Burned Wound Healing Effect of Prepared Pumpkin Seed Oil Nano Phytosome Loaded Lidocaine in Rabbit
Research Article
Burned Wound Healing Effect of Prepared Pumpkin Seed Oil Nano Phytosome Loaded Lidocaine in Rabbit
Wafa’a Mohammad Abduwhab1*, Waseem Ali Hasan2, Mohanad Abdulsataar AL-Bayati3*
1Department of Physiology, Biochemistry, Toxicology and Pharmacology, College of Veterinary Medicine, Tikrit University, Iraq; 2Department of Physiology, Biochemistry, Toxicology and Pharmacology, College of Veterinary Medicine, Tikrit University, Iraq; 3Department of Physiology, Biochemistry and Pharmacology, College of Veterinary Medicine, College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | The conversion of regular pumpkin seed oil into a nanotechnology-driven composition involves encapsulating Lidocaine within the phytosome structure of the oil. This process aims to bolster its physical resilience and therapeutic advantages while mitigating potential adverse reactions. The central objective of this research centers on assessing the curative impacts of the innovative Nano phytosome pumpkin-lidocaine gel in the context of third-degree burn wound healing. The reformulation of standard pumpkin seed oil involved the encapsulation of conventional Lidocaine within a phytosome structure, resulting in the formation of nanoparticles. This modification was undertaken to enhance both the physical stability of the oil and the therapeutic properties of pumpkin seed oil, while simultaneously mitigating the potential side effects associated with Lidocaine. Evaluation of the therapeutic effect of Nano phytosome pumpkin-lidocaine gel on the healing of third burn wounds. Twenty-five,10 to 18-week- old ,white male and female adult rabbits weighting 2.5-3 kg divided to5 groups (n=5) as following negative control group, positive control group, ordinary pumpkin seed oil group, Nano phytosome pumpkin-lidocaine 100% gel group, and Nano phytosome pumpkin 100% gel group. The Nano phytosome pumpkin-lidocaine gel at a concentration of 100% and the Nano phytosome pumpkin gel at a concentration of 100% showed a significant entrapment % and loaded efficiency%, entrapment % were 95.2 ± 9.41, and 87.32 ± 8.1 respectively, while loaded efficiency% were 86.84 ± 7.77, and 79.40± 3.72 respectively. As well as, both the Nano phytosome pumpkin-lidocaine gel at a concentration of 100% and the Nano phytosome pumpkin gel at a concentration of 100% revealed a significant closure rate of 0.216±0.05, and 0.215 ±0.03 mm/day respectively, compared to ordinary pumpkin seed oil treated group, and both positive and negative control group. From this study concluded the pumpkin seed oil loaded with Nano phytosome pumpkin-lidocaine demonstrated good physical stability, reducing the crystal nature of active extract substances, with good entrapment% and loaded efficiency %, and improving the wound healing of burn wound.
Keywords | Pumpkin seed oil, lidocaine, Phytosome, Nanotechnology, Burn wound, herbal medicine, antioxidants and biochemical indices.
Received | June 24, 2023; Accepted | July 20, 2023; Published | February 26, 2024
*Correspondence | Wafa’a Mohammad Abdulwahab, Mohanad Abdulsataar AL-Bayati, College of Veterinary Medicine, Tikrit University, Iraq; College of Veterinary Medicine, University of Baghdad, Iraq; Email: wafaadr619@gmail.com
Citation | Abduwhab WM, Hasan WA, Al-Bayati MA (2024). Burned wound healing effect of prepared pumpkin seed oil nano phytosome loaded lidocaine in rabbit. Adv. Anim. Vet. Sci. 12(4): 723-731.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.4.723.731
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The pumpkin plant originated in northern Mexico, Argentina, and Chile and has been spread to Western America, Europe, and Asia (Hancock, 2023). It known as “Kraa”, belongs to the family Cucurbitaceae (Prommaban et al., 2021). Pumpkin seeds, recognized as functional food by-products, contain a range of biologically active components, including flavonoids, phenolics, carotenoids, fatty acids, tocopherol, as well as vitamins like E and minerals (Hussain et al., 2021).
