Biological and Phytochemical Investigation of Heliotropium eichwaldii L.
Research Article
Biological and Phytochemical Investigation of Heliotropium eichwaldii L.
Abdur Rauf1*, Farooq Jan1, Muhammad Qayash2, Zia-ul-Islam3, Rizwanullah1, Ikramullah Khan1, Muhammad Shuaib4, Muhammad Khalid5 and Samrin Gul6
1Garden Campus Department of Botany, Abdul Wali Khan University, Mardan, Pakistan; 2Garden Campus Department of Zoology, Abdul Wali Khan University, Mardan, Pakistan; 3Garden Campus Department of Biotechnology, Abdul Wali Khan University, Mardan, Pakistan; 4School of Ecology and Environmental Science, Yunnan University, China; 5School of Agriculture & Biology Shanghai Jiao Tong University, Shanghai 200240, China; 6University of Sargodha, College of Agriculture, Pakistan.
Abstract | In this investigation, we have analyzed the biological and phytochemical activities of Heliotropium eichwaldii. The crude ethanolic extract (25, 20, and 15mg/ml) was used against bacterial and fungal species. The four pathogenic bacteria (Micrococcus luteus, E. coli, Staphylococcus aureus, Bacillus subtilis) and two fungi (Aspergillus flavus and Aspergillus niger) were examined. The above-mentioned various ethanolic crude extracts of H. eichwaldii revealed the highest potential (15.82±0.40), (18.12±0.56), and (17.41±0.51) against S. aureus, a moderate activity (13.31±0.64), (15.43±0.52), (14.35±0.64), (11.34±0.50), (13.47±0.55), (14.21±0.67), (9.53±0.51), (15.32±0.53), and (2.15±0.65) against E. coli, B. subtalus, and M. leutues. A 15mg/ml crude extract of H. eichwaldii exhibited the lowest potential (2.15±0.65) against M. leutues, while a 20mg/ml extract showed a very high potential against A. niger and A. flavus. Furthermore, the antioxidant potential of the ethanolic extract revealed the maximum at 400mg/ml (76.03%) and 300mg/ml (70.01%). The phytochemical analysis revealed the presence of alkaloids, saponins, flavonoids, and phenols in the ethanolic crude extract.
Received | March 01, 2023; Accepted | June 06, 2023; Published | June 22, 2023
*Correspondence | Abdur Rauf, Garden Campus Department of Botany, Abdul Wali Khan University, Mardan, Pakistan; Email: rauf77@awkum.edu.pk
Citation | Rauf, A., F. Jan, M. Qayash, Z. Islam, Rizwanullah, I. Khan, M. Shuaib, M. Khalid and S. Gul. 2023. Biological and phytochemical investigation of Heliotropium eichwaldii L. Pakistan Journal of Weed Science Research, 29(2): 88-94.
DOI | https://dx.doi.org/10.17582/journal.PJWSR/2023/29.2.88.94
Keywords | Biological activity, Phytochemicals, Ethanolic extract, Crude extract, Heliotropium eichwaldii
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Heliotropium eichwaldii is an erect herb, grows up to 15 to 20 inches and most commonly grow in August and September. H. eichwaldii is a common plant of sandy loam, found in a certain area of Pakistan (Karak, Bannu, and Lakki Marwat), north-western India (Rajasthan, Punjab, and Kashmir). H. eichwaldii is commonly known as shahdevi, chiraghas and nilkattai, belongs to family Boraginaceae. H. eichwaldii is an annual, inflorescences are commonly scorpiod cyme, ovary consists of 4-chembers stamens are five in number (El-Shazly and Wink, 2014).
Human being using plants as medicines for various diseases and also to fulfillment the basic requirements. The effort was started by human being to recognize toxic, edible, nutritive and medicinal plants (Rauf et al., 2012). The application of traditional medicines is safe and increasing day by day due to the potential negative effects of synthetic drugs. Moreover, traditional medicines either have less or no negative effects on health (Nair and Chanda, 2007; Siddique et al., 2016). The uses of medicinal plants increase gradually due to inexpensive and their curative properties (Bax and Mullan, 2000). It is recognized that some plants possess medicinal values such as antimicrobial and antiseptic (Rios and Recio, 2005).
