Calcium Carbide Induced Ethylene Regulates Seed Dormancy and Post-Germination Growth of Sweet Pepper
Research Article
Calcium Carbide Induced Ethylene Regulates Seed Dormancy and Post-Germination Growth of Sweet Pepper
Wazir Ahmed1*, Muhammad Naeem Akhtar2, Muhammad Waseem Akhtar3, Muhammad Nauman Hanif1, Aiman Salah ud Din4 and Ahmad Mahmood1
1Department of Soil and Environmental Sciences, MNS University of Agriculture Multan, Pakistan; 2Pesticide Quality Control Lab, Multan, Pakistan; 3Department of Plant Breeding and Genetics, University of Agriculture, Faisalabad. Pakistan; 4State Key Laboratory of Marine Food Processing & Safety Control, College of Food Science and Engineering, Ocean University of China, No.1299, Sansha Road, Qingdao, Shandong Province, 266404, P.R. China.
Abstract | Seed dormancy impacts seed germination success and seedling health. Ethylene plays a crucial role in facilitating the breaking of seed dormancy by initiating essential biochemical and physiological changes that are necessary for successful seed growth and development. It was hypothesized that acetylene (C2H2) from calcium carbide (CaC2) has the potential to induce an ethylene response in seed for breaking seed dormancy and better seedling growth. A study was conducted to evaluate the impacts of C2H2 released from ten CaC2 concentrations, i.e., 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 mg petri-plate-1, on seed dormancy and seedling growth in sweet pepper. Results indicated that CaC2 significantly induced 100% early seed germination, with significant improvements in post-germination variables. CaC2 applied at 14 mg petri-plate-1 was found to be an optimum dose for regulating seed dormancy and post-germination growth positively. However, CaC2 >16 mg petri-plate-1 proved lethal and suppressed the germination or growth of the seedlings. Compared to control treatment, improvements in seed sermination and growth variables, i.e., 8 to 37% in seed germination, 5 to 22% in seedling biomass, 5 to 46% in shoot length, and 3 to 37% in root length, suggest that calcium carbide, easily available on the market, can be used for seed dormancy breakage and better crop stand.
Received | May 13, 2024; Accepted | August 13, 2024; Published | August 27, 2024
*Correspondence | Wazir Ahmed, Department of Soil and Environmental Sciences, MNS University of Agriculture Multan, Pakistan; Email: [email protected]
Citation | Ahmed, W., M.N. Akhtar, M.W. Akhtar, M.N. Hanif, A.S. Din and A. Mahmood. 2024. Calcium carbide induced ethylene regulates seed dormancy and post-germination growth of sweet pepper. Pakistan Journal of Agricultural Research, 37(3): 223-231.
DOI | https://dx.doi.org/10.17582/journal.pjar/2024/37.3.223.231
Keywords | Acetylene, Calcium carbide, Ethylene, Germination, Sweet pepper
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Poor seed germination is still a top issue, particularly during off-season seeds usually remain dormant due to unfavorable environmental conditions. Germination processes are strictly regulated by the joint counteraction of different phytohormones (Wang et al., 2024). However, by regulating these hormones in seed physiology at molecular and genomic levels, a significant change in germination can be achieved (Miransari and Smith, 2014). Sweet pepper seeds germinate at 30°C, otherwise, these seeds remain dormant. During the off-season (October to November), the most of sweet pepper seeds remain dormant, and thus seedling vigor is very poor.
Ethylene (C2H4) is one of the phytohormones that play a key role in seed physiology and germination (Abeles et al., 2012; Sun et al., 2020; Baharudin and Osman, 2023). Plant physiologists believe that it is abscicic acid (ABA) that delays or prevents seed germination and determines the depth of dormancy during development (Sano and Marion-Poll, 2021; Kozaki and Aoyanagi, 2022). However, ethylene negatively regulates ABA accumulation in the seeds, and its signaling triggers seeds to germinate by accelerating endosperm weakening and testa rupturing (Kucera et al., 2005; Arc et al., 2013; Bhoi et al., 2023). During seed germination, the water uptake by seeds is triphasic; Phase I-imbibition (a rapid initial uptake), Phase II- plateau phase (uptake of water for metabolic preparation for germination), and Phase III uptake for hydraulic growth of the embryo (further increase in water uptake that occurs directly after germination to cause hydraulic growth of the embryo and the emerged seedling) whereas ethylene regulates these water uptake phases by interacting with ABA (Linkies et al., 2009). Stein et al. (2021) observed embryonic dormancy breakage in sweet cherry seeds due to exogenous C2H4. The efficacy of exogenous C2H4 for breaking dormancy in dormant and non-dormant seeds is well reported in the literature (Abeles et al., 2012; Shakar et al., 2016; Sun et al., 2020; Stein et al., 2021; Bailly et al., 2023). However, the rate and type of C2H4 source, C2H4 exposure time, and plant species/genotype are the factors that affect the efficacy of exogenous C2H4 for improving germination.