Nanotechnology is the use of science to enhance the effectiveness of medicine, with applications across biology, physics, chemistry, biotechnology, pharmaceuticals, and engineering. Nano, meaning “dwarf” in Greek, involves particles ranging from 1 to 100 nm in size (Demir, 2020; Hasan and Raheem, 2021). Phytosome, an advanced drug delivery system, is an evolved form of liposome particles and herbal formulation, it comprises phytoconstituents from plant extracts enclosed by a lipid layer (Mahmoudabad et al., 2023; Eftekhari et al., 2023). This system heightens the bioavailability of water-soluble phytoconstituents by enveloping them with phospholipids, leading to improved absorption in the intestinal lumen through simple diffusion. Consequently, it extends the drug’s action duration and enhances its delivery to target tissues (Yadav et al., 2010; Pawar and Bhangale, 2015). Phytosome formulation reduces herb extract toxicity; it counteracts the side effects of high-concentration herbs with equivalent amounts of phytosomes carrying the same extract. This reduces first-pass metabolism, resulting in decreased drug clearance and an extended half-life (t1/2) (Ghanbarzadeh et al., 2016; Singh et al., 2018). Burns, a severe form of trauma, can be caused by various sources, approximately 80% of burns stem from heat sources like hot liquids, solids, or fire, they are categorized into four degrees based on severity and depth: first-degree, second-degree, third-degree, and fourth-degree burns (Jeschke et al., 2020b). This present study aimed to transform pumpkin seed oil into a nanotechnology-based composition with encapsulated Lidocaine to enhance stability and therapeutic properties while minimizing side effects, and improving physical stability and enhanced entrapment efficiency. As well as evaluating the therapeutic efficacy of Nano phytosome pumpkin-lidocaine gel for healing third-degree burns.
MATERIALS AND METHODS
Experimental Animals
10 to 18-week- old ,white male and female adult rabbits weighting 2.5-3 kg the rabbit were given two weeks to adapt during which they were provided ad libitum access to food and water.
Institutional Animal Care And Use Committee (Iacuc)
Prior to conducting any experiment, the methodology and protocols used in this research were reviewed and endorsed in accordance with animal welfare ethical measurements by the Scientific Committee of the Department of Physiology, Biochemistry, Pharmacology, and Toxicology, College of Veterinary Medicine, University of Tikrit, and the Ethics Committee of the College of Veterinary Medicine, University of Tikrit, Salah Aldin– Iraq (IACUC#:.1285-18-7).
Animal Grouping
Twenty-five rabbits divided into 5 groups (n=5); each group treated separately as following:
First group: negative control rabbits leave without treatment. Second group: positive control rabbits treated with empty liposome. Third group: rabbits treated with ordinary pumpkin seed oil. Fourth group: rabbits treated with Nano phytosome pumpkin-lidocaine 100% gel. Fifth group: rabbits treated with Nano phytosome pumpkin 100% gel.
Preparation Of Nano Phytosome Pumpkin Seed
Cholesterol 0.5g, phosphatide choline 0.5g dissolved in mixed organic solvent chloroform 15 ml, methanol. The mixture was vortexed with a vacuum for 15 minutes, then incubated in a water bath at 40 ᵒC; the mixture was vortex and vacuumed for 20 minutes (Ghanbarzadeh et al., 2016). The blank liposome was created and then incubated in a water bath at 40 ᵒC for 5 minutes, 1 ml of pumpkin seed oil was mixed and vortexed for 2 hours, then removing the supernatant, the phytosome was formed (Lu et al., 2019).
Nano Phytosome Pumpkin Seed Encapsulated Lidocaine
The Nano phytosome pumpkin seed was incubated in a water bath at 40 ᵒC for 15 minutes. Lidocaine 0.5ml (0.2%) was added to pumpkin seed Nano phytosome 1 gm, then vortex for 1 hour, remove the supernatant, pumpkin seed Nano phytosome encapsulated Lidocaine was formed (Franz-Montan et al., 2015).
Entrapment Efficiency Procedure
The entrapment efficiency of Nano phytosome Pumpkin-lidocaine was measured by centrifugation by centrifugation technique. The Nano phytosome Pumpkin-lidocaine was separated in the high-speed centrifuge at 4000 rpm for 30 minutes. The sediment was separated from the supernatant. Sediment dissolved in methanol 10 ml, then centrifugation at 4000 rpm for 30 minutes. The entrapment percentage was calculated by the following equation (Hulla et al., 2015).
Entrapment efficiency % = (Entrapped drug /Total drug) ×100
Nano Phytosome Pumpkin-Lidocaine Loaded In The Gel
The gel was made by dissolving 0.125 g of Carbopol in 3 ml of pure distilled water for prepared the gel phase. The acidic component of the gel was then buffered by 1 ml of NaOH 0.1N, which was thoroughly manually blended until being appeared thick and clear. Lastly, the gel was heated for 15 minutes to remove air bubbles (Al-Shati and Ibrahim, 2019). The prepared phytosome was mixed with gel, and 100 mg of Nano phytosome Pumpkin-Lidocaine was mixed with gel 100 mg, to achieve a concentration of 100%.
Nano Phytosome Pumpkin–Lidocaine Standardization
Light Microscope
The light microscope use the visible light to explore the specimen details such as; size and shape, but its ability to resolve very small structures is limited by the wavelength of visible light (Bibi et al., 2011).
