materials and methods
Animals
According to the ethical approval No. 2, January 2023 of the animal and poultry health department, animal and poultry production division, Desert Research Center (DRC), Cairo, Egypt, this study was conducted, after the owners` agreements, on 40 Barki rams aged 3-4 years, randomly collected from different cities of Matrouh governorate (31.35 °N 27.23°E), Egypt. The owners depend on concentrates (14-16% protein) and grazing for the rams feeding. The rams were divided into two groups as follows:
Control group (CG): 20 apparently-healthy Barki rams (no abdominal pain, normal appetite, flying response, clean eyes and nostrils, physiological ranges of respiration and pulse rates, heavy musculature (60-65 kg), no problem in urination process).
Diseased group (DG): 20 Barki rams suffered from signs of urine retention (reluctance to move, legs stretched out, straining to urinate, little or no urine flow, arched back and bruxism, stretching and kicking at the abdomen), off-food and low body weights (50-55 kg).
All the rams in the diseased group were subjected to a urethral process amputation using sterile instruments, and all hygienic measures were taken into consideration during the operation. After the amputation, the urine flow confirmed the absence of any other stones (Riedi et al., 2018). The diseased animals were followed up for 2 weeks after amputation to ensure that they received appropriate postoperative care and to determine the dead and live animals for ROC analysis. The objective of the analysis was to evaluate the significance of the studied markers in predicting the outcomes of urethral process amputation.
Blood samples
Blood samples were collected from CG and DG (before amputation). Each sample was divided into three parts for analysis. Na2EDTA was added to the first part to stop the coagulation process, later it was used for manual estimation of the hemogram (red blood cells count (RBCs), hemoglobin concentration (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC)) and leukogram (total leukocytic count (TLC) and differential leukocytic count, (DLC)) parameters of rams of both groups according to the method mentioned by Feldman et al. (2000). Citrate was added to the second part (to prevent the coagulation cascade) and it was centrifuged to obtain plasma at 3000 r.p.m for 20 min for fibrinogen (Fb) measuring using ELISA kits from IBL International Crop (Canada)®. The third part was collected in a plain test tube, allowed to coagulate, and centrifuged at 3000 r.p.m. for 20 min to obtain serum for detection of various parameters including total protein (TP), albumin (Alb), kidney function tests (urea, creatinine (Cr)), hepatic enzymes (alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP)), minerals (calcium (Ca), phosphorus (P), magnesium (Mg)), electrolytes (sodium (Na), potassium (K), chloride (Cl)), trace elements (copper (Cu), zinc (Zn)), glucose, total lipids, phospholipids, triglycerides, T/LDL/HDL-cholesterol, free radicals (malondialdehyde (MDA), nitric oxide (NO)), total antioxidant capacity (TAC), antioxidants (catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR)), serum iron (SI) and total iron binding capacity (TIBC) spectrophotometrically using kits from Biodiagnostic Company®, serum pro-inflammatory cytokines (interleukin-1alpha (IL-1α), interleukin-1beta (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α)) and anti-inflammatory cytokine (interleukin-10 (IL-10)) using ELISA kits from MyBioSource Company®, serum amyloid A (SAA) and haptoglobin (Hp) using ELISA kits from IBL International Crop (Canada)®, caeruloplasmin (Cp) and transferrin (Tf) by a turbidimetric method using Elabscience USA® kits, and ferritin by CLIA method using Abnova® (Taipei) kits. All manual instructions were carefully followed.
- Transferrin saturation percent (Tf sat. %) = SI/TIBC*100.
- Unsaturated iron binding capacity (UIBC) = TIBC-SI.
Statistical analysis
Mean values of CG and DG were compared by independent-sample T test using SPSS® program version 23. A difference was considered significant at P< 0.05.
Graph pad prism version 8 program was used to evaluate the area under the curve (AUC), cut-off points, sensitivity, specificity, and likelihood ratio (LR) for TAC, the measured pro-inflammatory, and APPs of DG compared to CG, and dead group (De) compared to survive group (SG).
