Calliandra Calothyrsus as a Concentrate Substitution by Regarding its Phytochemicals and Productivity of Indonesian Peranakan Etawa Goats
Research Article
Calliandra Calothyrsus as a Concentrate Substitution by Regarding its Phytochemicals and Productivity of Indonesian Peranakan Etawa Goats
Faculty of Animal Science, Jenderal Soedirman University, Purwokerto, Indonesia.
Abstract | Calliandra calothyrsus is a common legume in Indonesia that has both high nutritional value and antinutritions. It is potent as a concentrate substitution regarding its phytochemicals and the effect on Indonesian Peranakan Etawa crossbreed Goats (PE), which needed to be comprehensively explored. Dried C. calothyrsus leaves were extracted with 70% ethanol, hexane, and chloroform. Twenty PE does on the third week of lactation with an average body weight of 35.22 kg and 1.5 to 2.0 years old were used. The experimental design of this research was a Completely Randomized Design (CRD). Animals were divided into four treatments, each using five lactating goats as replications. The animals were fed 3.5% of their body weight based on dry matter, then four levels of concentration substitution were T1 (0% dried C. calothyrsus), T2 (10% dried C. calothyrsus), T3 (20% dried C. calothyrsus), and T4 (30% dried C. calothyrsus). Descriptive analysis was used for phytochemical tests and bioactive identification; however, toxicity, productivity, and milk quality were analyzed by ANOVA, followed by Duncan’s Multiple Range Test. The research showed many active compounds in C. calothyrsus, such as lipids and fatty acids, steroids, triterpenoids, flavonoids, saponins, carbohydrates, and tannins. Few toxic compounds, such as tannins in C. calothyrsus, were not at harmful levels. The ethyl acetate fraction has higher toxicity 13.34±5.78; 50.00±10.00; 100.00±0.00% and hexane as lowest 6.67±5.78; 20.00±0.00; 60.00±10.00%. Fraction from 50% Ethanol extraction has the lowest LC50 (560.63ppm). Replacement of concentrate using dried C. calothyrsus in the diet of lactating PE goats had no effect (P>0.05) on productive performance such daily gain of T1-T2-T3-T4 respectively (0.20±.02;0.22±0.01;0.22±0.02;0.20±0.02kg/day), feed efficiency (12.03±1.31;13.02±0.7;12.59±0.78;11.87±1.53%), average milk production (662±55; 691±36; 732±72;738±65ml/day), pH (6.56±0.11; 6.60±0.10;6.57±0.16;6.59±0.12). In summary, C. calothyrsus has no harmful phytochemicals and can replace conventional concentrates up to 30% of goat feed without significantly affecting productivity and milk quality.
Keywords | Peranakan etawa goat, C. calothyrsus, Concentrate, Milk quality, Milk production, Phytochemical
Received | July 31, 2024; Accepted | August 29, 2024; Published | October 05, 2024
*Correspondence | Yusuf Subagyo, Faculty of Animal Science, Jenderal Soedirman University, Purwokerto, Indonesia; Email: yusuf.subagyo@unsoed.ac.id
Citation | Subagyo Y, Ifani M, Widodo HS (2024). Calliandra Calothyrsus as a concentrate substitution by regarding its phytochemicals and productivity of indonesian peranakan etawa goats. Adv. Anim. Vet. Sci. 12(11): 2221-2233.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.11.2221.2233
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Peranakan Etawa (PE) is a crossbreed resulting from Etawa and indigenous goats. It is recognized for its medium milk production capacity, strong ability to adapt to the local surroundings, and resistance against various diseases. The PE goat has become the preferred choice for dairy goat breeders in Indonesia. Wasiati and Faizal (2018), reported a significant and consistent growth in the development and enthusiasm of farmers towards PE goats over the years. The goat exhibits a substantial milk yield, ranging from 1.5 to 3 liters per day (Devendra and Liang, 2012). Providing the goat with adequate nourishment through high-quality feed is essential to optimize milk production.
In developing countries, particularly Indonesia, small ruminants rely only on ingesting low-grade feed for live. This problem hinders milk production since the amount of feed consumed and the ability to digest it are restricted. The primary factor impeding the utilization of low-grade fodder is the insufficient protein content. Protein is a crucial component that restricts animal productivity. Its insufficiency is evident in reduced total production, including slow weight gain, low reproductive rates, and decreased milk production (Devendra and Liang, 2012).
These disadvantages are due to the frequent challenges that dairy goat farmers encounter in supplying concentrate feed. The expense of acquiring concentrate for feed places a heavy financial burden on farmers, particularly those that manage on a small or medium scale and rely significantly on cost-effectiveness. Farmers must utilize alternative feed ingredients that are readily accessible, cost-effective, and rich in nutrients as a viable replacement for concentrate in order to address this issue (Devendra and Liang, 2012). Leguminous plants are a protein-rich feed component. Thus, legumes are highly appropriate as a complementary source for low-grade fodder. Legumes have been underestimated in ruminant feeding systems so far, primarily due to the need for knowledge of the diverse applications of legumes. Lately, there have been studies focused on enhancing innovative feeding methods (Abraham et al., 2023).
Calliandra calothyrsus is a tropical legume tree that can offer better quality feed, even in infertile and acidic soils. C. calothyrsus is a promising source of protein for animal feed and is commonly used due to its plentiful availability as a local feedstuff. C. calothyrsus is considered a nutritionally valuable plant due to its high protein content. According to Abqoriyah et al. (2015), C. calothyrsus has a crude protein content of 21%, crude fiber content of 15%, and TDN (Total Digestible Nutrients) content of 65%. C. calothyrsus is a suitable feed for dairy goat farming since it contains many nutrients and is readily available. It could be used as the primary protein source for these reasons. However, it has been discovered that C. calothyrsus possesses disadvantages, specifically in the presence of chemical compounds known as tannins.
Condensed tannin can protect the protein against bacterial degradation in the rumen (Waghorn, 2008), thereby enhancing the quantity of protein digested by the animal. Goats’ rumen bacteria have greater tolerance to tannin than cow’s rumen bacteria. According to Wahyuni et al. (2014), goats have bacteria that can tolerate tannins. These bacteria can be extracted from goats and adapted to grow in C. calothyrsus.
Further research is required to investigate the utilization of C. calothyrsus dry leaves as the primary protein source in dairy goat diets due to the varying levels of protein solubility and degradation in the rumen. The utilization of C. calothyrsus as a feed component for dairy goats in Indonesia has been extensively practiced. However, the study of C. calothyrsus as the primary protein source in PE goat diets has not been well examined, principally in phytochemical studies compared to the productivity of the animal.
The study begins by conducting phytochemical examinations on C. calothyrsus to ascertain its probable impact on the rumen microorganisms of PE goats. Following that, research was conducted to examine the utilization of C. calothyrsus as a protein source on the performance of PE goats, including its impact on milk productivity and the quality of milk that includes C. calothyrsus as the primary protein source.
MATERIALS AND METHODS
Materials
C. calothyrsus leaves were obtained from the East Baturraden Forest, Forest Management Unit of Banyumas, Indonesia, after being identified by botanists. The test was conducted at the Phytochemical Laboratory, Biology Research and Development Center, the Indonesian Research Institute (Lembaga Ilmu Pengetahuan Indonesia/LIPI), Bogor, Indonesia. The reagents were PA grade ethanol, n-hexane, acetic ethyl solution, TWEEN 80 (2000ppm), cyst of Artemia salina, KOH, diethyl ether, acetic acid, CHCl3, H2SO4, HCl, Meyer, Dragendorf, Wagner, Magnesium, FeCl3, and Molisch reagents.
The in vivo experiment was conducted at the Experimental Farm, Faculty of Animal Science, Jenderal Soedirman University, Purwokerto, Indonesia. Twenty lactating PE does, each animal was individually caged. The feed ingredients and nutritional composition of the rations in this experiment are presented in Table 1.