The phytosome smear was made; 50 μl of phytosome suspension was examined under the light microscope with an optical filter at an oil immersion magnification scale to characterize the phytosome shape and size. The examination was performed on both Nano phytosome pumpkin and Nano phytosome-pumpkin-lidocaine.
Electron Microscope (Scan And Transmission)
The fundamental principle underlying the electron microscope is akin to that of a light microscope. However, in contrast to utilizing visible light, it employs high-energy electrons as its illumination source (Peddie et al., 2022). Since the specimen interacts with the electron beam, yielding valuable information regarding the phytosome’s size, shape, crystalline structure, transmission for lamellar, and composition (Placzek and Kosela, 2016).
The Nano phytosome pumpkin-lidocaine samples were stored at deep freezing -18°C following packing in sealed Eppendorf tubes. Scanning and transmitted micrographs conducted by (AL-KAK) applied research laboratory submitted to Kashan University in Iran.
X-Ray Diffraction (Xrd)
XRD was used to analyze the crystallographic structure of Nano phytosome pumpkin-lidocaine and ordinary pumpkin seed oil and ordinary lidocaine using an X-ray diffractometer (2 to 40°C) (Udapurkar et al., 2018).
Zeta Potential
zeta potential was used to assess the stability of a dispersion of Nano phytosome pumpkin-lidocaine Nanoparticles (Babazadeh et al., 2018).
Induction Burn Wounds
First, according to the ethical fact, the rabbits were euthanized by infiltration general anesthesia. Clipped electrical shaver machine and shaved the rabbit’s dorsal region with a shaver machine and the prepared area was disinfected with alcohol 70% of the skin was labeled 0.8 cm, at Para-Medline labeled skin area and removed skin layers, burned by pressing the copper tip of an electric soldering tool heated rated to 95 °C and applied for 1 minute to burn wound skin and below to create identical burn wounds, This technique was done to prevent reaching the muscle layer under the skin layers (Giles et al., 2008).
Timeline Analysis Of Burn Wounds Healing
The morphological analysis of new tissue formation was performed at a specific time-point of healing. The closure indices were estimated using three altered parameters as follows:
- Closure rate: the wound closure area per unit time throughout the total growth phase (Jabbar and Al-Bayati, 2022).

- t½ of wound healing: the half-life of closure wound area time in the treated group
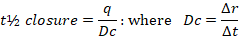
t= half-life, q= the intercept, Dc= rate of continuous linear healing, ΔA= alteration in the perceived burn wound area between two measurements, Δt: The time between two specific measurements consecutively
RESULTS AND DISCUSSION
Results
Entrapment %, And Loaded Efficiency % Of Prepared Phytosome
Table (1) showed the entrapment % of Nano phytosome pumpkin-lidocaine and Nano phytosome pumpkin were 95.2 ± 9.41, and 87.32 ± 8.1 respectively, while loaded efficiency% were 86.84 ± 7.77, and 79.40± 3.72 respectively.
Table 1: Entrapment%, loaded efficiency%, and Nano size of phytosome.
|
Phytosome types |
Entrapment % |
loaded efficiency % |
Nano size nm |
|
Nano physiome pumpkin-lidocaine |
95.2 ± 9.41a |
86.84 ± 7.77 a |
79.31 ± 7.20 |
|
Nano phytosome pumpkin |
87.32 ± 8.1 b |
79.40± 3.72 b |
71.48 ± 2.24 b |
The data presented mean ± SE n=5, The different letters denoted (p≤0.05) between types of phytosome.
Standardization And Characterization Of Nano Phytosome.
Light microscope and electron micrograph: Fig (1) A and B of the light microscope displayed the primary formation of Nano vesicular shapes of phytosome in each loaded pumpkin seed oil Fig (1) A and pumpkin seed oil with lidocaine formula Fig (1) B. The Nano phytosome pumpkin-lidocaine was examined by scanning electron microscopy (SEM) to determine the phytosome size and spherical shapes distributed homogeneously over a granulation surface representing the Nano phytosome pumpkin particles loaded with lidocaine in the core of the phytosome Fig (2) C, D and E. While Fig (2) A and B showed the transmission electron microscope (TEM) presented the regular distribution of the multilamellar phytosome. Table (1) showed the size of the Nano pumpkin phytosome loading lidocaine and Nano phytosome pumpkin.
X-Ray Diffraction (X-Rd)
The crystal structure and particle size of prepared crystalline Nanomaterials are determined using the nondestructive method known as XRD. The XRD analysis of ordinary Pumpkin seed oil (PSO) and ordinary lidocaine showed diffraction peaks of different intensities and high in the spectrum, and this is due to the higher crystallinity nature of lidocaine and some of PSO’s active compounds. The main diffraction peaks of ordinary pumpkin seed oil are 2θ =17.63o, 19.94o, 21.59o, 31.45o, 35.92o, 47.11o, 56.15o, 64.55o, and 67.60o while the main peaks of lidocaine are 2θ =23.129, 40.938, 44.432, and 73.958 Fig(3). It was observed that most of the distinctive main diffraction peaks of pumpkin seed oil and lidocaine disappeared from the diffractogram pattern of Nano phytosome pumpkin-lidocaine as compared with ordinary pumpkin seed oil (PSO) and lidocaine, moreover, PSO showed a decrease in the width of the peak diffraction at the range (2θ =17.63o-21.59o) Fig(3).