The positive predictive value (PPV), negative predictive value (NPV), and accuracy rate for them were calculated according to the next equations:
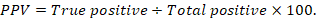
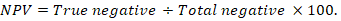
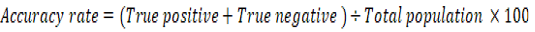
Results
The immunological parameters of DG (compared to CG) displayed a significant (P<0.05) increase in the pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α), APPs (Fb, Cp, SAA, Hp), and free radicals (MDA, NO,) concentrations and a significant (P<0.05) decrease of anti-inflammatory cytokine (IL-10), TAC, and antioxidants (CAT, GPx, GR) concentrations (Table 1).
The biochemical parameters of DG (in relation to CG) demonstrated a significant (P<0.05) decrease in serum levels of TP, Alb, A/G, glucose, total lipids, T/HDL/LDL-cholesterol, minerals (Ca, P, Mg), electrolytes (Na, K, Cl), and trace elements (Cu, Zn), while a significant (P<0.05) elevation in serum levels of Glob, triglycerides, kidney function tests, and liver enzymatic activity (Table 2).
Table 1: the immunological parameters of the diseased group (DG) compared to the control group (CG). Value= mean ±SD.
| Parameter |
CG |
DG |
|
IL-1α (Pg/ml) |
37.49±4.03 | 127.84±11.87* |
|
IL-1β (Pg/ml) |
34.03±1.87 | 128.58±4.28* |
| IL-6 (Pg/ml) | 34.44±1.48 | 89.78±5.01* |
|
TNF-α (Pg/ml) |
36.10±1.82 | 115.06±13.3* |
| IL-10 (Pg/ml) | 113.17±1.81 | 84.45±3.48* |
| Fb (mg/dl) | 127.50±5.74 | 298.50±16.23* |
| Cp (mg/dl) | 4.29±0.06 | 8.52±0.07* |
| Hp (g/dl) | 0.25±0.02 | 4.24±1.47* |
| SAA (mg/L) | 2.95±0.02 | 8.45±1.17* |
| MDA (nmol/ml) | 14.61±0.52 | 24.55±0.55* |
| NO (μmol/L) | 32.00±1.45 | 42.90±2.07* |
| TAC (Mm/L) | 1.44±0.02 | 0.37±0.06* |
| CAT (U/L) | 424.25±9.90 | 250.01±3.24* |
| GPx (mU/L) | 71.28±3.14 | 11.54±3.12* |
| GR(U/L) | 31.90±1.65 | 13.72±1.78* |
Significant differences in the values between the diseased group and the control group were indicated by (*) at P< 0.05.
IL-1α: interleukin-1alpha, IL-1β: interleukin-1beta, IL-6: interleukin-6, TNF-α: tumor necrosis factor-alpha, IL-10: interleukin-10, Fb: fibrinogen, Cp: caeruloplasmin, SAA: serum amyloid A, Hp: haptoglobin, MDA: malondialdehyde, NO: nitric oxide, TAC: total antioxidant capacity, CAT: catalase, GPx: glutathione peroxidase, GR: glutathione reductase.
The hematological parameters of DG clarified microcytic hypochromic anemia accompanied by neutrophilic leukocytosis and lymphocytopenia (indicated by the significant (P<0.05) decline in the red blood cell parameters and indices (RBCs, Hb, PCV, MCV, MCH, MCHC), and lymphocytes count and the significant (P<0.05) increase in TLC and neutrophils count depicted in DG in comparison with CG). The iron profile of DG (in relation to CG) showed a significant (P<0.05) hypoferremia, hypotransferrinemia, hyperferritinemia, with a significant (P<0.05) elevated TIBC, UIBC, and decreased Tf. Sat. % (Table 3).
After the urethral process amputation, two distinct outcomes were observed in the diseased rams. The urine flow resumed in ten rams, and a gradual improvement was noticed in their appetite and the clinical symptoms related to urine retention showed a marked regression (survive group (SG)). Conversely, the other ten rams although the urine flow returned, their appetite did not recover, and their clinical signs worsened over time, and finally died (dead group (DeG)).