Methods
The research was carried out in two stages. The first step is phytochemical, active compound identification, and toxicological test of C. calothyrsus leaves extract. The following experiment was in vivo test to collect information on the effects of different levels of C. calothyrsus fed on the productivity and milk production of PE does, as illustrated in Figure 1.
Table 1: Feed ingredients and nutrient composition of the diets (% DM).
|
Diet Ingredient |
T1 |
T2 |
T3 |
T3 |
|
Napier grass |
60 |
60 |
60 |
60 |
|
Coconut oil meal |
15 |
10 |
5 |
0 |
|
Soybean cake meal |
15 |
10 |
5 |
0 |
|
Dried C. calothyrsus leaves |
0 |
10 |
20 |
30 |
|
Corn meal |
9 |
9 |
9 |
9 |
|
Premix |
1 |
1 |
1 |
1 |
|
Nutrient content |
||||
|
CP (%) |
11.8 |
11.81 |
11.86 |
11.9 |
|
ME (MJ/kg DM) |
9.50 |
9.30 |
9.12 |
8.84 |
CP: Crude protein; ME: Metabolizable energy.
Identification of Phytochmical, Bioactive Compounds and Toxicological Test
Crude extract production: A quantity of one kilogram of freshly harvested C. calothyrsus leaves was dried in oven at a temperature of 60oC overnight. Subsequently, the dried leaves were crushed by a sieve with a mesh size of 1mm, then thoroughly mixed and immersed in 80% ethanol overnight. The extract was further heated to a temperature of 50oC until the solution clear, and then filtered into a flask. The residue and filter paper were rinsed with 80% ethanol and dried under vacuum conditions at 40oC using a rotary vacuum evaporator. The 60g residue was collected and completely dissolved in a solution of 50% ethanol. The resulting solution was then divided into three equal portions, each of which was mixed with either 100ml of n-hexane, chloroform, or ethyl acetate solution. The solutions were dried using a rotary vacuum evaporator until all liquid had evaporated. The extracts were used to test phytochemical, toxicological, and active compound analyses.
Phytochemical test: The phytochemicals were screened using various processes, including the crude extract, identification of volatile oils, sterols and monoterpenoids, alkaloids and triterpenoids, flavonoids, glycosides di/triterpenoids, saponins, and carbohydrates. The method of identification per compound was:
- Tannins: The crude extract was thoroughly shaken after 2ml of water and 2ml of FeCl3 were added to identify gallotannins as catechol tannins by their dark blue color appearance.
- Volatile oils, sterols, and monoterpenoids: The hexane fraction was dried until the smell was specifically aromatic, then dissolved in 10ml ethanol and divided into two parts. The first part was heated until dry and smelt of specific aromatic indicates volatile oils. The second part was heated and saponified in a water bath with 10ml 0.5N potassium hydroxide in ethanol until the solution contained no oils, then washed with 30ml hot water. After cooling, it was divided into two parts by washing with 16ml ether to get the ether extract and the alkali water. Ten milliliters of ether extract were then dried and dissolved in 0.5ml acetic acid and 0.5ml chloroform, then shaken. Two milliliters of sulfuric acid were added. A green or violet color appears, indicating sterols and monoterpenoids.
- Alkaloids and Triterpenoids: The chloroform fraction was dissolved with 1.5ml choleric acid 2% and distributed into three reaction tubes. The presence of alkaloids was indicated by the color of the solution, which would be opaque and have either a yellowish-white precipitate at the bottom of the tube when it is added the Meyer reagent or a violet precipitate when it is added the Dragendorf reagent. To identify triterpenoids, 0.5ml of the chloroform fraction was dissolved in 0.5ml anhydride acetic and 0.5ml chloroform. The solution was mixed thoroughly and placed into the third reaction tube and then added 2ml concentrated sulfuric acid. This solution is separated into two phases, with a brown or yellowish-green ring between the two phases. The upper phase was green or violet, indicating the presence of triterpenoids.
- Flavanoids: The flavanoids were identified by dissolving the ethyl acetate fraction in 2ml methanol and heating at 50°C for 1 hour, followed by adding 1g of magnesium and 5ml HCl. The development of red or violet color indicated the presence of flavonoids.
- Saponins and carbohydrates: Two milliliters of water were added to the 2ml of 50% ethanol fraction. The presence of voluminous foam indicates saponins. The carbohydrate was identified by adding 1ml of sulfuric acid into the dried filtrate fraction and left for 5 minutes. The development of a red color after Molisch reagent addition indicates carbohydrates.
Toxicological test: Brine Shrimp Lethality Test (BSLT) method was applied in the test. The previously dried extracts were diluted to concentrations of 1000, 100, and 10 as well as 0 ppm as blank or negative control. In order to achieve this concentration, a solution was prepared by diluting forty grams of extracted C. calothyrsus leaves with 20ml of seawater and 5ml of TWEEN-80. A 15 mg cyst of Artemia salina was immersed in filtered seawater. Afterward, the sample was aerated and stored for 48 hours in the hatchery chamber until all the cysts had hatched. Subsequently, the larvae were extracted using a pipette and employed for the toxicological examination. Thirty-nine groups, each consisting of ten larvae, were placed in Eppendorf vials containing each hexane, chloroform, ethyl acetate or 50% ethanol fraction at concentrations of 10, 100, and 1000 ppm, and a blank solution (only 10ml seawater and 4ml TWEEN-80). The larvae were kept in these solutions for 24 hours. The mortality was assessed by enumerating the quantity of dead larvae. Furthermore, the LC50 value was calculated to predict how much compound level will terminate 50% of the larvae population, indicating the presence of bioactive compounds. The LC50 value is calculated by regression of respective concentrations. Thus, the concentration to eliminate 50% of larvae could be determined.
Bioactive compound: The dried C. calothyrsus leaf crude extract was divided into ethanol, hexane, and chloroform fractions and then evaporated to create a thick extract fraction. The chromatogram and mass spectrum of the extract were determined using GC-MS, and the names and molecular structures of each compound were determined using the National Institute of Standards and Technology or the Willey library’s information database. The setting of gas chromatography as follows: Type of GC-MS: FINNIGAN-MAT; Column: (Liquid phase), TC-1, nonpolar; Length: 30m; Ø: 0.25mm; Carrier gas: He; Gas velocity: 40 mm/min; Initial temperature: 70℃; Final temperature: 250℃; Injection temperature:220C; Interface temperature:250°C; Temperature velocity:6°C/min.
Productivity and milk quality
The experimental design used in this research was a Completely Randomized Design (CRD). The does ages 1.5 to 2.0 years, with an average body weight of 35.22 kg, participate in this experiment during the third week of lactation. The does were grouped into four treatments, each consisting of five lactating does as replicates. The animals were given diets equivalent to 3.5% of their body weight (BW) based on dry matter. The proportion of Napier grass to concentrate was 60%:40%. The animals were provided with diets three times a day: at 08.00 AM for concentrates, 12.00 PM, and 5.00 PM for Napier grass. Water was given ad libitum.
The treatments were: T1 (substitution of 0% dry C. calothyrsus to diet), T2 (substitution of 10% dried C. calothyrsus), T3 (substitution of 20% dried C. calothyrsus), and T4 (substitution of 30% dried C. calothyrsus). Every diet is supplemented with a 1% premix, which is a valuable source of vitamins and minerals. This meal was provided to does from the beginning of its gestation.
The next phase was sample collection. The factors assessed to quantify productive performance in this experiment were limited to the body weight gain of goats. The body weight gain of goats was assessed by determining the difference between their final and initial body weight during the gestation. The milk production of goats was quantified by volumetric quantification. The does were manually milked twice daily, at 07.00 AM and 4.00 PM. Milking was conducted gently to minimize stress. Every two weeks, milk samples for quality analysis were collected. Sampling was conducted on daily production, with samples collected from the morning and afternoon milking and stored in 100ml bottles.