In addition to stimulating germination, ethylene also induces root proliferation (Dugardeyn and Van-Dser-Straeten, 2008) and is considered especially influential in fashioning the root system (Sun et al., 2020). Ethylene inhibits auxin activity during primary root cell elongation and initiates lateral root growth in seedlings (Patrick et al., 2009). Negi et al. (2008) confirmed that exogenous 1-amino cyclopropane 1-carboxylix acid (ACC) initiates lateral roots. Patrick et al. (2009) also corroborated root proliferation due to exogenous ACC. They observed inhibition of root elongation at higher rates of ACC but significant root proliferation with more root-hair. Various studies have elaborated on the role of exogenous C2H4 in improving germination and patterning root growth. Conclusively, exogenous C2H4 is an effective tool to improve the germination of dormant seeds.
Calcium carbide has recently been reported to increase the concentration of the plant hormone C2H4 in soil air as a result of microbial reduction of C2H2 into C2H4 (Arshad and Frankenberger, 2002). Acetylene (C2H2) liberated by the hydrolysis of CaC2 has poetential to induce C2H4 physiological role in seeds. Calcium carbide improved germination rate of different seeds, predominantly tomato (Siddiq et al., 2012), okra (Kashif et al., 2012), cucumber (Shakar et al., 2016a, b), etc. Seeds treated with coated CaC2 germinated much earlier than the untreated seeds. Moreover, CaC2 dependent release of C2H4 effectively induced the classical triple response in Pisum sativum seedlings (Khalid et al., 2006). Siddiq et al. (2012) reported that seeds of all tomato genotypes treated with CaC2 germinated earlier than untreated and led to maximum seed germination rate. Similarly, Yaseen et al. (2012) reported a 10 to 80% increase in the germination of tomato, cucumber, and okra seeds as a result of coated CaC2 application. In addition to the induction of C2H4 response, C2H2 released from CaC2 inhibits nitrification and denitrification, and thus improves nitrogen use efficiency in plants, resulting in better post-germination growth and crop stand (Kumar et al., 2015; Mahmood and Yaseen, 2016). This study indicates the potential of CaC2 to act as an C2H4 analog to break seed dormancy and improve post-germination growth in sweet pepper.
Materials and Methods
Seeds of sweet pepper (Capsicum anuum) cv. Yolo Wonder F-1 (85% germination rate at 25±2°C) were purchased from the market and stored at 25±2°C. The germination of the purchased seeds was assessed by a germination test. The response of seeds to ten rates of CaC2 (2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 CaC2 mg per petri-plate) was determined by placing sweet pepper seeds evenly in an 80 cc (cm3) petri-plate containing filter paper Whatman No. 1, moistened with 7 ml of distilled water. A treatment without CaC2 was also used as a control for comparison. The control treatment contained CaSO4 equivalent to Ca in 2 mg CaC2 just to nullify the Ca effect on seed germination and seedling vigor. The seeds were surface sterilized by dipping in 70% ethanol for 2 minutes, followed by five rinses with distilled water and drying with filter paper (Whatman No. 1) before the use in the experiment. Two autoclaved Whatman No. 1 filter papers were placed at the bottom of each petri-plate and then the required rate of CaC2 according to the treatment plan was placed in the center of it. The third filter paper was placed over it while sterilized seeds were spread on it. To inhibit the leakage of acetylene from petri-plates, each petri-plate was covered and tightly sealed with silicone and parafilm tape. After sealing the plates with silicone and parafilm tape, de-ionized water (7 mL, sufficient for moistening two filter papers) was injected into each petri-plate though a rubber septum by using a 12 mL disposable syringe. In each cover of perti-plate, a hole of 4 mm diameter near its edge was made, plugged with a rubber stopper, and sealed with silicone before use. All activities were performed in a laminar hood. All petri-plates were placed in an incubator (Sanyo Incubator MIR 253) under experimental conditions of 15±2oC temperature and 12/12 hours day and night, i.e. a light/dark period of four weeks. The light period was maintained with the help of cool white fluorescent bulbs (FL40SBR; National, Tokyo, Japan) fitted in the incubator that produced a photosynthetic photon flux density of 200 µmol m-2 s-1. In each petri-plate, the filter papers were kept wet on a weight basis by injecting de-ionized water through a rubber septum using a 12 ml disposable syringe. Germinated seeds with a radicle at least 2 mm long were counted after every 24 h interval for four weeks. At 528 h of incubation, the covers were removed, and data about seedling length, seedling weight, and chlorophyll contents were taken to calculate the means. The germination percentage was calculated as percent of the seeds germinated. The mean germination time (MGT) was calculated according to the equation of Ellis and Roberts (1981).