Zeta Potential
The zeta potential was used to clarify and identify the aggregation of Nano phytosome pumpkin-lidocaine due to their small sizes and wide surface area. Fig(4) showed the zeta potential of ordinary pumpkin seed oil, ordinary lidocaine, and Nano phytosome pumpkin-lidocaine were -52.3, -56.4, and -68.3 mV respectively.
Closure Rate And Closure T ½ Of Burn Wound Healing
In general, the wounds treated with Nano phytosome displayed a significantly (p≤0.05) faster closure rate per unit time than negative and positive control groups. The Nano phytosome pumpkin-lidocaine gel 100% and The Nano phytosome pumpkin gel showed significant (p≤0.05) fast values of closure rates 0.216±0.05 and 0.215±0.03 mm/day, respectively as shown in Table (2) and Fig (5&6). On the other hand, negative & positive control groups, and ordinary pumpkin seed oil treated revealed delayed closure rates of 0.021±0.002, 0.023±0.002, and 0.028±0.009 mm/day, respectively Table (2). while the closure t½ of the Nano phytosome pumpkin-lidocaine gel and Nano phytosome pumpkin gel treated groups showed a significant (p≤0.05) decrease in time values. as compared with both -ve &+ve control groups, and ordinary pumpkin seed oil treated group 20.4±1.7, 20.03±2.1, and 18.5±1.1 days, respectively Fig(5 & 6) and Table (2).
-ve control= negative control
+ve control= positive control
OPSE= ordinary pumpkin seed extract(oil)
Nano PPLG= Nano phytosome pumpkin-lidocaine gel
Nano PPG= Nano phytosome pumpkin gel
Table 2: Displays the closure rate in millimeters per day and the time required for half-closure (designated as “Closure t (½) day”) for both the control group and the group receiving treatment throughout 40 days focused on burn wound healing.
|
Groups |
Closure rate mm/day |
Closure t (½) day |
|
Negative Control |
0.021±0.002e |
20.4±1.7 d |
|
Positive Control |
0.023±0.002d |
20.03±2.1 d |
|
Ordinary pumpkin seed oil |
0.028±0.009c |
18.5±1.1 c |
|
Nano phytosome pumpkin-lidocaine G 100% |
0.216±0.05 a |
8.3±1.1 b |
|
Nano phytosome pumpkin G 100% |
0.215 ±0.03b |
9.1±1.13 a |
DISCUSSION
Entrapment %, And Loaded Efficiency% Of Prepared Phytosome
The entrapment efficiency serves as a metric to quantify the proportion of pumpkin seed oil (PSO) and lidocaine effectively encapsulated within the Nano phytosome structure. Optimal encapsulation was achieved by forming phytosomes at a 1:2 ratio of phosphatidylcholine (0.5) to pumpkin seed oil (1). This ratio facilitated the creation of electric hydrogen bonds within the polar region of phosphatidylcholine molecules, resulting in an enhanced entrapment efficiency of the pumpkin phytosome, measured at 87.3% ± 8.1. Simultaneously, it allowed for the successful encapsulation of lidocaine within the inner hydrophobic core, achieving an entrapment efficiency of 95.2% ± 9, consistent with the findings of Azeez et al. (2018).
It is important to note that several factors influence the effectiveness of drug encapsulation in phytosomes. These factors encompass characteristics related to both the phytosomes themselves and the drugs intended for encapsulation. Key phytosome-related factors include the preparation process, hydration duration, vesicle lamellarity, solvent properties (including solubility level and pH), membrane rigidity, phospholipid concentration, buffer composition, vesicle size, surface charge, and the nature of the drugs to be encapsulated, be they hydrophilic or lipophilic. These parameters collectively impact the entrapment efficiency of phytosomes, as highlighted by the studies of Lertsutthiwong et al. (2008) and Allawi and Al-Bayati (2020).
Light Microscope And Electron Micrograph
Pumpkin seed oil contains polyphenols, which feature two phenolic hydroxyl groups. These hydroxyl groups bind with the polar choline head of PC, while the lipophilic and nonpolar phosphatidyl group envelops the choline-hydroxyl complex. This unique arrangement results in various charges due to the positive charge of the choline ammonium group in pumpkin seed polyphenols and the negative charge of the phosphate group. This phenomenon leads to the formation of small spherical particles resembling cells (Ghanbarzadeh et al., 2016; Chen et al., 2022).