Milk quality test:
- Milk density: The density of milk was measured by a lactodensitymeter.
- Milk pH: The pH of milk was measured using a pH-meter.
- Milk fat: The fat content was quantified using the soxhlet extraction apparatus. Five grams of milk poured and dried in a flask, then petroleum ether was added. The extraction process lasted for a duration of 10 hours, following which the ether was evaporated completely. The quantity of fat was determined by calculating the difference in weight of the flask both before and after the removal of the ether (AOAC, 1990).
- Milk protein: Protein was determined by the Kjeldahl method (AOAC, 1990). To a 5g milk sample placed in a Kjeldahl flask was added 1g of mixed catalyst and 15ml of concentrated sulfuric acid. The flask was heated for 1 hour. After cooling, 20ml distilled water was added. A 5ml of the diluted sample then placed in a micro-Kjeldahl unit and added 10ml of sodium hydroxide then steam distilled, collecting the condensate into a 5ml solution of 4% boric acid. The collected condensate then titrated with 0.01M of HCl until red color appears. The procedure was repeated four times. A blank sample was treated similarly. The crude protein was then calculated by the equation:
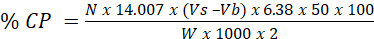
N= normality of standard HCl;Vs and Vb= volumes of standard HCl used to titrate sample and blank respectively; W= weight of sample.
- Milk lactose: The determination of lactose was conducted using a calorimetric method as specified by AOAC (1990). A volume of 1ml of milk sample was combined with 2ml of 10% sodium tungstate. The mixture was then gently mixed by adding 2ml of 0.33M sulfuric acid. The solution was diluted by 100ml of water and left for five minutes before filtered. Two ml of alkaline copper reagent was added to 1ml of the filtrate, and heated in a water bath for 8 minutes. A 4 ml of phosphomolybdic acid reagent was added and subsequently, the mixture was diluted to a volume of 25ml using diluted phosphomolybdic acid. The solution’s absorbance was measured at a wavelength of 600nm. The lactose concentration was determined using a standard curve.
Statistical Analysis
Descriptive analysis was deployed to explain qualitative data that were from phytochemical tests and bioactive identification. The remaining parameters were qualitative data toxicity, productivity, and milk quality, then analyzed using a one-way ANOVA (Analysis of Variance) in SPSS (IBM Statistic) with a model based on a Complete Randomized Design (CRD) to evaluate differences among treatments’ mean. A significance level of P<0.05 was set to emphasize the result as statistically significant differences. Duncan’s multiple range test (DMRT) as described by Steel and Torrie (1980), was applied to compare and rank these differences between treatment means.
RESULTS AND DISCUSSION
Toxicological Test of C. calothyrsus
The toxicological test in this investigation used Artemia salina larvae as an indicator or test object. The test was a preliminary step to determine whether C. calothyrsus contained bioactive compounds. Each fraction was evaluated for pharmacological activity. The pharmacological action of various compounds in C. calothyrsus was assessed using the percentage of larvae mortality. Table 2 shows the complete toxicological test results for each fraction (hexane, chloroform, ethyl acetate, 50% ethanol, and blank) on Artemia salina larvae using the Brine Shrimp Lethality Test (BSLT) method.
Table 2: Mortality rate (%) of Artemia salina by the Brine Shrimp Lethality Test (BSLT) method.
|
Fractions |
Concentration (ppm) |
P value |
||
|
10 |
100 |
1000 |
||
|
Hexane |
6.67±5.78a |
20.00±0.00b |
60.00±10.00c |
0.00 |
|
Chloroform |
6.67±5.78a |
26.67±5.78b |
76.67±5.78c |
0.00 |
|
Ethyl acetate |
13.34±5.78a |
50.00±10.00b |
100.00±0.00c |
0.00 |
|
50% Ethanol |
13.34±5.78a |
26.67±5.78b |
76.67±5.78c |
0.00 |
|
Blank |
0.00 |
0.00 |
0.00 |
|
Note: similar superscript in a row indicates the difference was not significant (P>0.05).
The ethyl acetate fraction exhibited the maximum percentage of Artemia salina mortality among the concentrations of 10, 100, and 1000 ppm, with 13.3, 50, and 100% being the average mortality rates in this fraction, respectively. Additionally, the hexane fraction exhibited the lowest mortality rate of Artemia salina among the concentrations, with 66.6%, 20%, and 60% as the average mortality rates of this fraction. Regression analysis was deployed to evaluate all data collected during this investigation to emphasize the result. Table 3 shows the result of the regression analysis. The results indicated that all C. calothyrsus fractions (from hexane, chloroform, ethyl ethanol, and 50% ethanol extraction) contained bioactive compounds, as the LC50 (Lethal Concentration 50) of all fractions was less than 1000 ppm. The LC50 value below 1000 ppm indicates bioactive compounds on a sample (Meyer et al., 1982).
Table 3: The LC50 value of the different fractions of C. calothyrsus.
|
Fractions |
LC50 (ppm) |
Regression |
|
Hexane |
818.50 |
X=(Y-10.370)/0.0501 |
|
Chloroform |
591.94 |
X=(Y-12.778)/0.0646 |
|
Ethyl Acetate |
100.00 |
X=(Y-26.851)/0.0746 |
|
50 % ethanol |
560.63 |
X=(Y-16.481)/0.0606 |
The LC50 represents the percentage concentration required to kill half of the total population of Artemia salina larvae. The LC50 value can be used to estimate the bioactive potential of certain substances in some samples. The compounds exhibit bioactive potential when LC50 is less than 1000 ppm (Throne et al., 1995). Table 3 reveals that the LC50 for all fractions was less than 1000 ppm. The LC50 value was highest in the hexane fraction (818.50 ppm) and lowest in the ethyl acetate fraction (100 ppm). Based on these findings, it was predicted that C. calothyrsus leaves contain some bioactive compounds, especially those contained in ethyl acetate fraction.
Phytochemical Test of C. calothyrsus
The phytochemical analysis of C. calothyrsus leaves was conducted following the toxicological test. The phytochemical screening was also performed on each individual fraction (hexane, chloroform, ethyl acetate, and 50% ethanol). The study found numerous bioactive compounds in C. calothyrsus leaves, including lipids and fatty acids, steroids and triterpenoids, flavonoids, saponins, carbohydrates, and tannins (Table 4). The hexane fraction contained lipids, fatty acids, and monoterpenoids. Hexanes, chloroform, and ethyl acetate fractions also included terpenoids and steroids. In this experiment, the saponin content of C. calothyrsus leaves was negligible. This compound proved positive in the 50% ethanol fraction.
This investigation aligns with the findings of Islam et al. (2023), which suggest that C. calothyrsus leaf extract is adequate in containing bioactive components such as saponins. Similarly, Messi et al. (2020) reported the presence of secondary metabolites in C. calothyrsus. The leaves of C. calothyrsus contain 9.2% condensed tannins and saponins, indicating that supplementation with these leaves may enhance sheep health and performance as well as reduce methane emissions (Mwangi et al., 2024). Additionally, pipecolic acid is a non-protein amino acid, and its derivatives have been isolated from C. calothyrsus leaves (Návarová et al., 2013), with these compounds exhibiting insecticidal properties (Somba et al., 2019). While the effects of these chemicals on sheep are not yet comprehensively understood, it is reasonable that some saponins, which are glycosides composed of a polycyclic aglycone structure—either a C27 steroid or a C30 triterpenoid (collectively known as sapogenins)—linked to carbohydrates, could play a role. These saponins are widely distributed in the plant kingdom and are characterized by their bitter taste and foaming properties (Aganga and Mosase n.d., 2001). Furthermore, saponins are known to lyse erythrocytes in solution and are toxic when administered intravenously.
