Ce-doped Nanobioactive Glass/ Collagen/ Chitosan Composite Scaffolds: Biocompatibility with Normal Rabbit’s Osteoblast Cells and Anticancer Activity Test
Ce-doped Nanobioactive Glass/ Collagen/ Chitosan Composite Scaffolds: Biocompatibility with Normal Rabbit’s Osteoblast Cells and Anticancer Activity Test
Hanan Fathy Hammouda1, Mohammad Mahmoud Farag2*, Mervet M.F. El Deftar3, Mohamed Abdel-Gabbar4, Basant M. Mohamed4
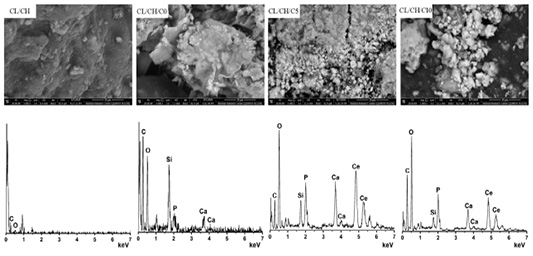
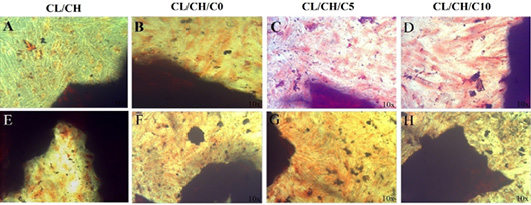
SEM and EDX of CL/CH, CL/CH/C0, CL/CH/C5 and CL/CH/C10 samples after immersion in SBF for 30 d.
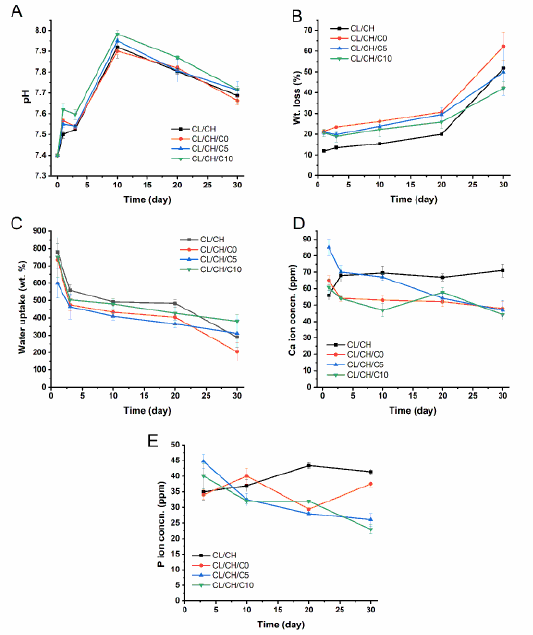
(A) pH, (B) weight loss (%), (C) water uptake (wt. %) (D) Ca2+ ion concentration (ppm), (E) phosphate ion concentration (ppm) of CL/CH, CL/CH/C0, CL/CH/C5 and CL/CH/C10 samples.