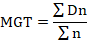
Where n is the number of seeds that germinated on day D, and D is the number of days counted from the beginning of the germination experiment. The germination index (GI) was calculated as described by the Association of Official Seed Analysis (1990) using the following formula:
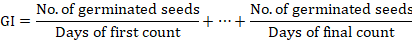
The vigor index (VI) was calculated according to the following formula (Dezfuli et al., 2008).
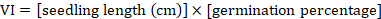
Chlorophyll content was measured from the seedlings of each petri-plate using a SPAD 502 Plus Chlorophyll Meter under light from cool white fluorescent bulbs fitted in the incubator. Shoot and root lengths were measured using measuring tape, while the shoot length was divided by the root length to calculate the shoot length to root length ratio (SL:RL). Seedlings were weighed using a top loading balance to calculate mean seedling weight.
Statistical analysis
A completely randomized design (CDR) with four replications was used. The data were subjected to an analysis of variance (ANOVA). The means were compared by the least significant difference (LSD) test at the 5% level of probability (Steel et al., 1997). The data were transformed in √arsine before the statistical analysis. Nonlinear regression analysis was used to determine how CaC2 affected the germination percentage. Germination (%) values at different rates of CaC2 were fitted to a functional three-parameter logistic model using SIGMA PLOT 2008 (version 11.0, SyStat Software GmbH, Schimmelbuschstrasse 25 D-40699 Erkrath, Germany). The model fitted was:
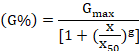
Where; G is the total germination (%) at concentration X, Gmax is the maximum germination (%), X50 is the rate of CaC2 for 50% of the maximum germination and g indicates the slope. Similarly, nonlinear regression analyses for MGT, GI, VI, shoot length, root length, and biomass at different rates of CaC2 were also determined and fitted to a cubic polynomial model using SIGMA PLOT 2008. The model fitted was:
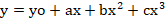
Whereas y is the response and x is the rate of CaC2.
Results and Discussion
Seed germination attributes
A three-parameter logistic model {G (%) = 89/[1 + (x/ 153)5.1], R2 = 0.988} was fitted to the germination (%) of C. annum obtained at different rates of CaC2 (Figure 3). The difference between the germination of treated and untreated seeds was significant at p < 0.05. The model indicates corresponding changes in
germination with increasing CaC2 rates. However, germination percentage declined sharply, signifying partial or complete inhibition of germination at ≥18 mg (Table 1, Figure 3). Although, the application of CaC2 improved the germination rate, a significant difference was witnessed at the mean germination time (MGT). The variations in MGT were according to cubic model that was fitted to it (Figure 3). Seeds in the control treatment had 10 days of MGT, which was reduced to 7 days due to the ethylene analog effect of CaC2. Application of CaC2 up to 14 mg plate-1 reduced MGT, but rates of CaC2 ≥16 mg plate-1 showed the reverse effect, observed at lower rates. A four-parameter Lorientzian model {y = 0.1+ 0.23/[(1+13.1)/0.4]2], R2 = 0.988} was fitted to the mean germination rate (MR) of C. annum under the influence of different rates of CaC2 (Figure 4). It was observed that peak MR occurred between 12 and 14 mg CaC2 plate-1 (Figure 4).