Table 4: Phytochemical analysis of the hexane, chloroform, ethyl acetate, and 50% ethanol extract fractions of C. calothyrsus leaves.
|
No. |
Compounds |
Extract Fraction |
|||
|
Hexane |
Choroform |
Ethyl acetate |
50% Ethanol |
||
|
1 |
Volatile oils |
- |
|||
|
2 |
Fat and Fatty Acids |
+ |
|||
|
3 |
Monoterpenoids |
+ |
|||
|
4 |
Alkaloids |
- |
|||
|
5 |
Sesqui/diterpenoids |
+ |
|||
|
6 |
Triterpenoids |
+ |
|||
|
7 |
Glycoside di/triterpenoids |
+ |
- |
||
|
8 |
Glycoside/Saponins |
+ |
|||
|
9 |
Carbohydrates |
+ |
|||
|
10 |
Tannins |
+ |
|||
(+): Detected; (-): Not detected.
Saponins are classified into two groups: steroidal saponins, found as glycosides in certain pasture plants, and triterpenoid saponins, which occur in soybean and alfalfa (Mwangi et al., 2024). The anti-nutritional effects of saponins have been primarily studied using alfalfa saponins. In ruminants, saponins were initially implicated in causing bloat (Setiawan et al., 2022). However, later studies have suggested that they are not involved in the bloat syndrome. Furthermore, saponins may affect bacterial growth in the rumen, which involves feed degradation; the growth of bacteria is not likely to be hindered. Nevertheless, recent studies have shown that microbial fermentation and synthesis in the rumen are inhibited by saponins (Kholif, 2023).
Saponins have the ability to modulate the ruminal microbial community and ruminal metabolites, thus affecting the rumen environment (Wang et al., 2019). However, the response depends on the composition of the basal diet. These results indicate that saponins from different plant species have varied biological effects, probably due to structural differences in their sapogenin fractions. Strategies to reduce anti-nutritional factors, including saponins, in plant-based foods include appropriate processing, such as repeated washing and fermentation, which can enhance palatability and reduce the content of toxic compounds (Samtiya et al., 2020).
Tannins are water-soluble phenolic compounds with a molecular weight of more than 500 Daltons and can precipitate proteins from water. Tannins occur in almost all vascular plants, and the leaves of trees or shrubs contain tannins which can be hydrolyzed and condensed. These two types differ in their nutritional effects. Condensed Tannins (CTs) are widely contained in legumes and grain forages. Goats are able to tolerate CTs better than cattle and sheep (Yisehak et al., 2016). CT can deliver nutritional benefits by increasing bypass protein availability and suppressing bloat in cattle at moderate levels (30 – 40 g/kg legume in dry matter). At higher levels (100 – 129 g/kg legume dry matter), a reduction in gastrointestinal parasitism in sheep has been reported Miles et al. (1969), mechanism of the effect of tannins on feed can be understood from their ability to integrate with proteins to make some complexes. Condensed tannins from feed (2 – 3%) are beneficial for ruminants because they reduce the degradation of proteins in the rumen by forming protein-tannin complexes (Ifani et al., 2021). Aganga and Mosase n.d.(2001), reported that the tannin content in the seeds is relatively low and may be beneficial for ruminants due to its effect in reducing forage protein degradation in the rumen, which can be overcome by increasing protein availability in the small intestine.
Shrubs and tree forage may contain alkaloids, terpenoids, oxalate, internecine, lignin, and other anti-nutrition factors (ANFs). Alkaloids such as N-methyl-a-phenethylamine cause locomotor ataxia of the hind quarters in sheep. Sesbania causes hemorrhagic diarrhoea. The terpenoids azadirachthin and lignin impart a bitter taste, and Acacia aneura leaves may have low Ca content. This study shows that the lignin content in feed is negatively correlated with dry matter digestibility (DMD), which impacts the nutritional value of the feed (Abraham et al., 2023). High lignin content can inhibit the decomposition of cellulose into glucose, reducing the efficiency of feed digestibility (Novaes et al., 2010).
Table 5: Identified bioactive compound by Gas Chromatography-Mass Spectrometry (GC-MS) of C. calothyrsus leaves.
|
Fraction |
R.T. (m:s) |
Area (%) |
Mol Weight (Da) |
Molecule Structure |
Predicted compound |
|
70% Ethanol 3 peaks: |
08:97 |
08.25 |
296.31 |
C20H40O |
2-Hexadecen-1-ol, 3,7,11,15-tetramethyl- [R- [R*,R'-(E)]]- (CAS) |
|
13:92 |
56.48 |
430.38 |
C29H50O2 |
Vitamin E |
|
|
10:42 |
00.67 |
102.05 |
C5H10S |
2-methyl-1-thia-cyclopentane |
|
|
Hexane 3 peaks |
04:56 |
67.66 |
296.31 |
C20H40O |
Phytol |
|
08:33 |
15.13 |
430.38 |
C29H50O2 |
Vitamin E, 2H-1-Benzopyran-6-ol, alpha-tocopherol |
|
|
10:13 |
03.79 |
412.37 |
C29H48O |
Stigmasta-5, 22-dien-3-ol, (3.beta.,22E) - (CAS),stigmasterol |
|
|
Chloroform 7 peaks: |
04:48 |
05.55 |
139.06 |
C7H9NO2 |
1H-Pyrrole-2,5 dione, 3-ethyl-4-methyl-me, 2-methoxy-4-aminophenol |
|
07:97 |
08.76 |
180.12 |
C11H1602 |
2 (4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4, 7a-trimethyl-(CAS) |
|
|
09:77 |
04.14 |
180.15 |
C12H20O |
Endo-7-Butyl-7-methoxybycyclo(4.1.0) hept-2-ene |
|
|
09:81 |
04.54 |
208.09 |
C11H16O2Si |
Acetophenone, 2'-(trimethylsiloxy)- (CAS), O-Hydroxyacetophenone-TMS- Ether, Ethanone, 1-[2-[(trimethylsilyl) oxy] phenyl]- |
|
|
10:59 |
56.14 |
196.11 |
C11H16O3 |
Loliolide, 2(4H)-Benzofuranone, digiprolactone, calend |
|
|
10:83 |
08.03 |
124.05 |
C7H8O2 |
1,3-Benzenediol, 5-methyl- (CAS), Orcinol, Orcin, Guaiacol glycerol ether |
|
|
17:44 |
11.21 |
390.28 |
C24H38O4 |
1,2-Benzenedicarboxylid acid, bis (2-ethylhexyl) ester (CAS) |
R.T: Retention time.
Bioative Compound Test of C. calothyrsus
The result of GC-MS analysis shows that the leaves of C. calothyrsus contain 12 substances, which may contribute to the biological effects resulting from synergistic or antagonistic effects of these substances. Many substances have been found in C. calothyrsus, including steroids, stigmasterol, vitamin E, isophytol, and antibacterials. The identified substances can be grouped into: 1. Vitamin E substances such as C20H40O and C29H50O2 in 50 % ethanol and hexane fraction; 2. Antimicrobial and insecticidal substances such as C11H16O2 and C11H16O3; 3. Stigmasterol; 4. Steroids such as C7H8O2 and C11H16O2Si. The quantity reflected by the chromatogram area was dominated by Vitamin E, Phytol and Loliolide for 50% ethanol, hexane, and chloroform fractions, respectively. Detailed compound identification is shown in Table 5.
The result of GC-MS analysis shows that the leaves of C. calothyrsus contain 12 substances, which may contribute to the biological effects resulting from synergistic or antagonistic effects of these substances. Many substances have been found in C. calothyrsus, including steroids, stigmasterol, vitamin E, isophytol, and antibacterials. The identified substances can be grouped into four groups: 1. Vitamin E substances such as C20H40O and C29H50O2 in 50 % ethanol and hexane fraction; 2. Antimicrobial and insecticidal substances such as C11H16O2 and C11H16O3; 3. Stigmasterol; 4. Steroids such as C7H8O2 and C11H16O2Si.The quantity reflected by the chromatogram area was dominated by Vitamin E, Phytol and Loliolide for ethanol, hexane, and chloroform fractions, respectively. Detailed compound identification is shown in Table 5.