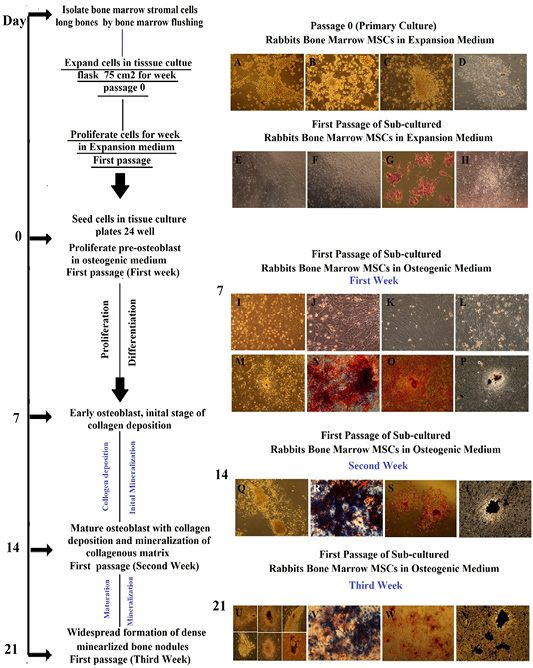
Morphology representation of cultured Rabbit BM-MSCs in expansion medium and their osteogenic differentiation potential with specific markers through first passage/ second and third week cultured in osteogenic medium; Morphology of cultured Rabbit BM-MSCs in expansion medium, passage 0 (primary cell culture). (A) at time 0, colonies of cells were seen by inverted phase contrast microscope in expansion medium, Cells were obtained by bone marrow flashing. (B) at day 2, elongated cells were appeared and selected by adherence to plastic, whereas floating cells are residual red blood cells and unattached mononuclear cells were maintained in expansion medium. (C) at day 3, following removal of non-adherent hematopoietic stem cell populations by changing medium for first time, adherent colonies. The number of colonies varied from 3-7 per flask. Central cells showed high nucleo cytoplamic ratio with little cytoplasm. (D) at day 7, fibroblastoid cell population proliferated and increased in density with time and nodular confluence (80-90%) was noticed. Confluence appeared as marked increase in fibroblastoid cell population in colonies (nodules). Morphology of sub-cultured Rabbit BM-MSCs in expansion medium, first passage. (E, F) thin and fiberoblast-like MSCs were seen and widespread in all culture plate after trypsinization. (G) By hematoxylin and eosin, the cells were polygonal with pink to light violet cytoplasm and blue nucleus. (H) Thin and fiberoblast-like MSCs were arranged and expanded in bundles and sheets at day 7 of first passage in expansion medium. Morphology of sub-cultured Rabbit BM-MSCs in osteogenic medium, first passage through first week of differentiation, alkaline phosphatase histochemical staining, Alizarin Red staining and von Kossa staining. (I-K) Rabbit BM-MSCs assumed a less elongated, polygonal, cubodial appearance with central rounded nuclei or multipolar after 48-72 h of subculture. (L-M) at day 7 showed coalescing cellular aggregates arranged in swirling sheets and bundles with interconnected multilayer foci showing central matrix-like substance like nodules. (N) Osteoblast expressing ALP enzyme were blue interspersed by negative red cells , Positivity of subculture for ALP staining was noticed as early as day 7 with percentage around 20%. (O) Orange red color was seen by positive cells in large amount and also, positive cells were interspersed with negative cells. (P) Black mineral deposits were seen in large amount and large size. Mineralized areas were interspersed by another un-calcified small cell colony. The characteristic halo, surrounding the bone nodule evidence of the enzymatic activity of the ALP, Inverted Phase Contrast Microscope Digital Camera Olympus, Magnification 10x, Morphology of sub-cultured Rabbit BM-MSCs in osteogenic medium, first passage through second week of differentiation, alkaline phosphatase histochemical staining, Alizarin Red staining and von Kossa staining. (Q) The number of nodules increased dramatically in size and becoming multilayered with deposition of collagen and mineralization of collagenous matrix(R) ALP staining was noticed with percentage around 60-80% at day14. (S)The mineralized nodules were labeled as red spots with alizarin stain and increased in size dependant on time and number of cells. (T) Mineral deposits were stained black with von kossa stain. Morphology of sub-cultured Rabbit BM-MSCs in osteogenic medium, first passage through thrid week of differentiation, alkaline phosphatase histochemical staining, Alizarin Red staining and von Kossa staining. (U) The number of nodules increased dramatically in size with widespread formation of dense mineralized bone structure. (V) Decline in staining intensity for ALP was noticed with percentage around 60% at day 21. (W) The mineralized nodules were labeled as red spots and increased in number and staining intensity in a time dependent manner covering more than 80 % of culture plate. (X) Mineral deposits were stained black with von kossa stain and almost of black mineral deposits areas were mostly located in the central confluent multilayered clusters of cells.