Many plants (herbs, shrubs, trees) parts are in use for human nutrition for a long (Tapsell, 2006; Lai and Roy, 2004). Phytochemicals such as phenols and flavonoids are very effective as antiviral, anti-carcinogenic, anti-inflammatory, antineoplastic, anti-proliferative, and anti-allergic (Carr et al., 2000). The exploration of medicinal herbs related to the Sumerians, which they used to make tables of clay with lists of medicinal plants like Curcuma longa and Commiphora myrrha.
Oxygen is needed for energy generation in a biological system. During this biological pathway, some oxidants or free radicals are also produced as byproducts. These free radicals cause damage to the cell, cell membrane, and tissue injury. Free radicals cause many diseases, including heart disease, aging, and diabetes (Velavan, 2011). The free radical scavengers nullify the harmful effects (such as aging and cancer) of free radicals (Ozsoy et al., 2008).
Many pathogenic bacteria and fungi cause infection in plants. Modifications during progressive phases including post-harvest are caused by these pathogenic organisms. Synthetic pesticides (bactericides/fungicides) are commonly used to inhibit the growth of pathogens. However, the application of synthetic fungicides produced harmful effects on human and their surroundings, it is therefore gradually controlled (Harris et al., 2001).
Materials and Methods
Plant material and preparation of crude extract
Whole plant of H. eichwaldii (voucher No. 481) was collected in August, 2018, from Bermi Khel, District Bannu, KP, Pakistan. The plant specimen was identified by Sultan Mehmmod in Taxonomy laboratory, University of Science and Technology Bannu. After shade dried for 10-15 days, the plants materials were powdered nearly 0.002 mm by electric mortar. The plant material (whole) was powdered and 200 grams of it was dissolved in 1 L of ethanol. The mixture was shake and filtered after 3 days for crude greenish extract.
Antibacterial tests
The anti-bacterial activity was screened through agar well diffusion method (Usman et al., 2013). The nutrient medium for bacteria was prepared and soaked in Petri plate up to a depth of 3mm and bacteria strains were swabbed on the plate after media solidification. Four wells were punched in the agar with bore. In each well, DMSO, Antibiotic (Ciprofloxacin) and 25mg/1ml, 20 mg/1ml and 15mg/1ml concentrations were added. DMSO was used as a standard while the antibiotic was used as a control. The Petri plates having agar were incubated for 24 hrs at 37 ℃.
Antifungal activity
The antifungal activity was conducted through tube dilution method (Islam et al., 2011). The fungal species including A. niger and A. flavus were selected for antifungal activities. The two autoclaved test tubes of 250ml were filled up from nutritive media and then the fungi colonies were introduced to each test tube individually. For stimulating the fungal strains, these test tubes were incubated at 28°C for two days.
Antioxidant activity
Radical scavenging activity of plant extract was conducted using DPPH method and ascorbic acid as positive control (Bibi et al., 2010). The samples were prepared by 1000mg/ml solution of plant extract. The fractions such as 100, 150, 200, 250, 300 and 400 µg/ml of dilute was prepared. Each sample was shake at room temperature for half an hour in the dark. The absorbance was measured at 517 nm. The antioxidant activity was calculated through given formula:
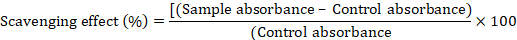
Phytochemical screening
The screening of both qualitative and quantitative analysis was carried out. The chemical constituents such as saponin, phenol, alkaloids, terpenoid, tannin, and resin were examined by using the protocol as designated by (Naz and Babo, 2013).
Qualitative analysis
Alkaloids: The crude plant extract (0.7 gm) were mixed in 12 ml of 2% HCl. The mixture was filtered and filtrate (4 ml) was treated separately with the reagents and precipitous were detected, which show alkaloids in the mixture (Trease and evans, 2002).
Terpenoids: The plant extract of 4 ml was dissolved in chloroform (2ml) plus 3 ml sulfuric acid, the reddish brown coloration shows presence of Terpenoid.