Table 2: the biochemical parameters of the diseased group (DG) compared to the control group (CG). Value= mean ±SD.
| Parameter |
CG |
DG |
| Total protein (g/dl) | 7.98±0.09 | 7.15±0.27* |
| Albumin (g/dl) | 4.68±0.05 | 2.50±0.06* |
| Globulin (g/dl) | 3.30±0.05 | 4.65 ±0.23* |
| A\G | 1.42±0.01 | 0.54±0.02 |
| Blood urea (mg/dl) | 31.63±0.90 | 43.18±2.22* |
| Cr (mg/dl) | 0.96±0.03 | 3.81±0.19* |
| AST (U/L) | 32.19±0.91 | 41.92±0.80* |
| ALT (U/L) | 40.49±0.47 | 47.06±1.27* |
| ALP (U/L) | 30.37±0.19 | 52.44±2.26* |
| Glucose (mg/dl) | 130.75±5.92 | 79.30±5.48* |
| Total lipids (mg/dl) | 381.29±5.86 | 369.13±5.87* |
| Triglycerides (mg/dl) | 82.67±1.99 | 103.11±2.04* |
| Phospholipids (mg/dl) | 166.04±6.42 | 166.04±4.23 |
| T-cholesterol (mg/dl) | 132.58±1.93 | 99.98±3.25* |
| HDL-cholesterol(mg/dl) | 38.68±1.49 | 26.46±3.47* |
| LDL-cholesterol (mg/dl) | 93.90±1.52 | 73.52±1.73* |
| Ca (mg/dl) | 11.80±0.22 | 8.71±0.06* |
| P (mg/dl) | 7.85±0.10 | 6.53±0.27* |
| Cl (mmol/L) | 112.97±1.79 | 93.97±2.80* |
| Na (mmol/L) | 155.00±3.75 | 103.94±2.06* |
| K (mmol/L) | 3.85±0.03 | 2.96±0.04* |
| Mg (mg/dl) | 4.11±0.50 | 3.13±0.09* |
| Cu (μmol/L) | 24.82±0.62 | 22.58±1.20* |
| Zn (μg/dl) | 174.63±2.59 | 114.63±2.59* |
Significant differences in the values between the diseased group and the control group were indicated by (*) at P< 0.05.
A/G: albumin/globulin: Cr: creatinine, ALT: alanine transaminase, AST: aspartate transaminase, ALP: alkaline phosphatase, Ca: calcium, P: phosphorus, Mg: magnesium, Na: sodium, K: potassium, Cl: chloride, Cu: copper, Zn: zinc.
Table 3: the hematological and iron profile parameters of the diseased group (DG) compared to the control group (CG). Value= mean ±SD.
| Parameter |
CG |
DG |
|
RBCs (×106/μl) |
14.59±0.11 | 10.24±0.11* |
| Hb (g/dl) | 16.44±0.30 | 9.27±0.11* |
| PCV (%) | 36.95±0.83 | 24.21±0.11* |
| MCV (fl) | 25.32±0.64 | 23.64±0.27* |
| MCH (pg) | 11.27±0.25 | 9.05±0.13* |
| MCHC (%) | 44.51±1.33 | 38.30±0.44* |
| SI (μg/dl) | 127.87±1.75 | 97.83±1.97* |
| TIBC (μg/dl) | 327.39±2.16 |
360.16±3.99* |
| UIBC (μg/dl) | 199.53±2.80 | 262.33±4.48* |
| Transferrin(mg/dl) | 135.50±2.95 | 105.90±2.80* |
| Tf sat. % | 39.06±0.60 | 27.17±0.62* |
| Ferritin (ng/ml) | 18.00±1.45 | 35.60±3.81* |
|
TLC (×103/μl) |
9.01±0.23 | 9.41±0.26* |
|
Neutrophils (×103/μl) |
3.15±0.02 | 6.29±0.19* |
|
Lymphocytes(×103/μl) |
4.80±0.19 | 2.06±0.04* |
|
Monocytes (×103/μl) |
0.56±0.06 | 0.56±0.02 |
|
Eosinophils (×103/μl) |
0.46±0.06 | 0.46±0.03 |
|
Basophils (×103/μl) |
0.02±0.004 | 0.02±0.004 |
Significant differences in the values between the diseased group and the control group were indicated by (*) at P< 0.05.