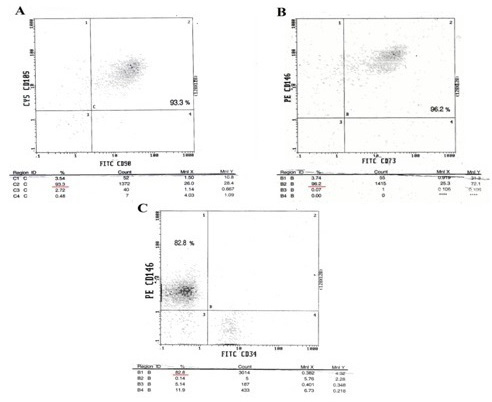
Immuno-phenotype of MSC-derived multipotent cells. Cells were isolated, expanded and analyzed by flow cytometric analysis with antibodies against the indicated antigens of hematopoietic and mesenchymal lineage markers (CD34, CD90, CD146, CD73 and CD 105), the rate positive staining for (A) CD 105 and CD 90 93.3%, (B) CD146 and CD73 96.2% and (C) CD146 82.8%, which confirms their mesenchymal characteristics but negative for CD34.
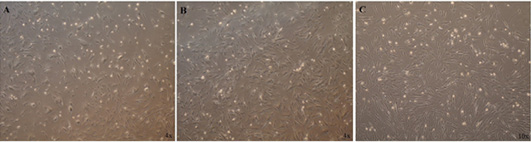
Morphology of MSC-derived multipotent cells by inverted microscope, Cells were isolated by flow cytometric analysis expanded in complete expansion medium (A-C) Condensation of thin and elongated mesenchymal stem cells with morphology of fibroblast through the period of culture and subculture in expansion medium.
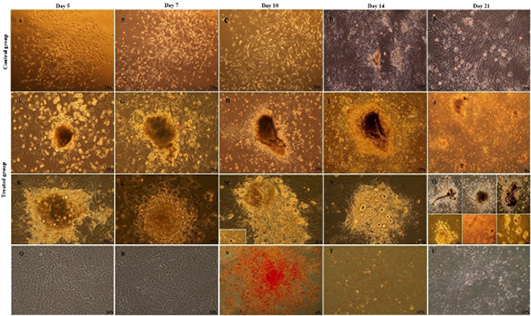
Morphology of MSCs control group subcultured in complete expansion medium and other treated subgroup sub-cultured in osteogenic medium supplemented with b-FGF during three weeks after subculture: (A-E) control group, thin and fiberoblast-like MSCs were arranged in bundles and sheets after subculture at d (A) 5, (B) 7, (C) 10 and (D, E) network pattern of growth was seen. Networks were connected to each other’s via long cellular processes at day 14 and 21(F-R) Nodule formation was observed at day 5 in osteogenic medium, and increased in size and number progressively over time during three weeks of subculture. (Q, R) Morphology of MSCs completely different from morphology of osteoblast cells, black arrow,(S) Alkaline phosphatase histochemical staining of control group; the cells were negative for ALP staining (red) at d 7,14, and 21,(T,U) control group was negative for both Alizarin red and Von Kossa staining through three week of differentiation.
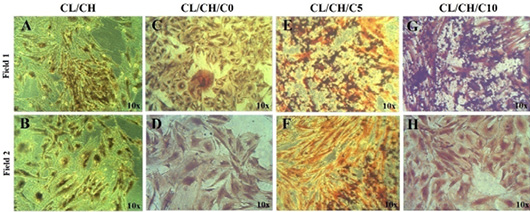
Alizarin Red and von Kossa staining of osteoblastic like-cells of first passage/ second and third week cultured in osteogenic medium with the scaffolds. (A-D) (Second week, orange red color was seen by positive cells in large amount in the vicinity of the scaffold surfaces and stained alizarin red stain at day 14. (E-H) Third week, orange red color was seen by positive cells in large amount interspersed with black mineral deposits with both (Alizarin Red/ von Kossa Staining) together at day 21. Inverted Phase Contrast Microscope Digital Camera Olympus, Magnification 10x.
von Kossa staining of osteoblastic cells of first passage/ third week at day 21 cultured in osteogenic medium barrel to cells were seeded with the various of scaffolds extract (A-H) two fields for each scaffold extract brown -black mineral deposits (Mineralized areas) were seen by large cell colony in different fields of culture well, Inverted Phase Contrast Microscope Digital Camera Olympus, Magnification 10x.