Phenols: The plan extract was dissolved in 4 ml of 2% iron chloride solution. A black coloration shown phenols existence (Archana et al., 2012).
Tannins: A 0.20 gm of crude extract was dissolved in 12 ml distilled water and filtered. One percent (1%) Ferric Chloride was mixed with the filtrate and a blue color in mixture shows tannins occurrence (Trease and Evans, 2002).
Flavonoids: The crude extract (0.4 gm) was mixed in petroleum ether for the purpose to remove the oily constituents of the solution. The oil free component was mixed in 12 ml of 95% ethanol. The filtrate was subjected for flavonoids test. The filtrate (5 ml) was mixed in 6 ml of 2% potassium hydroxide in a test tube and the yellow color in the sample shows the presence of flavonoids (Trease and Evans, 2002).
Resins: A 0.7 gm of plant extract was dissolved in 10 ml of distilled water and the formation of the precipitation shows resins.
Saponins: A 0.10 gm extract of the plant was mixed in 8 ml water (distill) and shaken. The precipitation shows the existence of saponins in the sample (Ejikeme and Ezeonu, 2014).
Sterols: The crude extracts (4ml) were mixed with Chloroform (6ml) and 2 ml of Sulfuric acid was dissolved in the mixture. The presence of greenish-red color shows sterols (Sheel et al., 2014).
Protein: A 3 ml of plant extract was dissolved in six drops of Sulfuric acid, and the formation of white precipitation shows the existence of protein in the tested mixture (Trease and Evans, 2002).
Quantitative analysis
Total flavonoids contents: The quantitative analysis of flavonoids was done by using 0.8g of crude extract. The extract was dissolved in 5 ml ethanol along with Aluminum tri chloride (0.3 ml). A 0.3 ml Potassium acetate was mixed, and the volume was raised to 4 ml. Then the solution was shaken and placed at 25oC for 28 minutes. The absorption was checked at 417nm. In the quantitative analysis of flavonoids, the Quercetin was used as a standard (Daffodil et al., 2013).
Total phenolic contents: The quantitative analysis of phenolic was performed by the addition of 0.8 gm crude extract of H. eichwaldii to 1 ml of 95% Ethanol. The solution was centrifuged for 25 minutes and then it was soaked in distil water for the dryness. Its volume raised to 5 ml with the addition of 3 ml 25% Sodium carbonate. The reagent folin-ciocalteau (0.5 ml) was added and it was supplemented with 2ml 25% Sodium carbonate. The solutions were mixed and treated with boiling water. The absorbance was tested at 645 nm. Pyrocatechol chemical was used as a standard in the quantitative analysis of phenolic contents (Hagerman et al., 2004).
Results and Discussion
The present research work was conducted to examine the phytochemical analysis, antimicrobial and free radical scavenging capacity of H. eichwaldii.
Antibacterial activity
The antibacterial activity of H. eichwaldii was analyzed against four bacterial species including S. aureus, E. coli, B. subtalus and M. leutues. The result obtained revealed that the applied concentrations of the crude extract of H. eichwaldii were highly effective. The zone of inhibition in case of S. aureus was (15.82±0.40), (18.12±0.56) and (17.41±0.51) at 25mg/ml, 20mg/ml and 15mg/ml, respectively (Figure 1, Table 1). The negative control did not showed inhibition. The zones of inhibition against E. coli at (15mg/ml), (20mg/ml) and 25mg/ml were (13.31±0.64), (15.43±0.52) and (14.35±0.12) respectively (Figure 1, Table 1). The zones of inhibition were (11.34±0.63), (13.47±0.50) and (14.21±0.55) at 25mg/ml, 20mg/ml and 15mg/ml concentration against B. subtalus respectively (Figure 1, Table 1). The zones of inhibition were (2.15±0.65), (9.53±0.70), and (11.19±0.53) against M. leutues at 15mg/ml, 20mg/ml and 25mg/ml concentrations respectively. The zones of inhibition in case of Ciprofloxacin 25mg/ml (standard) were (36.66±0.81), (33.12±0.52), (23.07±0.58) and (26.13±0.47) against S. aureus, E. coli, B. subtalus and M. leutues (Figure 1, Table 1). Dimethyl Sulfoxide (negative control) did not inhibit the growth of any strain.