RBCs: red blood cell count, Hb: hemoglobin concentration, PCV: packed cell volume, MCV: mean corpuscular volume, MCH; mean corpuscular hemoglobin, MCHC: mean corpuscular hemoglobin concentration, SI: serum iron, TIBC: total iron binding capacity, UIBC: unsaturated iron binding capacity, Tf sat. %: transferrin saturation percent, TLC: total leukocytic count.
Table 4: Cut-off points, sensitivity%, specificity%, likelihood ratio (LR), PPV%, NPV% and accuracy rate (AR), of the estimated TAC, pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α) and acute phase proteins (Fb, Cp, Hp, SAA, transferrin, ferritin) in diseased rams compared to the control group, and in the dead group (DeG) compared to the survive group (SG).
|
Parameter |
Group |
Cut-off |
Sensitivity% |
Specificity% |
LR |
PPV% |
NPV% |
AR |
|
TAC (Mm/L) |
DG |
1.41 |
100% |
95% |
20 |
95.24% |
100% |
97.5% |
|
DeG |
0.38 |
100% |
80% |
5 |
83.33% |
100% |
90% |
|
|
IL-1α (Pg/ml) |
DG |
41.95 |
100% |
85% |
6.67 |
86.96% |
100% |
92.5% |
|
DeG |
132 |
100% |
90% |
10 |
90.91% |
100% |
95% |
|
|
IL-1β (Pg/ml) |
DG |
36.40 |
100% |
95% |
20 |
95.24% |
100% |
97.5% |
|
DeG |
129.30 |
100% |
80% |
5 |
83.33% |
100% |
90% |
|
|
IL-6 (Pg/ml) |
DG |
35.97 |
100% |
80% |
5 |
83.33% |
100% |
90% |
|
DeG |
87.47 |
100% |
80% |
5 |
83.33% |
100% |
90% |
|
|
TNF-α (Pg/ml) |
DG |
38.85 |
100% |
85% |
6.67 |
86.96% |
100% |
92.5% |
|
DeG |
116.80 |
100% |
90% |
10 |
90.91% |
100% |
95% |
|
|
Fb (mg/dl) |
DG |
132.5 |
100% |
75% |
4 |
80% |
100% |
87.50% |
|
DeG |
292 |
100% |
90% |
10 |
90.91% |
100% |
95% |
|
|
Cp (mg/dl) |
DG |
4.37 |
100% |
90% |
10 |
90.91% |
100% |
95% |
|
DeG |
8.50 |
100% |
80% |
5 |
83.33% |
100% |
90% |
|
|
Hp (g/dl) |
DG |
0.28 |
100% |
90% |
10 |
90.91% |
100% |
95% |
|
DeG |
3.74 |
100% |
80% |
5 |
83.33% |
100% |
90% |
|
|
SAA (mg/L) |
DG |
2.97 |
100% |
75% |
4 |
80% |
100% |
87.5% |
|
DeG |
7.43 |
100% |
50% |
2 |
66.67% |
100% |
75% |
|
|
Transferrin(mg/dl) |
DG |
132 |
100% |
90% |
10 |
90.91% |
100% |
95% |
|
DeG |
105 |
100% |
60% |
2.50 |
83.33% |
100% |
86.67% |
|
|
Ferritin (ng/ml) |
DG |
19.50 |
100% |
80% |
5 |
83.33% |
100% |
90% |
|
DeG |
35 |
100% |
90% |
10 |
90.91% |
100% |
95% |
LR= 0.5-5: low; LR=5-10: moderate; LR>10: high.