Germination and vigorous index
Significant differences were also noted in the germination index (GI) and vigorous index (VI) of treated and untreated seeds of C. annum. To make a difference more comprehensive, polynomial cubic models {y=0.0146-8.86x+0.001x2+ 0.00006x3} and [{y=125-6.69x+2.261x2-011x3}] were fitted to GI and VI, respectively (Figure 5). Both indices vary in cubic trends, with an increasing rate of CaC2 (Figure 6). However, maximum GI and VI were found in treatments containing 14 mg CaC2. An antagonistic effect of CaC2 on GI and VI was observed in treatments having CaC2>14 mg plate-1 i.e. 16, 18, and 20 mg CaC2 plate-1 (Figure 5).
Seedling length
Seedlings of sweet pepper in CaC2 containing petri-plates had longer roots and shoots compared to the control treatment (Figure 1). However, great variations originated due to differences in rates of CaC2 applied. The polynomial cubic model {y = 1.234-0.084x+0.233x2-0.001x3} was fitted to shoot length data (Figure 6) which indicated a gradual increase in shoot length with an increase in CaC2 rate (Figure 6). However, 18 and 20 mg plate-1 suppressed shoot length by 0.233 times with each incremental unit of CaC2. The model also suggests 12, 14 and 16 mg plate-1 as optimum rates of CaC2 for improving shoot length. Like shoot length, variations in root length are also the best described by polynomial cubic model {y=1.50-0.055x+0.06x2-0.0008x3} (Figure 6). Those rates of CaC2, that accelerated shoot length, also improved root length of sweet pepper (Figure 6). According to fitted model {y=1.50-0.055x+0.06x2-0.0008x3}, a strong synergistic relationship between 12 and 14 mg CaC2 and root length exists. Furthermore, it also predicted an antagonistic effect of CaC2 applied at rates ≥ 15 mg plate-1.
Seedling biomass and chlorophyll contents
Calcium carbide improved the seedling biomass of sweet pepper compared to the control (Figure 2). According to the polynomial cubic model {y=64.74-0.38x+0.4045x2-0.023x3} fitted to biomass, CaC2 improved biomass in cubic trend quite similar to other variables (Figure 7). However, maximum biomass was observed at 12 and 14 mg CaC2 plate-1 (Figure 7). he model fitted to biomass simulates complete growth inhibition upon > 20 mg plate-1 CaC2 application. Similar to biomass, a significant difference in leaf chlorophyll contents was observed due to CaC2 application (Figure 2).
Seed germination is considered a model system for the analysis of plant growth and development (Jia et al., 2024). It is the germination that decides when and where all further developments in a plant have to take place. Thus, germination is a process of decisive significance, and as such, it must be optimally regulated (Jia et al., 2024). Seeds possess sensing mechanisms that enable them to get information about the environment. On the basis of signals from these sensing mechanisms, seeds decide whether to germinate or not (Sajeev et al., 2024). These sensing mechanisms induce different types of seed dormancies by up- or down regulating ABA and GA (Kozaki and Aoyanagi, 2022). The ABA/GA ratio is considered the most critical factor during the germination of dormant or non-dormant seed (Kozaki and Aoyanagi, 2022). According to Finch-Savage and Leubner-Metzger (2006) and Sun et al. (2024), ABA-GA interaction decides the state and type of dormancy of a seed because embryonic dormancy persists if the ABA/GA ratio is higher. Similarly, if ABA/GA ratio gets lower, the seed dormancy comes to an end, which can be either because of an increase in GA level or a reduction in ABA level in the seed (Finch-Savage and Leubner-Metzger, 2006; Zuo and Xu, 2020; Kozaki and Aoyanagi, 2022; Sun et al., 2024).