Table 1: Inhibition zones of H. eichwaldii ethanolic crude extract against the four examined bacteria species (Value mean+SEM).
|
Bacterial species |
Control |
Crude concentrations |
||
|
(Ciprofloxacin) 25mg/ml |
25mg/ml |
20mg/ml |
15mg/ml |
|
|
S. arureus |
36.66±0.81 |
15.82±0.40 |
18.12±0.56 |
17.41±0.51 |
|
E. coli |
33.12±0.52 |
13.31±0.64 |
15.43±0.52 |
14.35±0.12 |
|
B. subtalus |
23.07±0.58 |
11.34±0.64 |
13.47±0.50 |
14.21±0.55 |
|
M. leutues |
26.13±0.47 |
2.15±0.65 |
9.53±0.70 |
11.19±0.51 |
Antifungal activity
Different concentrations of crude extract such as 5mg/ml, 10mg/ml, 15mg/ml and 20mg/ml were used against A. flavus and A. niger. When the inhibition of fungus was studied at different concentrations of applied crude extract, it was shown that the concentration of 20mg/ml was highly active against the examined fungi (Figure 2, Table 2). The 5mg/ml concentration was least active against A. flavus. The 20mg/ml and 15mg/ml concentrations have shown the highest activity against A. niger. The 10mg/ml and 5mg/ml concentrations of the crude extract inhibit the growth of A. niger at low degree as (27.6%) and (35.8%) (Figure 2, Table 2).
Table 2: Effect of different concentrations of crude extract of H. eichwaldii on the linear growth of A. niger and A. flavus.
|
Concentration (mg/ml) |
Inhibition |
|
|
A. flavus |
A. niger |
|
|
20 |
59.3% |
44.9% |
|
15 |
54.6% |
39.3% |
|
10 |
30.9% |
27.6% |
|
5 |
24.7% |
35.8% |
Antioxidant activity
We used DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) radical scavenging assay, to compare the anti-oxidant activity. The free radical scavenging potential of the plant extract along with the standard (Ascorbic acid) was noted. We found that the scavenging potential of the plant extract is less than the standard (Table 3). The highest scavenging activity was showed at 400µg/ml (76.3%) and 300µg/ml (70.1%) inhibition, as compared to the standard ascorbic acid which showed 89.1 ± 0.1 and 85.3 ± 0.0 % DPPH inhibition in the assay (Figure 3, Table 3).
Table 3: Antioxidant activity of Ethanolic crude extract of H. eichwaldii. DPPH (2, 2-diphenyl-1-picryl-hydrazyl-hydrate). Data are mean±SD.
|
Concentration (µg/ml) |
Extract (DPPH % inhibition) |
% Ascorbic acid scavenging |
|
100 µg/ml 150 µg/ml 200 µg/ml 250 µg/ml 300 µg/ml 400 µg/ml |
42.2 ± 0.2 51.4 ± 0.1 60.1 ± 0.1 66.2 ± 0.1 70.1 ± 0.2 76.3 ± 0.1 |
70.0 ± 0.0 74.2 ± 0.1 78.0 ± 0.0 82.1 ± 0.1 85.3 ± 0.0 89.1 ± 0.1 |
Qualitative phytochemical analysis
Qualitative phytochemical screening of H. eichwaldii showed the presence of flavonoids, alkaloids, terpenoids, sterols, tannins and phenols in the tested sample, while protein, saponins and resins were absent in the examined sample (Table 4).
Table 4: Qualitative investigation of ethanolic crude extract of H. eichwaldii.
|
Terpenoids |
Phenol |
Saponins |
Sterols |
Protein |
Flavonoid |
Resins |
Alkaloids |
Tannins |
|
+ |
+ |
̶ |
+ |
̶ |
+ |
̶ |
+ |
+ |
Quantitative phytochemical analysis
The crude extract of H. eichwaldii was analyzed for the quantitative study of total phenolic and flavonoids contents (Figure 4, Table 5). The mean of total phenolic was (1.1319±0.532379), while that of flavonoid was (1.528±0.553376).