Concerning the diagnostic value of the estimated pro-inflammatory cytokines, APPs, and TAC for urine retention,
all of them yielded sensitivity and NPV as 100 % in DG (compared to CG)and high (more than 70%) specificity, PPV, and AR, but TAC and IL-1β only had high LR as 20 followed by Cp, Hp, and Tf with moderate LR as 10. In regard to their importance in the prediction of the urethral process amputation outcomes, they also yielded sensitivity and NPV as 100 % in DeG (compared to SG) and high (more than 70%) specificity, PPV, and AR (except SAA had low specificity and PPV and Tf had low specificity), but they scored moderate to low LRs. The best LRs were for IL-1α, TNF-α, Fb, and ferritin as 10 (moderate LR) (Table 4).
Discussion
Urolithiasis is an economically important disease that has considerable significance for sheep farming. It has a mixed physiological, managemental, and nutritional etiology and may be affected by season (Sickinger and Windhorst, 2022). In the current work, urine retention led to a cascade of inflammatory immune responses that began with pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, TNF-α) activation. This data agreed with Suen et al. (2010), and Thongboonkerd et al. (2021) who noted elevated levels of urinary chemokines and cytokines in patients with urolithiasis and nephrolithiasis. They referred to their role in linking innate and adaptive immunity. They confirmed their importance as potential biomarkers for the detection of urolithiasis, especially in health screening and clinical follow-up. Contrariwise, the anti-inflammatory cytokine (IL-10) showed diminished levels in DG, this explains the exacerbation of the pro-inflammatory cytokines activation consequences (Salem et al., 2020).
One of the pro-inflammatory cytokines activation outcomes in this study is the noted increase in positive acute phase proteins (APPs) serum levels in DG. APPs are a group of immune proteins, synthesized by the liver under the above-mentioned pro-inflammatory cytokines stimuli. Their blood concentrations upregulate (positive APPs) and downregulate (negative APPs) in response to various conditions (infection, inflammation, ex……). Although they are not specific to a particular disease, they are highly sensitive to subclinical infections or inflammation. Their persistence in the blood reflects the infection severity and underlying tissue damage. In addition to their immunomodulatory role, Fb has an important role in blood coagulation and neutrophils recruitment, Cp has anti-inflammatory anti-oxidants action by oxidizing toxic ferrous into nontoxic ferric, Hp binds free hemoglobin, thus depriving bacteria of iron required for their growth and SAA participates in bacterial opsonization. APPs elevated concentrations were noticed before in bovine urinary tract infections (El-Deeb and Elmoslemany, 2016), and obstructive urolithiasis in sheep (Maciel et al., 2017).
Another effect of the activation of the pro-inflammatory cytokine in the present work is free radical liberation and accumulation. Free radicals are a part of the host’s innate immunity, they are released from different immune cells to attack invaders. The imbalance between free radicals production (MDA, NO) and antioxidant capacity (TAC, CAT, GR, GPx) here, induces oxidative stress which potentially damages biomolecules leading to cell injury and death. Oxidative stress has a great involvement in tissue destruction and different diseases pathogenesis including urolithiasis (Boonla, 2018, Abdallah et al., 2021, Saenz-Medina et al., 2022).
Regarding biochemical parameters of DG in the present data, the protein profile of DG revealed a marked hyperglobulinemia and hypoalbuminemia. These changes were mainly assigned to bladder inflammation (cystitis) because of decreased urination frequency, incomplete voiding, and urine stagnation. This inflammatory condition enhances the synthesis of different globulin fractions and inhibits albumin production (negative APP declines during inflammation) (Sarker et al., 2020, Ismail, 2018). The above-described increase in the pro-inflammatory cytokines and positive APPs blood levels which are α and β globulins in DG in this research supported this assumption. Oxidative stress noted in DG in the present data may take part in the recorded hypoalbuminemia, as albumin is anti-oxidant and the accumulated free radicals consumed it. In parallel, slight hypoproteinemia and decreased A/G were reported in DG here (Sarker, 2020, Ismail, 2018).