Several factors have the potential to influence the morphology and configuration of phytosomes. These factors include preparation conditions and time for formation (timely formation being crucial), temperature optimization for enhanced phospholipid binding, the specific medium used for hydration-dehydration, and the exclusive involvement of methanol and chloroform as primary solvents for the formation of large multilamellar Nano formulations. Additionally, the lamellarity of Nano phytosome pumpkin-lidocaine with multilamellar vesicle shape was observed. To achieve multilamellar and stable Nano phytosome formulations, phosphatidylcholine to pumpkin seed oil ratio of 1:2 was selected due to the establishment of electric hydrogen bonds between phosphatidylcholine and pumpkin seed oil, following findings by Saha et al. (2013). Furthermore, temperature and hydration conditions were presumed to impact phytosome lamellarity, as suggested by Gaikwad et al. (2021).
X-Ray Diffraction (X-Rd)
The X-ray diffraction (XRD) profile provides valuable insights into the structural characteristics of Nano phytosome pumpkin-lidocaine. Notably, it reveals the amorphous nature of both pumpkin seed oil (PSO) and lidocaine when encapsulated within the Nano phytosome structure. This is evident in the broadening of the peak signals observed in the XRD pattern. The loss of well-defined peaks for PSO, along with the reduction in their peak width, suggests the formation of a network involving interconnected carbon atoms between the active compounds and phosphatidylcholine within the Nano phytosome (Ghanbarzadeh et al., 2016).
Interestingly, the XRD analysis of Nano phytosome pumpkin-lidocaine exhibited a noteworthy feature—the presence of a significant peak associated with the extract. However, this peak had a lower diffraction intensity compared to ordinary PSO. This observation implies that the interaction between phosphatidylcholine and certain active compounds from the extract may have influenced the degree of crystallinity and intensity within the Nano phytosome, as previously suggested (Pourhojat et al., 2017). This suggests that the PSO loaded in the phytosome structure may have undergone partial recrystallization, resulting in less orderly crystal formation.
Furthermore, the X-ray diffraction spectrum of the loaded lidocaine within Nano phytosome pumpkin-lidocaine displayed considerably weaker diffraction signals compared to ordinary lidocaine. This observation suggests that lidocaine, when encapsulated in the phytosome, assumed a less crystalline structure, aligning with previous findings (Jabbar and Al-Bayati, 2022). This implies that lidocaine within the Nano phytosome is more evenly and consistently distributed within the lipid core at a molecular level, contributing to its reduced crystallinity
Zeta Potential
The zeta potential of nanoparticles is a critical parameter influenced by the chemical nature of stabilizing agents and the pH of the surrounding medium (Lertsutthiwong et al., 2008). In the case of Nano phytosome pumpkin-lidocaine, the measured zeta potential value was -68.3 mV. This value suggests that the combination of pumpkin seed oil (PSO) and lidocaine contributes to a high zeta potential, likely due to an increased number of carboxyl groups. These carboxyl groups have a direct impact on both the size and the negative charge of the nanoparticles (Hatanaka et al., 2010).
The shift towards a negative zeta potential value can be attributed to the incorporation of PSO into the phosphatidylcholine structure. This shift results in a significant increase in the electrostatic repulsion forces between negatively charged particles, effectively preventing particle aggregation. Consequently, this contributes to the exceptional stability of the phytosome and enhances its ability to penetrate the skin (Mora-Huertas et al., 2010;).
Documents noted that other studies have suggested that liposomes loaded with lidocaine, possessing a zeta potential of less than 20 mV, can enhance system stability, reduce the tendency of lidocaine nanoparticles to aggregate, and result in a more controlled release of lidocaine. These findings align with the results obtained in our zeta potential analysis (Jabbar and Al-Bayati, 2022).
Closure Rate, And Closure T ½ Of Burn Wound
Numerous studies have documented the positive impact of pumpkin seed oil (PSO) on wound healing, particularly in accelerating the initial stages of the process (Rezk et al., 2023). The mechanisms through which PSO facilitates wound healing when applied topically are multifaceted. These mechanisms likely involve its potent antioxidant activity, anti-inflammatory properties, promotion of re-epithelialization, stimulation of fibroblast differentiation and migration, up-regulation of growth factors such as TGF-beta, neovascularization, promotion of collagen synthesis, wound shrinkage, and tissue remodeling (Fahmi et al., 2014;).
One noteworthy component of PSO is its high content of linoleic acid, which serves as the precursor to arachidonic acid (Siano et al., 2016). Arachidonic acid is a primary polyunsaturated fatty acid found in the skin and plays a pivotal role in modulating and expediting the inflammatory process, it achieves this by generating key inflammatory mediators like leukotrienes, thromboxane, and prostaglandins. These mediators, in turn, enhance wound healing by facilitating local neovascularization, fibroblast differentiation, cellular migration, and extracellular matrix remodeling (Bardaa et al., 2016b).