Table 5: Quantitative investigation of ethanolic crude extract of H. eichwaldii.
|
Sample |
Flavonoid contents |
Phenol contents |
|
H. eichwaldii |
1.528±0.553376 |
1.1319±0.532379 |
In present research, we have evaluated pharmacological significance and phytochemical ability of the plant crude extract. The results of current examination showed that extract of plant have antibacterial potential. Different concentrations (25 mg/ml, 20 mg/ml and 15mg/ml) of the ethanolic extract were effective, when applied on the bacterial strains. The inhibition zone diameter in case of S. aureus was the maximum such as (18.12±0.56), (15.82±0.40). While M. leutues showed the lowest result such as (2.15±0.65), (9.53±0.70) and (11.19±0.51) at concentrations 25mg/ml, 20mg/ml and 15mg/ml, respectively. Various concentration of ethanolic extract of H. eichwaldii was used against two fungal species including A. flavus and A. niger. The concentration (20mg/ml) was more effective against the fungal species A. flavus. While the concentration (5mg/ml) of the ethanolic crude extract was the least effective against Aspergillus flavus. Similarly, the concentration (10mg/ml) was least effective against A. niger. While the concentration (20mg/ml) was the more effective against A. niger.
The maximum scavenging potential of our tested crude extract found at 400 µg/ml (76.03%) and 300 µg/ml (70.01%) the standard ascorbic acid showed 89.1% DPPH inhibition. The investigation of phytochemical show that it inhibits oxidative reactions and protect DNA from destruction. antioxidant activity is very important in the cure of free radical causing disorders. Our result showed similarities with the result of antioxidant activity as reported (Saeed et al., 2018).
In present investigation the phytochemicals such as flavonoid, sterols, terpenoids, alkaloid, phenols and tannins were identified in extract of the studied plant. While the phytochemicals including resin, protein and saponins were absent in the examined sample. The mean of total phenolic was calculated as (1.1319±0.532379) and the mean of flavonoid was noted as (1.528±0.553376), respectively. Alkaloids are any heterocyclic bases organic constituent in which the ring contain nitrogen atoms. The application of flavonoids can change the response of body to germ and allergies. Flavonoids have antimicrobial, pharmacological and antiallergic capability. Leucorrhoea, diarrohea and burn piles are most commonly treated via tannins (Buzzini et al., 2008; Sharif et al., 2022). The shapes phenol may be aromatic ring to polymeric arrangements but glycoside is the most common forms (Williamson, 2005). The chemical process such as metabolism may result in formation of oxidant trigger oxidative destruction to of many essential molecules e.g., proteins, lipids and DNA. Various parts of plant and their products are used because they possess highly effective properties such as analgesic, anticancer and antioxidant activities. The medicinal property of the studied are due to the presence of alkaloids, terpenoid and phenols. These phytochemicals are known as the common source for the cure of many diseases. Some phytochemicals perform important function in development of immune system. These phytochemicals are responsible for protection of body from pathogens. Alkaloids tannin, flavonoids and phenolic compounds are the bioactive phytochemicals of H. eichwaldii. The antiseptic potential of plants is because of the phytochemical constituents, which the plants possess (El-Astal et al., 2005). The results of current experiments showed that extract of the examine plant contain bioactive compounds.
Conclusions and Recommendations
The extract of H. eichwaldii shown antimicrobial, phytochemical and antioxidant potential. The extract of the examine plant has potential for preparation of traditional drugs. The result of our study displayed the presence of medicinal components in the examined plant. The previous studied researches resolute the presence of these curative phytochemical, which provide pharmaceutical feature to the studied plant. We concluded that the extract from H. eichwaldii is effective source of medicine against the examine microbe and possess high antioxidant activity.
It is suggested that further experiments will encourage to examine of the possible phytotoxic effect of this extract on cereal crops.
Acknowledgement
We are thankful to the Department of Botany Abdul Wali Khan University Mardan, for providing chemicals and facilitation during the execution of this research project.