The seed germination rate of C. annum cv. Yolo Wonder F-1 was 85%, indicating 15% seed dormancy at room temperature. However, achieving a 100% germination rate in CaC2 seeds revealed that CaC2 induced brekage of seed dormancy (Table 1). Seed dormancy might be broken because of the physiological role of C2H4 in seed germination at molecular and genomic levels because ethylene down-regulates ABA, resulting in a narrow ABA/GA ratio, as reported by Finch-Savage and Leubner-Metzger (2006), Sano and Marion-Poll (2021), Kozaki and Aoyanagi (2022), and Sun et al. (2024). The findings of Table 1 showed the early emergence of seeds in CaC2 containing petri-plates, which might be a result of the breakage of dormancy induced by C2H4, as reported in apple seeds (Gniazdowska et al., 2007) and in cucumber seeds (Shakar et al., 2016). Calcium carbide releases C2H2 upon hydrolysis, which is well reported to give the C2H4 reaction (Abeles et al., 2012). In a nutshell, the better germination of the CaC2 treated seeds than the seeds under control is just because of CaC2-induced C2H4 response. In later studies, C2H4 was measured and was found that CaC2 containing seedings had higher C2H4 concentrations compared to control (data is not given). Moreover, CaC2 induced C2H2 might have positively regulated C2H4 biosynthesis, which is then perceived by receptors such as ETR1 and EIN2 in order to stimulate seed germination (Arc et al., 2013). However, a significant reduction in seed germination compared to control was observed when CaC2 was applied at ≥16 mg CaC2 plate-1 (Table 1, Figure 3). Higher rates of Ethephon suppressed seed germination of Echinacea angustifolia and Echinacea pallida with Ethephon, a CaC2 based product (Qu et al., 2000). This negative effect of Ethephon might be a result of excessive C2H4 biosynthesis. Sano and Marion-Poll (2021) and Kozaki and Aoyanagi (2022) have proposed that any increased level of ABA in seeds negatively regulates GA in seeds and prolongs seed dormancy. So, it can be suggested that the ethylene response of 18 and 20 mg CaC2 negatively regulated GA instead of ABA, caused an upsurge in the ABA/GA ratio, and thus prolonged the state of dormancy compared to the control. Linkies et al. (2009) have also reported C2H4 induced endosperm cap weakening and endosperm rupture in Lepidium sativum and Arabidopsis thaliana. All these physiological changes caused a decrease in MGT (Figure 3).
Water is responsible for remarkable changes in the metabolism of a germinating seed. Seed absorbs water in a triphasic pattern. Water uptake by Phases I and II is usually utilized in enzyme activation, but Phase III is accountable for the hydraulic growth of an embryo. Thus, Phase III is the most critical for controlling seedling vigor. Although, ABA has no significant role in phases I and II, it strictly regulates phase III water uptake in seeds (Manz et al., 2005). Manz et al. (2005) suggested that ABA negatively regulates phase III water uptake. They further suggested that phase III water uptake inhibits the transition from germination to postgermination growth. According to the data shown in Figure 4, the treated seeds had more GI and VI compared to the control which might be happening due to the CaC2-induced C2H4 response. The seeds treated with 2 to 14 mg CaC2 might have less ABA than the seeds in control as a result of the counteraction of C2H4 with ABA. This lessening ABA ultimately accelerated phase III water uptake and post-germination growth in terms of improved vigorous index (Figure 6). However, poor GI and VI at CaC2 > 14 mg plate-1 might be happening due to the loss of the ethylene physiological role. Kashif et al. (2012) and Shakar et al. (2016) also found such C2H4 responses in CaC2 treated okra and cucumber seeds, respectively, because of higher rates of CaC2. Inhibition of post-germination growth at comparatively higher CaC2 rates might happen due to C2H4 biosynthesis above the permissible limit or the lethal effects of C2H2 as all the other conditions were the same. An excessive C2H2 might have blocked the sensing mechanism of seeds, which diminished CaC2 induced by the C2H4 response. Conclusively, all improvements in different variables shown in Figures 1 and 2 are the result of C2H4 giving response potential of CaC2. However, these results advocate investigating the CaC2, induced C2H4 response under rhizosphere conditions.
Significant improvements in other seedling vigor attributes such as root and shoot length (Figure 1), seedling biomass, and chlorophyll contents (Figure 2) are also evidence of the CaC2-induced response. An increase in seedling length and weight in CaC2 containing petri-plates is a result of C2H4 based root proliferation and root formation patterns. Adequate phase III water uptake might have triggered the embryo axes elongation by testa rupturing and finally resulted in root elongation. Ethylene induced phase III water uptake, although, triggers endosperm weakening and rupturing by inhibiting ABA (Linkies et al., 2009) but it also inhibits root elongation due to its physiological role under submerged conditions. Ethylene induces root proliferation by inhibiting root elongation, which leads to an increase in the SL:RL ratio. Ethylene inhibits cellular elongation by counteracting with auxin, induces adventitious or lateral roots in the seedlings, and thus causes the proliferation of roots in plants. The SL:RL clearly elucidates the pattern of root and shoot growth in the presence and absence of a C2H4 source (Figure 2). Similarly, an increase in biomass and leaf chlorophyll contents in treated seedlings (Table 1) might have occurred due to the triggering of C2H4 based phase III water uptake. Finally, the results predict CaC2 as an effective compound to induce C2H4 response for early germination and seedling emergence. Moreover, this information provides foundations to explore the role of CaC2 based gases in different aspects of seed physiology and the practicality of CaC2 for vegetable growers, where they need early and fast growth of vegetables to fetch more benefit from the market.