Moreover, the antioxidant properties of PSO likely contribute to wound healing by safeguarding cells against damage, increasing vascularity, and strengthening collagen fibers (Kaur et al., 2019). PSO may further enhance wound healing by stimulating and increasing fibroblast formation, migration, and mitogenesis during the proliferation phase. Consequently, as fibroblasts increase, there is a corresponding rise in collagen deposition within the dermis, particularly collagen types I and II (Al Chalabi et al., 2021).
PSO may expedite wound closure by promoting re-epithelization through the elevation of growth factors such as Keratinocyte Growth Factor (KGF) and interleukin-6 (Bardaa et al., 2016). When PSO is formulated into nanoparticles, it appears to intensify its therapeutic effects, achieving comparable results to those obtained with conventional PSO. This enhancement could be attributed to the small size of nanoparticles, which facilitates deeper penetration into the skin with increased permeability, targeting the active site and enabling a higher concentration (Susilawati et al., 2021).
Additionally, the incorporation of lidocaine into the core of the phytosome structure provides a sedative effect and offers short-term relief from itching during the treatment period. This, in turn, contributes to improved wound healing and reduces the frequency of dressing changes (Jabbar and Al-Bayati, 2022).
CONCLUSIONS AND RECOMMENDATIONS
Reformulated the pumpkin seed oil to Nano phytosome pumpkin-lidocaine demonstrated good physical stability, Reducing the crystal nature of active extract substances and improving the entrapment efficiency %. The Nano phytosome pumpkin-lidocaine gel 100% and Nano phytosome pumpkin gel 100% showed significant acceleration and diminished closure rate and closure t ½ of the burned wound as compared with ordinary pumpkin oil treated group and both positive, and negative control groups.
Application of the formula to humans and large animals for burned wounds, as well as can be used for recovery during the remodeling phase in different degrees of external wound
ACKNOWLEDGEMENTS
We do thank and praise Allah as thankers and praisers thanking and praising for the guidance and aid in finishing this paper. He is the first and last deserver of thinking and praising. A special thanks and gratitude to the pharmacology and toxicology department, college of veterinary medicine/the University of Baghdad, State Company for Drug and medical appliance/Samarra Department of Pharmacology, for consultation and for facilitating particular challenges of the work.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
DATA AVAILABILITY
Authors will provide all data at the reasonable request.
NOVELTY STATEMENT
The preliminary study formulated Nano-phytosomes with Pumpkin Seed Oil in a burn wound in the full third stage on the skin with analgesic lidocaine for accelerating healing with low adverse effects during the healing phase and remodeling phase.
AUTHORS’ CONTRIBUTION
First author: practical work and search writing
Second author: Implant and suggested project and methodology writing
Third author: final arrangement and styling.
REFERENCES
Afifi NM, Elhabiby MM, El-Mehi AES, Faried MA (2022). Effect of Pumpkin Seed Oil on Experimentally Induced Early Liver Fibrosis in the Adult Male Albino Rat. Egyptian J. Histol., 45(4):1-18 https://doi.org/10.21608/EJH.2022.106873.1594
Al Chalabi SM, Sail AI, Al-Azzaw KS (2021). Study the physiological and immunological effects of pumpkin extract on wound healing in diabetic rats. Ann. Trop. Med. Pub. Health, 24(4):362-370. https://doi.org/10.36295/ASRO.2021.24441
Allawi HM, Al-Bayati MA (2020). Formulation of Camellia sinensis phytosome encapsulated Diclofenac and effect on analgesia and inflammation in mice. Online J. Vet. Res., 24(3):175-190.
Al-Shati I, Ibrahim N (2019). Muco-adhesive Gel of Liposomal Progesterone and Liposomal PMSG Vaginal Formula Characterization and Preparation In vitro and in situ in Vaginal Mucous of Ewe. J. Nanopart. Res., 18(2):109-146
Azeezn A, deepa VS, Sivapriya V (2018). Phytosomes: emergent promising nano vesicular drug delivery system for targeted tumor therapy. Adv. Nat. Sci. Nanosci. Nanotechnol., 9(3):1-6. https://doi.org/ 10.1088/2043-6254/aadc50
Babazadeh A, Zeinali M, Hamishehkar H (2018). Nano-phytosome: a developing platform for herbal anti-cancer agents in cancer therapy. Curr. Drug Targets., 19(2):170-180. https://doi.org/10.2174/1389450118666170508095250
Bardaa S, Ben Halima N, Aloui F, Ben Mansour R, Jabeur H, Bouaziz M (2016). Oil from pumpkin (Cucurbita pepo L.) seeds: evaluation of its functional properties on wound healing in rats. Lipids Health Dis., 73:1-12. https://doi.org/10.1186/s12944-016-0237-0
Bardaa S, Chabchoub N, Jridi M, Moalla D, MseddI M, Rebai T (2016b). The effect of natural extracts on laser burn wound healing. J. Surg. Res., 201(2):464-472. https://doi.org/10.1016/j.jss.2015.11.052
Bibi S, Kaur R, Henriksen-Lacey M, Mcneil SE, Wilkhu J, Lattmann E (2011). Microscopy imaging of liposomes: from coverslips to environmental SEM. Int. J. Pharmaceut., 417(1-2):138-150. https://doi.org/10.1016/j.ijpharm.2010.12.021
Chen RP, Chavda VP, Patel AB, Chen ZS (2022). Phytochemical delivery through transferosome (phytosome): an advanced transdermal drug delivery for complementary medicines. Front. Pharmacol., 13:1-6. https://doi.org/10.3389/fphar.2022.850862
Demir N (2021). Nanotechnology in cosmetics: opportunities and challenges. NanoEra.1(1):19-23.