Novelty Statement
Our analysis revealed that the Heliotropium eichwaldii L. crude ethanolic extract has antibacterial and antifungal phytochemicals against pathogenic bacteria (Micrococcus luteus, E. coli, Staphylococcus aureus, Bacillus subtilis) and fungi (Aspergillus flavus and Aspergillus niger) respectively. Furthermore, the phytochemical analysis revealed the presence of alkaloids, saponins, flavonoids, and phenols in the ethanolic crude extract of Heliotropium eichwaldii L.
Author’s Contribution
The authors are grateful to the following for their contributions to this M.Phil. research project: Dr. Abdur Rauf and Mr. Rizwanullah (research design); Mr. Rizwanullah (execution); Dr. Abdur Rauf, Dr. Ikramullah Khan, Dr. Farooq Jan, Dr. Samrin Gul and Mr. Rizwanullah (manuscript writing and formatting), Dr. Muhammad Qayash, Dr. Muhammad Khalid, Mr. Muhammad Shoaib and Dr. Zia-ul-Islam (statistical analysis).
Conflict of interest
The authors have declared no conflict of interest.
References
Archana, P., T. Samatha, B. Mahitha and N.R. Chamundeswari. 2012. Preliminary phytochemical screening from leaf and seed extracts of Senna alata L. Roxb-an ethno medicinal plant. Int. J. Pharm. Biol. Res., 3(3): 82-89.
Bax, R., N. Mullan and J. Verhoef. 2000. The millennium bugs the need for and development of new antibacterials. Int. J. Antimicrob. Agents, 16(1): 51-59. https://doi.org/10.1016/S0924-8579(00)00189-8
Bibi, Y., S. Nisa, A. Waheed, M. Zia, S. Sarwar, S. Ahmed and M.F. Chaudhary. 2010. Evaluation of Viburnum foetens for anticancer and antibacterial potential and phytochemical analysis. Afr. J. Biotechnol., 9: 5611.
Buzzini, P., P. Arapitsas, M. Goretti, E. Branda, B. Turchetti, P. Pinelli, F. Ieri and A. Romani. 2008. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem., 8(12): 1179. https://doi.org/10.2174/138955708786140990
Carr, A.C., B.Z. Zhu and B. Frei. 2000. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and α-tocopherol (vitamin E). Circ. Res., 87(5): 349-354. https://doi.org/10.1161/01.RES.87.5.349
Daffodil, E.D., K. Rajalakshmi and V.R. Mohan. 2013. Antioxidant activity, total phenolics and flavonoids of Salicornia brachiata Roxb. Leaf extracts (Chenopodiaceae). World J. Pharm. Pharm. Sci., 2(1): 352-366.
Ejikeme, C., C.S. Ezeonu and A.N. Eboatu. 2014. Determination of physical and phytochemical constituents of some tropical timbers indigenous to Nigerdelta area of Nigeria. Eur. Sci. J., 10(18): 247-270.
El-Astal, Z.Y., A.E.R.A. Ashour and A.A.M. Kerrit. 2005. Antimicrobial activity of some medicinal plant extracts in Palestine. Pak. J. Med. Sci., 21(2): 187-193.
El-Shazly, A. and M. Wink. 2014. Diversity of pyrrolizidine alkaloids in the Boraginaceae structures, distribution, and biological properties. Diversity, 6(2): 188-282. https://doi.org/10.3390/d6020188
Hagerman, R.J., B.R. Leavitt, F. Farzin, S. Jacquemont, C.M. Greco, J.A. Brunberg, F. Tassone, D. Hessl, S.W. Harris, L. Zhang and T. Jardini. 2004. Fragile-X–associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 permutation. Am. J. Hum. Genet., 74(5): 1051-1056. https://doi.org/10.1086/420700
Harris, C.A., M.J. Renfrew and M.W. Woolridge. 2001. Assessing the risks of pesticide residues to consumers: Recent and future developments. Food Addit. Contam., 18(12): 1124-1129. https://doi.org/10.1080/02652030110050122
Islam, M.S., R. Zahan, M.B. Alam, M. Nazin, C.S. Gopal, M.A. Mosaddik and E.M. Haque. 2011. Studies on antibacterial and insecticidal activities of Suregada multiflora. Libyan Agric. Res. Center J. Int., 2(2): 62-67.