The renal and hepatic function tests of DG in this work displayed raised values. These results were attributed to the prolonged urine stagnation in the urinary bladder (due to obstruction) which led to an increased rate of creatinine and urea reabsorption into the systemic circulation. In addition, renal insufficiency due to hydronephrosis (urine flow back and accumulation inside the kidneys) reduced urine production by decreasing glomerular filtration rate and ultimately decreasing urea and creatinine secretion in urine. This excessive accumulation of urea and other nitrogenous waste products logically leads to the development of uremic syndrome, which in turn causes hepatic dysfunction. Consequently, an elevation in the liver enzymatic activities was observed in DG during this investigation. Similar observations were reported in buffalo calves and calves with urine retention (Aref and Abd El-hakiem, 2013, Ismail, 2018), goats with urolithiasis (Sarker et al., 2020), ruminants with obstructive urolithiasis (Makhdoomi and Gazi, 2013, Mahajan et al., 2017, Riedi et al., 2018), sheep (Maciel et al., 2017, Batista et al., 2020), and mare with urine retention (Hussein, 2014). It is worth mentioning that, ALP activity was recommended before as an early diagnostic biomarker for renal pathological conditions and renal injury, this may be a more specific reason for ALP elevated activities in DG in the current research (Ismail, 2018).
The pain and anorexia usually observed with urolithiasis are rational causes for the noticed hypoglycemia, hypolipidemia, T/HDL/LDL-hypocholesterolemia, hypocalcemia, hypophosphatemia, hypomagnesemia, hyponatremia, hypokalemia, hypochloremia, hypocupremia, and hypozincemia in DG in the present study (Radostitis et al., 2007). Renal insufficiency and impairment of tubular reabsorption of minerals and electrolytes are additional reasons for the decreased minerals and electrolytes serum concentrations in DG. Lack of renal hydroxylation of 25-hydroxycholecalciferol due to renal dysfunction interferes with intestinal calcium absorption and magnifies the hypocalcemia in the diseased rams. (Makhdoomi and Gazi, 2013, Ismail, 2018, Sarker et al., 2020). In contrast, the pronounced hypertriglyceridemia detected in DG in this data pointed to enhanced adipose tissue lipolysis to get the energy required for animal survival (Radostitis et al., 2007).
The hemogram of the diseased rams in the current study was concurrent with the immunological findings, as DG suffered from microcytic hypochromic anemia. Whereas the prior activated pro-inflammatory cytokines inhibit erythropoiesis by interfering with iron intestinal absorption, enhancing ferritin formation (positive APP), decreasing transferrin synthesis (negative APP), and increasing hepcidin production (compete with transferrin for iron). These measures prevent iron access to invading microorganisms during infection and inflammation to inhibit their growth. They also reduce iron access to the host bone marrow thus producing the microcytic hypochromic anemia depicted in DG here (Suen et al., 2010, El-Deeb and Elmoslemany, 2016, Maciel et al., 2017, Thongboonkerd et al., 2021). The iron profile of DG confirmed this theory, it revealed hypoferremia, hypotransferrinemia, and hyperferritinemia with subsequent elevated TIBC, UIBC, and decreased Tf Sat. %. Downregulation of erythropoietin production due to renal injury is another cause for the determined anemia in DG in this work (Radositites et al., 2007). Similarly, the leukogram of DG in this research reflected the inflammatory condition accompanying urine retention. As the invigorated pro-inflammatory cytokines stimulated bone marrow to produce more neutrophils in the diseased animal circulation and enhance lymphocytes migration to the inflammation site. This action caused the obtained neutrophilic leukocytosis and lymphocytopenia obtained in DG in the current data (Suen et al., 2010, Thongboonkerd et al., 2021).
Table (4), revealed that urine retention in sheep can be detected using pro-inflammatory cytokines, APPs, and TAC. Among the estimated markers, TAC and IL-1β were found to be the most effective indicators for the disease. Additionally, these biomarkers demonstrated potential for predicting mortality after the urethral process resection, particularly IL-1α, TNF-α, Fb, and ferritin. These findings align with previous studies that recommended pro-inflammatory cytokines, APPs, and oxidative stress markers as reliable diagnostic and prognostic tools for urine retention in various species, including sheep (Suen et al., 2010; El-Deeb and Elmoslemany, 2016; Boonla, 2018; Maciel et al., 2017; Abdallah et al., 2021; Thongboonkerd et al., 2021; Saenz-Medina et al., 2022). However, this study differed in terms of marker prioritization due to variations in species, disease stage, and methodology employed.