Conclusions and Recommendations
This study suggests that use of 14 mg CaC2 per plate is an optimum rate of CaC2 to break seed dormancy of sweet pepper. Moreover, results suggest that the seed germination and seedling vigor of vegetable seeds can be improved by the use of CaC2. Application of CaC2 as soil amendment can result in a successful crop stand and provide a strong foundation for the better growth and yield of vegetables, particularly of sweet pepper. However, there is a need to use some inhibitors of ethylene biosynthesis to make CaC2 a more effective tool for improving seed germination and seedling growth, as a significant reduction in seed germination and different seedling parameters was observed due to excessive release of ethylene from CaC2.
Acknowledgements
We acknowledge the financial support provided by Higher Education Commission (HEC), Islamabad, Pakistan.
Novelty Statement
This highlights the importance of using calcium carbide for off-season vegetable cultivation, addressing the issue of poor tomato germination rates. The use of CaC2 as a soil amendment leads to improved crop stand, promoting better growth and yield of vegetables, especially sweet peppers.
Author’s Contribution
Wazir Ahmed: Planned, supervised the study, collected data on time, and presented it in written form.
Muhammad Naeem Akhtar: Analyzed the data statistically and calculated secondary parameters from the primary data.
Muhammad Waseem Akhtar: Contributed to the results and discussion.
Muhammad Nauman Hanif: Contributed to the references, materials, and methods write-up.
Ahmad Mahmood and Aiman Salah ud Din: Conducted an overall review and analysis of the data.
Conflict of interest
The authors have declared no conflict of interest.
References
Abeles, F.B., P.W. Morgan and M.E. Saltveit Jr. 2012. Ethylene in plant biology. Academic press. New York (USA).
Arc, E., J. Sechet, F. Corbineau, L. Rajjou and Marion-Poll. 2013. ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination. Front. Plant Sci., 4: 63-67.
Arshad, M. and W.T. Frankenberger Jr. 2012. Ethylene: Agricultural sources and applications. Springer science and Business Media, New York.
Baharudin, N.F. and N.I. Osman. 2023. Plant development, stress responses and secondary metabolism under ethylene regulation. Plant Stress, 7: 100146.
Bailly, C., R. Jurdak and F. Corbineau. 2023. Ethylene in the regulation of seed dormancy and germination: molecular mechanisms. In: (eds. A. Ferrante, S. Munné-Bosch and N.A. Khan). The plant hormone ethylene: Stress acclimation and agricultural applications. Academic Press. pp. 41-60.
Bhoi, A., B. Yadu, J. Chandra and S. Keshavkant. 2024. Cross-talk of strigolactones with abscisic acid, gibberellins, ethylene, and other hormones. In: Strigolactones. Academic Press. pp. 103-126.
Dugardeyn, J. and D. Van-Der-Straeten. 2008. Ethylene: Fine-tuning plant growth and development by stimulation and inhibition of elongation. Plant Sci., 175: 59-70.
Finch-Savage, W.E. and G. Leubner-Metzger. 2006. Seed dormancy and the control of germination. New Phytologist, 171: 501-523.
Gniazdowska, A., U. Dobrzyńska, T. Babańczyk and R. Bogatek. 2007. Breaking the apple embryo dormancy by nitric oxide involves the stimulation of ethylene production. Planta, 225: 1051-1057.
Jia, Y., J.M. Barrero, J. Wang, M.J. Considine, S. Nakamura and C. Li. 2024. Seed dormancy, germination, and pre-harvest sprouting, volume II. Front. Plant Sci., 15: 1399510.
Kashif, S.U.R., M. Yaseen, H. Raza and A. Kirn. 2012. Improving seed germination and green pod yield in okra (Hibiscus esculentus L.) using calcium carbide- a new source of ethylene. J. Plant Nutr., 35(13): 2024-2036.
Khalid, A., M.J. Akhtar, M.H. Mahmoud and M. Arshad. 2006. Effect of substrate-dependent microbial produced ethylene on plant growth. Microbiol., 75: 231-236.