C. calothyrsus as Concentrate Replacement on Peranakan Etawa (PE) Does Productivity
The productive performance of Indonesian Peranakan Ettawah crossbreed goats fed diets containing different levels of C. calothyrsus leaves is shown in Table 6. The average gain and dry matter intake of goats throughout the gestation period were not affected by treatment (P>0.05). The result means that the level substitution of dried C. calothyrsus leaves up to 30 % of concentrate in the diets did not affect all parameters of productive performance of Indonesian PE goats.
Table 6 shows that body weight gain and dry matter intake of all does during the gestation period were almost similar throughout diets, although there tended to increase from T1 (0% C. calothyrsus) to T4 (30 % C. calothyrsus). However, treatments had no significant difference (P>0.05). The results implicate that the dried C. calothyrsus leaves could replace coconut oil meal and soybean cake meal by up to 30% in the diets without any adverse effect on body weight gain and dry matter intake, FCR and feed efficiency of pregnant does. The response curve showing the effect of treatments on the increase in body weight due to the substitution of conventional protein sources by C. calothyrsus leaves is illustrated in Figure 2.
Figure 2 displays that replacing conventional protein sources with C. calothyrsus leaves did not significantly affect body weight gain in PE does. The average body weight gain during pregnancy of T1-T4 was recorded as follows: 14.28±1.59; 15.22±0.53; 15.28±1.33; 14.14±1.47kg with no significant differences observed (P>0.05). These findings
Table 6: The productive performance of Indonesian PE lactating does diets containing different levels of dried C. calothyrsus leaves (0 – 30 %) during experiment.
|
Parameters |
Diets |
P value |
|||
|
T1 |
T2 |
T3 |
T4 |
||
|
During gestation: |
|||||
|
Initial weight (kg) |
34.80±1.02 |
33.40±0.78 |
34.75±0.82 |
34.60±0.91 |
0.12 |
|
Final weight (kg) |
49.00±0.92a |
48.62±0.67a |
50.03±0.77a |
48.74±0.61a |
0.07 |
|
Gain (kg) |
14.28±1.59a |
15.22±0.53a |
15.28±1.33a |
14.14±1.47a |
0.45 |
|
Average daily gain (kg/day) |
0.20±0.02a |
0.22±0.01a |
0.22±0.02a |
0.20±0.02a |
0.48 |
|
DMI (g/day) |
1.68±0.04a |
1.68±0.05a |
1.73±0.07a |
1.71±0.03a |
0.44 |
|
DMI/body weight (%) |
3.43±0.04a |
3.45±0.07a |
3.46±0.11a |
3.50±0.09a |
0.66 |
|
12.03±1.31a |
13.02±0.7a |
12.59±0.78a |
11.87±1.53a |
0.48 |
|
|
Feed conversion ratio (FCR) |
8.36±0.77a |
7.73±0.41a |
7.79±0.48a |
8.54±0.93a |
0.36 |
Note: similar superscript in a row indicates the difference was not significant (P>0.05); T1: 0% supplementation; T2: 10% suppl; T3: 20% suppl; T4: 30% suppl; DMI: Dry matter intake.
align with the results reported by Norton and Waterfall (2000), where legume leaves were commonly used to improve the digestibility of low-quality grasses and crop by-products in tropical regions. In Zambia, body weight loss (-20 g/day) was observed in goats fed with poor-quality hay; however, body weight gain (24 g/day) was noted following supplementation with C. calothyrsus at 140 g/day in dry matter. Furthermore, the nutritional status of pregnant sheep was improved by C. calothyrsus leaves (Mwangi et al., 2024). No significant differences in body weight gain and wool production were found when sheep were fed fresh or wilted C. calothyrsus leaves as a 28% supplement to Buffel grass (Palmer and Ibrahim, 1996). The composition of the basal diet influences the response of animals to feed supplements, the type of fodder tree leaves, and the level of supplementation (Mwangi et al., 2024). Additionally, increasing the level of fresh C. calothyrsus leaves from 0% to 35% in a diet of low-quality hay resulted in an increase in the daily body weight gain of sheep from 27 to 52 g/day (Palmer and Ibrahim, 1996).
The dry matter intake of T4 (3.50±0.09%) was the highest, and the lowest dry matter intake was T1 (3.43±0.04%), although there was no significant difference among treatments (P>0.05) resulting similar FCR and feed efficiency. The result was probably caused by the bulkiness of the diets by supplementation. In the present study, the bulkiness diet on T1 was probably because of the fat (lipid) content in the coconut oil meal and water content in the soybean cake meal; therefore, the feed intake of goats fed T1 was lower than that of the others. This finding is in agreement with a report noted that dry matter intake is influenced by many factors, such as the environment, the physical capacity of the intestine, the bulkiness of the diet, and the need to avoid imbalances or excesses of a particular nutrient (Huhtanen et al., 2008). Furthermore, Miles et al. (1969) noted that the diet’s composition and availability of nutrients are significant factors influencing voluntary intake. The protein concentration, the balance of amino acids, and deficiency or excess of minerals or vitamins can all affect intake. The significant parameter of a feed that determines intake quantity is the concentration of available energy.
Table 7 gives the average data of milk production, pH, and density during the ten weeks of the experiment. The different diets of treatment did not affect milk production, pH, or density (P>0.05), although the milk yield tended to increase from T1 to T4. The finding indicates that substituting dried C. calothyrsus leaves for coconut oil meal and soybean cake meal as concentrate in the diets did not affect milk production, pH, or density.
Variability in results for milk production is caused by genetics, parity, and climate, in addition to the rate and extent of carbohydrate fermentation and microbial protein synthesis in the rumen. Increased milk production can result from one or a combination of the following raseon: 1) increased microbial protein synthesis, 2) increased propionate concentrations (glucogenic precursor), 3) increased post-ruminal digestion of starch, or 4) increased amount of bypass protein (Ekinci and Broderick, 1997). The response curve showing the effect on milk production due to replacing conventional protein sources with leaves from Calliandra calothyrsus can be seen in Figure 3.
Figure 3 shows no significant response to milk production due to replacing conventional protein sources with Calliandra calothyrsus leaves. The mean overall milk production from Indonesian PE goats in this study was 662±55; 691±36; 732±72 and 738±65ml/day for does receiving T1, T2, T3, and T4, respectively. The mean daily milk yield of these goats was low. These observations are similar to other reports on PE goats in Indonesia. PE goats produced 0.45 - 2.2 L/day (Amiri Ghanatsaman et al., 2023), besides this, also found this goat produced 1.83 kg/day (Pazla and Arief, 2023). The difference is due to the type of forage and concentrates used. The range of milk production of these goats is wide and varied, because most goats in the tropics are meat or dual-purpose breeds and have not been intensively selected for milk production. Indeed, milk production showed that the phenotypic performance of Peranakan Etawah (PE) goats varied and was affected by genotypic variance. Therefore, it is better to consider tropical goats as dual-purpose meat and milk producers. The milk yield of goats in all treatments of this experiment was almost similar, but there was a tendency to increase of milk production in does fed with more C. calothyrsus. The effect of C. calothyrsus in improving milk production is typically indicated by its relatively high crude protein (CP) intake (Kholif et al., 2014; Widodo et al., 2019).
This study agrees with (Paterson et al., 2000), who found that dairy cows under East African conditions given 3 kg fresh C. calothyrsus leaves responded similarly with cows fed 1 kg of commercial concentrate in milk production and butterfat percentage. Likely, this feeding control does not provide a source of tannin-protected protein that affects milk yield and quality by increasing the amount of intestinally absorbed protein in these cows. Furthermore, milk production is primarily influenced by the pre and postpartum nutritional status of the does (Martins et al., 2022). The dry matter level and energy intake were positively related to milk production (Harvey et al., 2021). Any improvement in nutritional management, particularly in the forage quality in the ration, increases milk production (Fernández et al., 2021).