Conclusion
Urine retention in sheep is associated with several hemato-biochemical changes which have an immunological origin. These changes should be followed-up and reversed by the appropriate supportive treatment. Pro-inflammatory cytokines, APPs, and TAC are sensitive biomarkers for urine retention especially (TAC and IL-1β) and good predictors of the urethral process surgical removal results especially (IL-1α, TNF-α, Fb, and ferritin).
Acknowledgments
All members of the animal and poultry health department, Desert Research Center (DRC), Cairo, Egypt.
Conflict of interest
The author declared that she had no conflict of interest.
novelty statement
This research was the first research to investigate the immunological and clinicopathological alterations associated with urine retention in Barki rams, with the evaluation of the pro-inflammatory cytokines, APPs, and TAC importance in urine retention diagnosis and urethral process amputation outcomes prediction.
Author Contributions
AAD made the study design, collected the samples, performed the immunological and clinicopathological, and statistical analysis, and wrote the paper.
References
Abdallah A., Ezzeldein S., Eisa E., Abd El Raouf M., Bayoumi Y. (2021). Obstructive urolithiasis in buffalo calves (Bubalus bubalis): Serum changes of Vitamins A and D and efficacy of surgical management using tube cystostomy. Vet. World. 14(1); 129-136. https://doi.org/10.14202/vetworld.2021.129-136.
Aref N., Abd El-hakiem M. (2013). Azotemia and metabolic alkalosis in calves with urolithiasis associated with urinary bladder and urethral rupture. Assiut Vet. Med. J. 59(137): 86-92. https://doi.org/10.21608/avmj.2013.171495.
Batista A., Morais L., Silva W., Reis A. (2020). Obstructive urolithiasis in sheep- case report.17. https://doi.org/10.18677/EnciBio_2020D38.
Boonla C. (2018). Oxidative Stress in Urolithiasis. Reactive Oxygen Species (ROS) in Living Cells. In Tech. https://doi.org/10.5772/intechopen.75366.
El-Deeb W. M., Elmoslemany A. M. (2016). Acute phase proteins as biomarkers of urinary tract infection in dairy cows: diagnostic and prognostic accuracy. Japanese J. Vet. Res. 64(1): 57–66.
Ewoldt J.M., Jones M.L., Miesner M.D. (2008). Surgery of obstructive urolithiasis in ruminants. Vet. Clin. North Am. Food Anim. Pract. 24(3): 455-65. v. https://doi.org/10.1016/j.cvfa.2008.06.003. PMID: 18929952.
Feldman B.F., Zinkl J.C., Jain N.C. (2000). “Schalm’s Veterinary Hematology”, 5th (ed.), Lippincott Williams & Wilkins, Philadelphia, London.
Gazi M.A., Makhdoomi D.M., Parrah J.D., Ganai A.M., Shiekh G.N., Mir S.A. (2014). Recent advances in surgical management of urolithiasis in sheep and goat. African J. Agr. Res. 9: 2055-2061. https://doi.org/10.5897/AJAR2014.8643
Hussein H.A. (2014). Ultrasonographic and clinicopathological findings in a 7-year-old mare with urine retention. Turkish J. Vet. Anim. Sci. 38: 110-115.