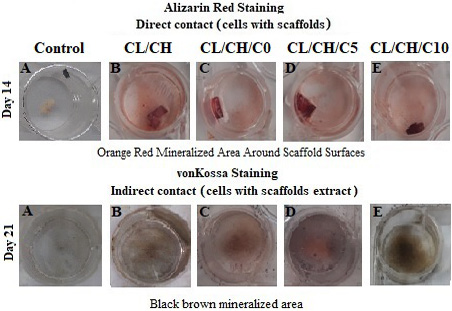
Alizarin Red and von Kossa staining of osteoblastic cells of first passage/ second and third week sub-cultured in osteogenic medium direct and indirect with the scaffolds by naked eye. (A-E) Direct contact at day 14, orange red color was seen by positive cells in large amount in the vicinity of the scaffold surfaces by alizarin red staining. (A-E) Indirect contact at day 21, brown -black mineral deposits (Mineralized areas) were seen by large cell colony in different fields of culture well with scaffolds extract by von Kossa staining.
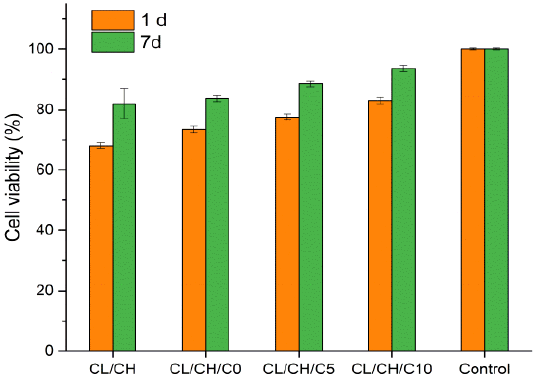
Effect of Cl/CH, Cl/CH/C0, Cl/CH/C5 and Cl/CH/C10 scaffolds evaluated by proliferation of osteoblast cells in osteogenic medium after 1 and 7 d. All scaffolds showed proliferation inhibition < 25% reference to final cell number of control group, (zero inhibition to proliferation).
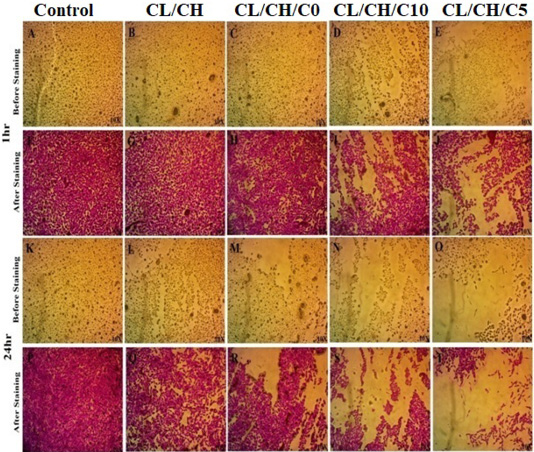
The viability results of the Ce-doped bioactive glass nanoparticles composite scaffolds on osteosarcoma cells. (A-T) The results were shown for Cl/CH, Cl/CH/C0, Cl/CH/C5 and Cl/CH/C10, respectively before and after staining by SRB stain from three independent experiments at exposure time 1h and 24h of treatment. Cell viability decreased respectively and the composite with low level of cerium concentration 5 was significantly greater than all other groups and control cells and caused growth inhibition to osteosarcoma cells more than 24%, Inverted Phase Contrast Microscope Digital Camera Olympus, Magnification 10x.