Lai, P.K. and J. Roy. 2004. Antimicrobial and chemo preventive properties of herbs and spices. Curr. Med. Chem., 11(11): 1451-1460. https://doi.org/10.2174/0929867043365107
Nair, R. and S. Chanda. 2007. Antibacterial activities of some medicinal plants of the western region of India. Turk. J. Biol., 31(4): 231-236.
Naz, R. and A. Bano. 2013. Phytochemical screening, antioxidants and antimicrobial potential of Lantana camara in different solvents. Asian Pac. J. Trop. Dis., 3(6): 480-486. https://doi.org/10.1016/S2222-1808(13)60104-8
Ozsoy, N., A. Can, R. Yanardag and N. Akev. 2008. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem., 110(3): 571-583. https://doi.org/10.1016/j.foodchem.2008.02.037
Rauf, A., N. Muhammad, A. Khan, N. Uddin and M. Atif. 2012. Antibacterial and phytotoxic profile of selected Pakistani medicinal plants. World Appl. Sci. J., 20(4): 540-544.
Rios, J.L. and M.C. Recio. 2005. Medicinal plants and antimicrobial activity. J. Ethnopharmacol., 100(1-2): 80-84. https://doi.org/10.1016/j.jep.2005.04.025
Saeed, A., M.S. Marwat, A.M. Chohan, A.H. Shah, R. Naz, J. Gul, M.Z. Bhatti and A. Saeed. 2018. Antioxidant activity in seeds of Avena fatua and Chenopodium album weeds associated with wheat crop. Pak. J. Weed Sci. Res., 24(3). https://doi.org/10.28941/24-3(2018)-2
Sharif, A., N.A. Shah, A. Rauf, N. Hadayat, A. Gul, G. Nawaz, S. Sakhi, M. Iqbal, M.R. Khan, A.A. Shah and N. Azam. 2022. Ethnomedicinal uses of plants for various diseases in the remote areas of Changa Manga Forest, Pakistan. Braz. J. Biol., 84. https://doi.org/10.1590/1519-6984.255916
Sheel, R., K. Nisha and J. Kumar. 2014. Preliminary phytochemical screening of methanolic extract of Clerodendron infortunatum. IOSR J. Appl. Chem., 7(1): 10-13. https://doi.org/10.9790/5736-07121013
Siddique, A., N. Akhtar, M.S. Khan, M. Anwar, J. Samin and W.M. Khan. 2016. Diversity, distribution and indigenous uses of the medicinal plants of district Karak, Khyber Pakhtunkhwa, Pakistan. Pak. J. Weed Sci. Res., 22(2).
Tapsell, L.C., I. Hemphill, L. Cobiac, D.R. Sullivan, M. Fenech, C.S. Patch, S. Roodenrys, J.B Keogh, P.M., Clifton, P.G. Williams and V.A. Fazio. 2006. Health benefits of herbs and spices: The past, the present, the future. https://doi.org/10.5694/j.1326-5377.2006.tb00548.x
Trease, G.E. and W.C. Evans. 2002. Pharmacognosy. 15th Ed. London: Saunders Publishers, pp. 42–44, 221–229, 246–249, 304–306, 331– 332, 391–393.
Usman, U.A., H. Rahman, Z. Niaz, M. Qasim, J. Khan, Tayyaba and B. Rehman. 2013. Antibacterial activity of some medicinal plants against selected human pathogenic bacteria. Eur. J. Microbiol. Immunol., 3: 272-274. https://doi.org/10.1556/EuJMI.3.2013.4.6
Velavan, S., 2011. Free radicals in health and diseases. A mini review. Pharmacology online Newsletter, 1: 1062-1077.
Williamson, G. and C. Manach. 2005. Bioavailability and bio efficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr., 81(1): 243S-255S. https://doi.org/10.1093/ajcn/81.1.243S
To share on other social networks, click on any share button. What are these?