Ismail H.T.H. (2018). Hemato-biochemical parameters as comparative tools and prognostic indicators in urine retention cases with intact or ruptured urinary bladder in buffalo calves. Adv. Anim. Vet. Sci. 6(4): 148-155. DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.4.148.155
Maciel T.A., Ramos I.A., Silva R.J., Soares P.C., Carvalho C.C., Junior R.J., Amoroso L., Artoni S.M., Afonso J.A., Oliveira D. (2017). Clinical and Biochemical Profile of Obstructive Urolithiasis in Sheep. Acta Scient. Vet., 15.https://www.redalyc.org/articulo.oa?id=289053641089
Mahajan A., Gupta A.K., Bhadwal M.S., Bhat M.A., Bhardwaj H.R. (2017). Occurrence and Management of Obstructive Urolithiasis in Ruminants. J. Anim. Res. 7(4), 723-731. August 2017 https://doi.org/10.5958/2277-940X.2017.00111.5
Makhdoomi D.M., Gazi M.A. (2013). Obstructive urolithiasis in ruminants – A review Vet. World. 6(4): 233-238. https://doi.org/10.5455/vetworld.2013.233-238.
Radostitis O.M., Gay C.C., Hinchcliff K.W., Constable P.D. (2007). “Veterinary Medicine”: A text book of the diseases of cattle, sheep, pigs, goats and horses, 10th (ed.) Saunders Company, Philadelphia, U.S.A.
Riedi A. K., Nathues C., Knubben-Schweizer G., Nuss K, Meylan M. (2018). Variables of initial examination and clinical management associated with survival in small ruminants with obstructive urolithiasis. J. Vet. Inter. Med. 32(6): 2105–2114. https://doi.org/10.1111/jvim.15336
Saenz-Medina J., Muñoz M., Rodriguez C., Contreras C., Sánchez A., Coronado M.J., Ramil E., Santos M., Carballido J., Prieto D. (2022). Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress. Cells. 11(15): 2306. https://doi.org/10.3390/cells11152306. PMID: 35954150; PMCID: PMC9367519.
Salem P.P.O., Vieira N.B., Garcia D.A., Nicácio K.J., Dias D.F., de Paula A.C.C., Assis D.M., Caldas I.S., Novaes R.D., Marinho M.V., Rosa I.M.L., Soares M.G., Chagas-Paula D.A. (2020). Anti-urolithiatic and anti-inflammatory activities through a different mechanism of actions of Cissus gongylodes corroborated its ethnopharmacological historic. J. Ethnopharmacol. 253: 112655. https://doi.org/10.1016/j.jep.2020.112655. Epub 2020 Feb 8. PMID: 32045681.
Santarosa B.P., Ferreira D.O., Surian S.R., Tremori T.M., Hooper H.P., Silva P.S., Coelho M.R., Santos V.H., Gonçalves R.C. (2021). Clinical and anatomopathological study of urolithiasis in feedlot lambs subjected to diets with different phosphorus concentrations. Cienc. Anim. Bras. 22: 67849 https://doi.org/10.1590/1809-6891v22e-67849
Sarker D., Akter M.A., Rahman M.S., Yesmin N., Alam M.M. (2020). Clinicopathological Consequences of Urinary Retention due to Urolithiasis in Indigenous Goats. PSM Vet. Res. 5(2): 28-37.
Sharun K., Manjusha K. M., Kumar R., Pawde A. M., Malik Y., Kinjavdekar P., Maiti S. K., Amarpal A. (2021). Prevalence of obstructive urolithiasis in domestic animals: An interplay between seasonal predisposition and dietary imbalance. Iraqi J. Vet. Sci. 35(2): 227-232. https://doi.org/10.33899/ijvs.2020.126662.1358
Sickinger M., Windhorst A. (2022). A systematic review on urolithiasis in small ruminants according to nutrition-dependent prevalence and outcome after surgery, Vet. World. 15(3): 809-817. doi: https://doi.org/10.14202/vetworld.2022.809-817
Suen J. L., Liu C. C., Lin Y. S., Tsai Y. F., Juo S. H., Chou Y. H. (2010). Urinary chemokines/cytokines are elevated in patients with urolithiasis. Urol. Res. 38: 81–87. https://doi.org/10.1007/s00240-010-0260-y
Thongboonkerd V., Yasui T., Khan S.R. (2021). Editorial: Immunity and Inflammatory Response in Kidney Stone Disease. Front. Immunol. 12:795559. https://doi.org/10.3389/fimmu.2021.795559
To share on other social networks, click on any share button. What are these?