Kozaki, A. and T. Aoyanagi. 2022. Molecular aspects of seed development controlled by gibberellins and abscisic acids. Int. J. Mol. Sci., 23(3): 1876.
Kucera, B., M.A. Cohn and G. Leubner-Metzger. 2005. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res., 15: 281-307.
Kumar, R., B.S. Parmar, S. Walia and S. Saha. 2015. Nitrification inhibitors: classes and its use in nitrification management. In: (eds. A. Rakshit, H.B. Singh and A. Sen). Nutrient use efficiency: From basics to advances. Springer Nature, pp. 103-122.
Linkies, A., K. Müller, K. Morris, V. Turečková, M. Wenk, C.S. Cadman and G. Leubner-Metzger. 2009. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: A comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell Online, 21: 3803-3822.
Mahmood, R. and M. Yaseen. 2016. Influence of calcium carbide formulations on growth, yield and nitrogen uptake of wheat under field conditions. J. Plant Nutr., 39(5): 609-619.
Manz, B., K. Müller, B. Kucera, F. Volke and G. Leubner-Metzger. 2005. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol., 138: 1538-1551.
Miransari, M. and D.L. Smith. 2014. Plant hormones and seed germination. Environ. Exp. Bot., 99: 110-121.
Negi, S., M.G. Ivanchenko and G.K. Muday. 2008. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J., 55: 175-187.
Patrick, B., L. Antonin, L.L. Servane, C. Deleu and E. Le-Deunff. 2009. Ethylene modifies architecture of root system in response to stomatal opening and water allocation changes between root and shoot. Plant Signal. Behav., 4: 44-46.
Qu, L., X. Wang, E. Hood and R. Scalzo. 2004. Ethephon promotes germination of Echinacea angustifolia and E. pallida in darkness. HortScience, 39: 1101-1103.
Sajeev, N., M. Koornneef and L. Bentsink. 2024. A commitment for life: Decades of unraveling the molecular mechanisms behind seed dormancy and germination. Plant Cell, 36(5): 1358-1376.
Sano, N. and A. Marion-Poll. 2021. ABA metabolism and homeostasis in seed dormancy and germination. Int. J. Mol. Sci., 22(10): 5069.
Shakar, M., M. Yaseen, A. Niaz, R. Mahmood, M.M. Iqbal and T. Naz. 2016. Calcium carbide-induced changes in germination, morpho-phenological and yield traits in cucumber (Cucumis sativus). Int. J. Agric. Biol., 18(4): 703-709.
Shakar, M., M. Yaseen, R. Mahmood and I. Ahmad. 2016. Calcium carbide induced ethylene modulate biochemical profile of Cucumis sativus at seed germination stage to alleviate salt stress. Sci. Hortic., 213: 179-185.
Siddiq, S., M. Yaseen, M. Arshad and N. Ahmed. 2012. Effect of calcium carbide on photosynthetic characteristics, growth and yield of tomato cultivars. Pak. J. Agric. Sci., 49(4): 505-510.
Steel, R.G.D., J.H. Torrie and D.A. Deekey. 1997. Principles and procedures of statistics- A biometrical approach (3rd edition). McGraw Hill Book Company Incorporation, New York, pp. 400-428.
Stein, M., C. Serban and P. McCord. 2021. Exogenous ethylene precursors and hydrogen peroxide aid in early seed dormancy release in sweet cherry. J. Am. Soc. Hortic. Sci., 146: 50-55.
Sun, M., P.A. Tuan, M.S. Izydorczyk and B.T. Ayele. 2020. Ethylene regulates post-germination seedling growth in wheat through spatial and temporal modulation of ABA/GA balance. J. Exp. Bot., 71(6): 1985-2004.
Wang, Y., X. Sun, J. Peng, F. Li, F. Ali and Z. Wang. 2024. Regulation of seed germination: ROS, epigenetic, and hormonal aspects. J. Adv. Res.,
Yaseen, M., M. Arshad and W. Ahmed. 2012. Effect of calcium carbide dependent release of acetylene and ethylene on nitrification in soil to improve nitrogen use efficiency and yield of vegetables. Lambert Academic Publishing, Germany.
Zuo, Y. and Y. Xu. 2020. Research progress on the mechanism of GA and ABA during seed germination. Mol. Plant Breed., 11: 20.
To share on other social networks, click on any share button. What are these?