Table 7 shows that neither the density nor the pH of milk was significantly different in the different diets of this experiment (P>0.05). It has been reported that the density of milk is a function of the fat and total solids contents in the sample (Martins et al., 2022). The pH values of the milk from all treatments on expected results because the normal range of goats’ milk is 6.3 – 6.7. The composition of ewe and does milk varies over a wide range because of genetic differences between species, between breeds within species, and between breeds. These genetic differences considerably influence this milk’s cheese-making process and human digestion (Widodo et al., 2023). Furthermore, the variation in the stage of lactation, season, parity, type of feed, physiological status, health of the udder, and processing procedure change the quality of milk and its products (Erduran and Dag, 2021; Fernández et al., 2021).
The percentage of milk components measured during the experiment is given in Table 8. The study showed that the milk fat content in this experiment was influenced by treatments (P<0.05). The fat content of milk from diet T1 was significantly lower (P<0.05) compared to the fat content of milk of T2, T3, and T4, respectively; however, among T2,T3, and T4, there was no significant difference (P>0.05). Contrary to the fat content of goat’s milk, milk
Table 7: Average milk yields (ml), pH and density of Indonesian PE lactating does fed diets containing different levels of dried C. calothyrsus (0 – 30 %) during experiment.
|
Parameters |
Diets |
P value |
|||
|
T1 |
T2 |
T3 |
T4 |
||
|
Average milk production (ml/day) |
0.25 |
||||
|
pH |
6.56±0.11 |
6.60±0.10a |
6.57±0.16a |
6.59±0.12a |
0.96 |
|
Density |
1.032±0.003a |
1.033±0.003a |
1.033±0.003a |
1.033±0.002a |
0.92 |
Note: similar superscript in a row indicates the difference was not significant (P>0.05); T1: 0% supplementation; T2:10% suppl; T3: 20% suppl; T4: 30% suppl.
Table 8: Average milk composition of Indonesian PE lactating does fed diets containing different levels of dried C. calothyrsus (0 – 30 %) during 10 weeks of experiment.
|
Milk component |
T1 |
T2 |
T3 |
T4 |
P value |
|
Fat (%) |
4.31±0.11a |
5.83±0.16b |
5.83±0.15b |
5.83±0.18b |
0.00 |
|
Protein (%) |
5.24±0.36a |
0.57 |
|||
|
Lactose (%) |
4.52±0.25a |
4.65±0.31a |
4.65±0.37a |
4.65±0.36a |
0.92 |
Note: similar superscript in a row indicates the difference was not significant (P>0.05).
protein and lactose were not affected (P > 0.05) by the treatment of diets. The finding means that the substitution level of C. calothyrsus for coconut oil meal and soybean cake meal as concentrate in the diets did not affect the protein and lactose percentage of the milk. Variability in results for milk production may caused by genetics, parity, and climate, in addition to the rate and time of carbohydrate fermentation and microbial protein synthesis in the rumen. Increased milk production could be a result of one or a combination of the following:
- Increased microbial protein synthesis.
- Increased propionic acid concentrations in fermentation (glucogenic precursor).
- Increased post-ruminal digestion of starch.
- Increased amount of bypass protein (Ekinci and Broderick, 1997).
The different diets affected the fat contents of does’s milk in this experiment (P<0.05), probably caused by the intake of crude fibre of goats fed dried C. calothyrsus leaves. Fibrous concentrates tend to be associated with higher milk fat percentage and high starch concentrates with higher milk protein percentage (Carmo et al., 2015). This finding is consistent with (Paterson et al., 2000), who reported that C. calothyrsus had an observed positive effect of about 10% increment on the butterfat percentage of milk cows.
Due to the variability of milk fat and protein content, the possibility of altering milk composition by feeding is higher for fat than protein and casein percentage (Gross et al., 2015; John et al., 2019). Furthermore, milk fat percentage is affected by physiological and environmental factors, in which physiological factors generally involve changes in energy balance (Habimana et al., 2023). However, nutrition is the predominant factor affecting milk fat and represents a practical tool to alter its yield and composition. Energy deficiency in the diet can alter the fatty acid composition of milk fat towards higher medium-chain fatty acids, while daily milk yield may decrease but high in fat percentage (Goetsch et al., 2011; Laroche et al., 2022). Another theory causing lower milk fat is a shortage of lipid precursors available in the mammary gland. When starch in the rumen increases from the feed, ruminal propionate concentration may increase, causing accumulations of gluconeogenesis, stimulating insulin secretion, and decreasing the number of fatty acids that are released from adipose tissue for milk fat synthesis (Piantoni and VandeHaar, 2023). Another theory of milk fat depression is the direct inhibition of one or more stages in the synthesis of fat in the mammary gland (Osorio et al., 2016). The appearance of trans fatty acids, particularly trans-18 fatty acids, may also play a role in milk fat depression.
Substituting dried C. calothyrsus leaves for coconut oil meal and soybean cake meal as concentrate in the diet had no effect (P>0.05) on the protein and lactose of milk of lactating does. In this experiment, the protein content of milk was T1 (5.56±0.45%); T2 (5.24±0.31%), T3 (5.24±0.42%), and T4 (5.24±0.36%). The milk lactose was T1 (4.52±0.25%), T2 (4.65±0.31%), T3 (4.65±0.37%), and T4 (4.65±0.36%). These milk constituents were almost similar and not influenced by different levels of substitution of dried C. calothyrsus in the diets. The results are probably related to the amounts of energy and crude protein in the diets, which were similar in T1 to T4.
The level of nutrition, precisely the level of energy or feed intake, is the main positive factor affecting milk yield and milk composition in dairy ruminants (Francois and Caja, 2004). The effects of nutrition on milk composition are still unclear because there was a natural change caused by the lactation stage and nutrition supply from feeding. Furthermore, in the middle and end of lactation, changes in nutrition mainly affect the persistence of production and usage of body fat as reserves; this is why biased effects is still considered on milk yield or composition (Herve et al., 2019). Other factors, including breed, stage of lactation, milking system, and feeding, are considered for the production and quality of the milk. In addition, consideration of milk yield to milk composition as fat, protein, casein, and serum proteins are negatively correlated, except the lactose (Tong et al., 2012).
Milk protein percentage is the direct consequence of two main processes, one resulting from the expression of milk-encoding genes in the mammary epithelial cell and the other resulting from the transfer of amino acids from plasma proteins (Bionaz et al., 2012; Zhou et al., 2018). During lactation, milk protein in healthy cows’ mammary glands resulting from both processes is carried through distinct intracellular compartments and the endocytotic-transcytotic compartments. Intracellular membranes separate the different pools in which different biochemical reactions take place. Since the intracellular membrane is strictly dependent on cell differentiation, which is controlled by the physiological and biochemical reactions occurring in these intracellular compartments. Milk protein composition is directly influenced by mammary epithelial cell physiology (Rosen et al., 1999).
CONCLUSIONS AND RECOMMENDATIONS
The Calliandra calothyrsus leaves contain bioactive compounds. Based on the phytochemical test, the compounds in the C. calothyrsus leaves were lipid and fatty acids, monoterpenoids, sesqui/diterpenoids, triterpenoids, glycosides/triterpenoids, saponins, carbohydrate, and tannin, respectively. The chemical compounds of the C. calothyrsus in this experiment were C20H40O, C29H50O2, C5H10S, C29H48O, C7H9NO2, C11H16O2, C12H20O, C11H16O2Si, C11H16O3, C7H8O2, and C24H38O4. The phytochemical compounds of C. calothyrsus leaves are in safe level.
The dried C. calothyrsus leaves could replace conventional/traditional concentrate (coconut oil meal and soybean cake meal) by up to 30 % of the goat diet without any significant effect on their productive and quality of milk. This shows that it is possible to replace concentrate using C. calothyrsus without affecting productive performance such milk production and composition.