Eftekhari A, Kryschi C, Pamies D, Gulec S, Ahmadian E, Janas D (2023). Natural and synthetic nanovectors for cancer therapy. Nanotheranostics., 7(3): 236–257. https://doi.org/ 10.7150/ntno.77564
El-Menshawe SF, Ali AA, Rabeh MA, Khalil NM (2018). Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: an approach for acceptable level of evidence of an effective novel herbal weight loss product. Int, J. Nanomed., 13:307-318. https://doi.org/10.2147/IJN.S153429
Fahmi ZM, Khadeem EJ, Hasan HF, Luaibi OK (2014). The Antibacterial Effect of Phytosterols Isolated from Echinops heterophyllus in Comparison with MEBO® and Standard Antimicrobial Agents. AJPS., 14(2): 116-128.
Franz-Montan M, Baroni D, Brunetto G, Sobral VRV, Dasilva C MG, Venancio P (2015). Liposomal lidocaine gel for topical use at the oral mucosa: characterisation, in vitro assays and in vivo anesthetic efficacy in humans. J. Liposome Res., 25(1):11-19. https://doi.org/10.13109/08982104.2014.911315
Gaikwad AR, Ahire KD, Gosavi AA, Salunkhe K, Khalkar A (2021). Phytosome as a novel drug delivery system for bioavailability enhancement of phytoconstituents and its applications: a review. J. Drug Delivery Therapeut., 11(3): 138-152. https://doi.org/10.22270/jddt.v11i3.4847
Ghanbarzadeh B, Babazadeh a, Hamishehkar H (2016). Nano-phytosome as a potential food-grade delivery system. Food Biosci. 15:126-135. https://doi.org/10.1016/j.fbio.2016.07.006
Giles N, Rea S, Beer T, Wood FM, Fear MW (2008). A peptide inhibitor of c-Jun promotes wound healing in a mouse full-thickness burn model. Wound Repair Regenerat., 16(1):58-64. https://doi.org/10.1111/j.1524-475X.2007.00331.x
Hancock JF (2023). Fifty Years Later—The Legacy of Alfred Crosby’s. The Columbian Exchange: Biolog. Cultur. Consequences of 1492, 77: 82-102. https://doi.org/10.1007/s12231-022-09563-6
Hasan HF, Raheem SS (2021). Preparation of poly (Lactic-co-glycolic Acid)-loaded pentoxyfilline by nanoparticipation technique. Med. J. Babylon., 18(1): 12-17.
Hatanaka J, Chikamori H, Sato H, Uchida S, Debari K, Onoue S (2010). Physicochemical and pharmacological characterization of α-tocopherol-loaded nano-emulsion system. Int. J. Pharmaceut., 396(1-2):188-193. https://doi.org/10.1016/j.ijpharm.2010.06.017
Hulla J, Sahu S, Hayes A (2015). Nanotechnology: History and future. Human Exp. Toxicol., 34(12):1318-1321. https://doi.org/10.1177/0960327115603588
Hussain A, Kausar T, Din A, Murtaza M A, Jamil MA, Noreen S, et al.(2021). Determination of total phenolic, flavonoid, carotenoid, and mineral contents in peel, flesh, and seeds of pumpkin (Cucurbita maxima). J. Food Process. Preserv., 45(6): 15542. https://doi.org/10.1111/jfpp.15542
Jabbar RK, AL-Bayati MA (2022). The Local Anesthetic Effect of SNL Lidocaine and Conventional Lidocaine on Skin Wound Healing in Rabbits. Egyptian J. Hosp. Med., 89(2):7526-7534. https://doi.org/10.21608/EJHM.2022.276679
Jeschke MG, Van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S (2020b). Burn injury. Nat. Rev. Dis. Primers.6(1): 1-25. https://doi.org/10.1038/s41572-020-0145-5
Kaur S, Panghal A, Garg M, Mann S, Khatkar SK, Sharma P (2019). Functional and nutraceutical properties of pumpkin–a review. Nutrit. Food Sci., 50(2):384-401. https://doi.org/10.1108/NFS-05-2019-0143
Lertsutthiwong P, Noomun K, Rojsitthisak P, Nimmannit U (2008). Preparation of alginate nanocapsules containing turmeric oil. Carbohydr. Polymers, 74(2): 209-214. https://doi.org/10.1016/j.carbpol.2008.02.009
Lu M, Qiu Q, Luo X, Liu X (2019). Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J. Pharmaceut. Sci., 14(3):265-274. https://doi.org/10.1016/j.ajps.2018.05.011
Mahmoudabad AG, Shirshahi V, Mehrabi M, Gheybi F, Gharravi AM, Salehi M (2023). Phytosome: An Effective Transdermal Drug Delivery System for Phytoconstituents. Lett. Drug Design Discovery, 20(8):1020-1030(1). https://doi.org/10.2174/1570180819666220615092854
Mora-Huertas CE, Fessi H, Elaissari A (2010). Polymer-based nanocapsules for drug delivery. Int. J. Pharmaceut., 385(1-2):113-142
Patel S, Rauf A (2017). Edible seeds from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother., 91:330-337. https://doi.org/10.1016/j.biopha.2017.04.090
Pawar HA, Bhangale BD (2015). Phytosome as a novel biomedicine: a microencapsulated drug delivery system. J. Bioanal. Biomed., 7(1):6-12. https://doi.org/ 10.4172/1948-593X.1000116
Peddie C J, GenoudC, Kreshuk A, Meechan K, Micheva KD, Narayan K (2022). Volume electron microscopy. Nat. Rev. Methods Primers, 51(2): 22-25. https://doi.org/10.1038/s43586-022-00131-9
Placzek M, Kosela M (2016). Microscopic methods in analysis of submicron phospholipid dispersions. Acta Pharmaceut., 66(1): 1-22. https://doi.org/10.1515/acph-2016-0003
Pourhojat F, Sohrabi M, Shariati S, Mahdavi H, Asadpour L (2017). Evaluation of poly ε-caprolactone electrospun nanofibers loaded with Hypericum perforatum extract as a wound dressing. Res Chem. Intermediat., 43:297-320. https://doi.org/10.1007/s11164-016-2623-7
Prommaban A, Kuanchoom R, Seepuan N, Chaiyana W (2021). Evaluation of fatty acid compositions, s.antioxidant, and pharmacological activities of pumpkin (Cucurbita moschata) seed oil from aqueous enzymatic extraction. Plants., 10(8):1582. https://doi.org/10.3390/plants10081582
Rezk MY, Ibrahim S, Khalil EA, Saba D, Abdellatif M, Abdellatif A (2023). Pumpkin Seed Oil-Loaded Chitosan/Polyvinyl Alcohol Electrospun Nanofiber Scaffold for Dermal and Oral Wound Dressing. Chem. Select, 8(26): e202300722. https://doi.org/10.1002/slct.202300722
Saha S, Sarma A, Saikia P, Chakrabarty T (2013). Phytosome: A brief overview. Scholars academic J. Pharm., 2(1):12-20.
Siano F, Straccia MC, Paolucci M, Fasulo G, Boscaino F, Volpe MG (2016). Physico-chemical properties and fatty acid composition of pomegranate, cherry and pumpkin seed oils. J. Sci. Food Agricult., 96(5):1730-1735. https://doi.org/10.1002/jsfa.7279
Singh b, Awasthi R, Ahmad A, Saifi A (2018). Phytosome: Most significant tool for herbal drug delivery to enhance the therapeutic benefits of phytoconstituents. J. Drug Delive. Therapeut., 8(1):98-102. https://doi.org/10.22270/jddt.v8i1.1559
Susilawatiy, ChaerunisA AY, Purwaningsih H (2021). Phytosome drug delivery system for natural cosmeceutical compounds: Whitening agent and skin antioxidant agent. J. Adv. Pharmaceut. Technol. Res., 12(4):327-334. https://doi.org/10.4103/japtr.JAPTR_100_20
Udapurkar PP, Bhusnure OG, Kamble SR (2018). Diosmin Phytosomes: development, optimization and physicochemical characterization. Indian J. Pharm. Educ. Res., 52(4):29-36.https://doi.org/ 10.5530/ijper.52.4s.73
Yadav M, Jain S, Tomar R, Prasad G, Yadav H (2010). Medicinal and biological potential of pumpkin: an updated review. Nutri. Res. Rev. 23(2):184-190. https://doi.org/10.1017/S0954422410000107
To share on other social networks, click on any share button. What are these?