ACKNOWLEDGEMENTS
The authors express their gratitude to the Faculty of Animal Science at Jenderal Soedirman University and LIPI Bogor for granting permission.
NOVELTY STATEMENT
The results of the study showed that dried C. calothyrsus leaves could replace conventional/traditional concentrates (coconut meal and soybean meal) in up to 30% of goats without significantly affecting milk productivity and quality. The concentrate substitution is helpful in gaining PE goat’s potency as dairy animals in Indonesia. This research also proved that C. calothyrsus contains some phytochemical compounds at safe levels for supplementation which was not previously studied.
AUTHOR’S CONTRIBUTIONS
Yusuf Subagyo: Data collection, analysis and manuscript drafting.
Merryafinola Ifani: Data analysis and manuscript drafting.
Hermawan Setyo Widodo: Manuscript drafting.
Conflict of Interest
The authors declared no conflict of interest.
REFERENCES
Abqoriyah A, Utomo R, Suwignyo B (2015). Productivity of Calliandra plants (Calliandra calothyrsus) as forage at different defoliation ages. Bul. Peternak., 39(2): 103. https://doi.org/10.21059/buletinpeternak.v39i2.6714
Abraham G, Kechero Y, Andualem D (2023). Nutritional quality of indigenous legume browse in southern Ethiopia: farmers’ preference and correlation of local valuation of feed value with scientific indicators. Front. Vet. Sci., 10: 1198212. https://doi.org/10.3389/fvets.2023.1198212
Aganga AA, Mosase KW n.d.(2001). Tannin content, nutritive value and dry matter digestibility of Lonchocarpus capassa, Zizyphus mucronata, Sclerocarya birrea, Kirkia acuminata and Rhus lancea seeds. Anim. Feed Sci. Technol., 91(1–2): 107–113. https://doi.org/10.1016/S0377-8401(01)00235-8
Amiri Ghanatsaman Z, Ayatolahi Mehrgardi A, Asadollahpour Nanaei H, Esmailizadeh A (2023). Comparative genomic analysis uncovers candidate genes related with milk production and adaptive traits in goat breeds. Sci. Rep., 13(1): 8722. https://doi.org/10.1038/s41598-023-35973-0
AOAC (1990). Official methods of analysis. 13 Ed). Assoc. Off. Anal. Chem., Washington DC: USA.
Bionaz M, Hurley W, Loor J (2012). Milk Protein Synthesis in the Lactating Mammary Gland: Insights from Transcriptomics Analyses. In Milk Protein InTech, https://doi.org/10.5772/46054
Carmo CA, Batistel F, de Souza J, Martinez JC, Correa P, Pedroso AM, Santos FAP (2015). Starch levels on performance, milk composition and energy balance of lactating dairy cows. Trop. Anim. Health Prod., 47(1): 179–184. https://doi.org/10.1007/s11250-014-0704-4
Devendra C, Liang JB (2012). Conference summary of dairy goats in Asia: Current status, multifunctional contribution to food security and potential improvements. Small Rumin. Res., 108(1–3): 1–11. https://doi.org/10.1016/J.SMALLRUMRES.2012.08.012
Ekinci C, Broderick GA (1997). Effect of Processing High Moisture Ear Corn on Ruminai Fermentation and Milk Yield. J. Dairy Sci., 80(12): 3298–3307. https://doi.org/10.3168/jds.S0022-0302(97)76305-7
Erduran H, Dag B (2021). Determination of factors affecting milk yield, composition and udder morphometry of Hair and cross-bred dairy goats in a semi-intensive system. J. Dairy Res., 88(3): 265–269. https://doi.org/10.1017/S0022029921000637
Fernández N, Carmen Beltrán M, Romero G, Amparo Roca M, Rodríguez M, Balasch S (2021). Pointing out some issues regarding reproduction management in murciano-granadina goats. Animals, 11(6): 1781. https://doi.org/10.3390/ANI11061781
Francois B, Caja G (2004). Effects of nutrition on ewe’s milk quality. In Principles of sheep dairying in North America (B. Y, B. P, B. F, C. G, C. A, M. B, M. P-G, and T. D (Eds.), (pp. 51–61) University of Wisconsin-Extension Service, United Kingdom. https://www.researchgate.net/publication/256343773
Goetsch AL, Zeng SS, Gipson TA (2011). Factors affecting goat milk production and quality. Small Rumin. Res., 101(1–3): 55–63. https://doi.org/10.1016/j.smallrumres.2011.09.025
Gross JJ, Kessler EC, Albrecht C, Bruckmaier RM (2015). Response of the Cholesterol Metabolism to a Negative Energy Balance in Dairy Cows Depends on the Lactational Stage. PLoS One, 10(6): e0121956. https://doi.org/10.1371/journal.pone.0121956
Habimana V, Nguluma AS, Nziku ZC, Ekine-Dzivenu CC, Morota G, Mrode R, Chenyambuga SW (2023). Heat stress effects on milk yield traits and metabolites and mitigation strategies for dairy cattle breeds reared in tropical and sub-tropical countries. Front. Vet. Sci., 10: 1121499. https://doi.org/10.3389/fvets.2023.1121499
Harvey KM, Cooke RF, Moriel P (2021). Impacts of Nutritional Management During Early Postnatal Life on Long-Term Physiological and Productive Responses of Beef Cattle. Front. Anim. Sci., 2: 730356. https://doi.org/10.3389/fanim.2021.730356
Herve L, Quesnel H, Veron M, Portanguen J, Gross JJ, Bruckmaier RM, Boutinaud M (2019). Milk yield loss in response to feed restriction is associated with mammary epithelial cell exfoliation in dairy cows. J. Dairy Sci., 102(3): 2670–2685. https://doi.org/10.3168/jds.2018-15398
Huhtanen P, Rinne M, Nousiainen J (2008). Evaluation of concentrate factors affecting silage intake of dairy cows: A development of the relative total diet intake index. Animal, 2(6): 942–953. https://doi.org/10.1017/S1751731108001924
Ifani M, Suhartati FM, Rimbawanto EA (2021). Effect of Protection of Soybean Meal Using Mahogany Leaf Extract in Ruminant Diet on Rumen Fermentation Products. J. Ilmu Ternak Dan Vet., 26(3): 96–107. https://doi.org/10.14334/jitv.v26i3.2829
Islam M, Malakar S, Rao M V, Kumar N, Sahu JK (2023). Recent advancement in ultrasound-assisted novel technologies for the extraction of bioactive compounds from herbal plants: a review. Food Sci. Biotechnol., 32(13): 1763–1782. https://doi.org/10.1007/s10068-023-01346-6
John A, Sun R, Maillart L, Schaefer A, Spence EH, Perrin MT (2019). Macronutrient variability in human milk from donors to a milk bank: Implications for feeding preterm infants. PLoS One, 14(1): 0210610. https://doi.org/10.1371/journal.pone.0210610
Kholif AE (2023). A Review of Effect of Saponins on Ruminal Fermentation, Health and Performance of Ruminants. Vet. Sci., 10(7): 450. https://doi.org/10.3390/vetsci10070450
Kholif AE, Khattab HM, El-Shewy AA, Salem AZM, Kholif AM, El-Sayed MM, Gado HM, Mariezcurrena MD (2014). Nutrient digestibility, ruminal fermentation activities, serum parameters and milk production and composition of lactating goats fed diets containing rice straw treated with Pleurotus ostreatus. Asian-Australasian J. Anim. Sci., 27(3): 357–364. https://doi.org/10.5713/AJAS.2013.13405
Laroche J-P, Gervais R, Lapierre H, Ouellet DR, Tremblay GF, Halde C, Boucher M-S, Charbonneau É (2022). Milk production and efficiency of utilization of nitrogen, metabolizable protein, and amino acids are affected by protein and energy supplies in dairy cows fed alfalfa-based diets. J. Dairy Sci., 105(1): 329–346. https://doi.org/10.3168/jds.2021-20923
Martins LF, Wasson DE, Hristov AN (2022). Feeding dairy cows for improved metabolism and health. Anim. Front., 12(5): 29–36. https://doi.org/10.1093/af/vfac059
Messi LM, Noté OP, Mbing JN, Lavedan P, Vedrenne M, Ouedraogo N, Carraz M, Bourgeade-Delmas S, Pegnyemb DE, Haddad M (2020). Triterpenoid saponins from Calliandra calothyrsus Meisn. and their antiproliferative activity against two digestive carcinoma human cell lines. Fitoterapia, 146: 104669. https://doi.org/10.1016/j.fitote.2020.104669
Meyer B, Ferrigni N, Putnam J, Jacobsen L, Nichols D, McLaughlin J (1982). Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med., 45(05): 31–34. https://doi.org/10.1055/s-2007-971236
Miles DG, Walters RJK, Evans EM (1969). Dry-matter intake and live-weight gain of cattle and sheep offered different grass varieties with and without clover. Anim. Prod., 11(1): 19–28. https://doi.org/10.1017/S000335610002657X
Mwangi PM, Eckard R, Gluecks I, Merbold L, Mulat DG, Gakige J, Marquardt S, Pinares-Patino CS (2024). Supplementation of a tropical low-quality forage with Calliandra calothyrsus improves sheep health and performance, and reduces methane emission. Front. Anim. Sci., 5: 1296203. https://doi.org/10.3389/fanim.2024.1296203
Návarová H, Bernsdorff F, Döring AC, Zeier J (2013). Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell, 24(12): 5123–5141. https://doi.org/10.1105/tpc.112.103564
Norton B., Waterfall M (2000). The nutritive value of Tipuana tipu and Calliandra calothyrsus as supplements to low-quality straw for goats. Small Rumin. Res., 38(2): 175–182. https://doi.org/10.1016/S0921-4488(00)00147-4
Novaes E, Kirst M, Chiang V, Winter-Sederoff H, Sederoff R (2010). Lignin and biomass: A negative correlation for wood formation and lignin content in trees. Plant Physiol., 154(2): 555–561. https://doi.org/10.1104/pp.110.161281
Osorio JS, Lohakare J, Bionaz M (2016). Biosynthesis of milk fat, protein, and lactose: roles of transcriptional and posttranscriptional regulation. Physiol. Genomics, 48(4): 231–256. https://doi.org/10.1152/physiolgenomics.00016.2015
Palmer B, Ibrahim TM (1996). Calliandra calothyrsus forage for the tropics -- a current assessment. In D. O. Evans (Ed.), Proceedings of the International Workshop on the Genus Calliandra. Forest, farm and Community Tree Research Reports (Special Issue) (pp. 183–194)Winrock International
Paterson RT, Roothaert RL, Kiruiro E (2000). The feeding of leaf meal of Calliandra calothyrsus to laying hens. Trop. Anim. Health Prod., 32(1): 51–61. https://doi.org/10.1023/A:1005293019581
Pazla R, Arief (2023). Milk Production and Quality of Etawa Crossbred Goats with Non-Conventional Forages and Palm Concentrates. Am. J. Anim. Vet. Sci., 18(1): 9–18. https://doi.org/10.3844/ajavsp.2023.9.18
Piantoni P, VandeHaar MJ (2023). Symposium review: The impact of absorbed nutrients on energy partitioning throughout lactation. J. Dairy Sci., 106(3): 2167–2180. https://doi.org/10.3168/jds.2022-22500
Rosen JM, Wyszomierski SL, Hadsell D (1999). Regulation of milk gene expression. Annu. Rev. Nutr., 19(1): 407–436. https://doi.org/10.1146/annurev.nutr.19.1.407
Samtiya M, Aluko RE, Dhewa T (2020). Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod. Process. Nutr., 2(1): 6. https://doi.org/10.1186/s43014-020-0020-5
Setiawan F, Erwanto E, Suharyati S, Siswanto S (2022). The effect of purslane flour (Portulaca oleracea) supplementation on total white blood cells and white cell differentation of Jawarandu goat. J. Ris. Dan Inov. Peternak., 6(1): 234–241. https://doi.org/10.23960//jrip.2022.6.1.58-65
Somba GC., Edy HJ, Siampa JP (2019). Antibacterial effectiveness test of ethanol extract cream preparation of Calliandra leaves (Calliandra surinamensis) against Staphylococcus aureus bacteria. J. MIPA, 8(3): 105. https://doi.org/10.35799/jmuo.8.3.2019.25776
Steel RGD, Torrie JH (1980). Principles and Procedures of Statistics. A Biometrical Approach. (2nd ed.)McGraw Hill
Throne JE, Weaver DK, Baker JE (1995). Probit Analysis: Assessing Goodness-of-Fit Based on Backtransformation and Residuals. J. Econ. Entomol., 88(5): 1513–1516. https://doi.org/10.1093/jee/88.5.1513
Tong HL, Li QZ, Gao XJ, Yin EY (2012). Establishment and characterization of a lactating dairy goat mammary gland epithelial cell line. Vitr. Cell. Dev. Biol. - Anim., 48(3): 149–155. https://doi.org/10.1007/s11626-012-9481-4
Waghorn G (2008). Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Technol., 147(1–3): 116–139. https://doi.org/10.1016/j.anifeedsci.2007.09.013
Wahyuni IMD, Muktiani A, Christianto M (2014). Determination of tannin and saponin dosage for defaunation improvement feed fermentability. J. Ilmu Dan Teknol. Peternak., 3(3): 133–140. https://doi.org/https://doi.org/10.20956/jitp.v3i3.788
Wang B, Ma MP, Diao QY, Tu Y (2019). Saponin-Induced Shifts in the Rumen Microbiome and Metabolome of Young Cattle. Front. Microbiol., 10: 00356. https://doi.org/10.3389/fmicb.2019.00356
Wasiati H, Faizal E (2018). Peternakan kambig Peranakan Etawa di kabupaten Bantul (Peranakan Etawa goat farms in Bantul Regency). J. Pengabdi. Masy. Univ. Merdeka Malang, 3(1): 8–14. https://doi.org/10.26905/abdimas.v3i1.2242
Widodo HS, Murti TW, Agus A, Pertiwiningrum A (2023). Identification of CSN1S1 Gene Variations Between Dairy Goat Breeds and its Influence on Milk Protein Fractions in Indonesia. Adv. Anim. Vet. Sci., 11(12): 1936–1944. https://doi.org/10.17582/journal.aavs/2023/11.12.1936.1944
Widodo HS, Sudjatmogo, Muktiani A, Nuswantoro LK, Harjanti DW, Syamsi AN (2019). Contribution of Different Feeding Method and Protein Source on Blood Urea as well as Urinal Nitrogen Excretion of Ettawah Crossbreed Goats. IOP Conf. Ser. Earth Environ. Sci., 372(1): 012063. https://doi.org/10.1088/1755-1315/372/1/012063
Yisehak K, Kibreab Y, Taye T, Lourenço MLA, Janssens GPJ (2016). Response to dietary tannin challenges in view of the browser/grazer dichotomy in an Ethiopian setting: Bonga sheep versus Kaffa goats. Trop. Anim. Health Prod., 48(1): 125–131. https://doi.org/10.1007/s11250-015-0931-3
Zhou Y, Zhou Z, Peng J, Loor JJ (2018). Methionine and valine activate the mammalian target of rapamycin complex 1 pathway through heterodimeric amino acid taste receptor (TAS1R1/TAS1R3) and intracellular Ca2+ in bovine mammary epithelial cells. J. Dairy Sci., 101(12): 11354–11363. https://doi.org/10.3168/jds.2018-14461
To share on other social networks, click on any share button. What are these?






