Assessment of Physicochemical and Sensory Characteristics of Stevia Sweetened, Low-Caloric Orange Drink
Research Article
Assessment of Physicochemical and Sensory Characteristics of Stevia Sweetened, Low-Caloric Orange Drink
Muhammad Usman1, Majid S. Hashmi1, Ayaz Ahmad1*, Fawad Ahmad2 and Zahid Alam1
1Department of Food Science and Technology, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Food Science and Technology, Abdul Wali Khan University, Mardan, Pakistan.
Abstract | Excessive consumption of food products laden with sugar pose threat to health of consumers generally and diabetes patients particularly. Moreover, some low-calorie products did not meet sensory preferences of consumers. Therefore, this research was conducted to evaluate the quality and acceptability of a low-caloric orange drink by incorporating various concentrations of stevia powder and storing it within a temperature range of 20±0.4°C. The investigated treatments included: Orange Drink (OD)0 (Juice: 15%, Sugar: 13%, Stevia: 0%, Sodium Benzoate: 0.1%, Water: 72%), OD1 (Juice: 15%, Sugar: 0%, Stevia: 0.065%, Sodium Benzoate: 0.1%, Water: 84.83%), OD2 (Juice: 15%, Sugar: 0%, Stevia: 0.070%, Sodium Benzoate: 0.1%, Water: 84.83%), and OD3 (Juice: 15%, Sugar: 0%, Stevia: 0.075%, Sodium Benzoate: 0.1%, Water: 84.82%). These treatments were assessed over a three-month storage period at 15-day intervals, with a focus on various physicochemical parameters, including total soluble solids, pH, titratable acidity, and vitamin C content. Furthermore, organoleptic attributes such as color, taste, aroma, and overall acceptability were subject to evaluation. The findings indicated a decrease in pH values and ascorbic acid content over time, while total soluble solids, as well as titratable acidity, increased. Notably, the OD2 formulation exhibited significantly (P≤0.05) higher pH and ascorbic acid content while having lower TSS and TA content compared to the sugar-based sample and other stevia-containing samples. During the organoleptic assessment, OD2 consistently received higher scores from the panel of judges throughout the storage period. Statistical analysis confirmed that this particular formulation had a substantial impact on both the physicochemical and sensory characteristics of the drink. For commercialization of the product large scale evaluation should be done to validate these findings in addition to microbial analysis.
Received | October 19, 2023; Accepted | January 31, 2024; Published | April 12, 2024
*Correspondence | Ayaz Ahmad, Department of Food Science and Technology, The University of Agriculture Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: ayaz@aup.edu.pk
Citation | Usman, M., M.S. Hashmi, A. Ahmad, F. Ahmad and Z. Alam. 2024. Assessment of physicochemical and sensory characteristics of stevia sweetened, low-caloric orange drink. Sarhad Journal of Agriculture, 40(2): 354-361.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.2.354.361
Keywords | Sweet orange, Stevia powder, RTS drink, Low-calorie, Physicochemical, Organoleptic analysis
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Globally oranges contribute to more than half of the citrus production and account for 40% of the total export making it a significant contributor to the agricultural landscape (FAO, 2021). Citrus fruits generally and oranges are renowned for their mineral and ascorbic acid content, endowing them with notable health benefits (Sarvarian et al., 2022). Furthermore, there exists substantial potential for the development of value-added products, such as Ready to Serve (RTS) drinks, which utilize orange juice containing biologically active substances (Ambreen et al., 2023). RTS drinks, enriched with a high-water content, offer hydration benefits to consumers while also serving as a source of essential nutrients and minerals (Rathinasamy et al., 2021).
It is crucial to acknowledge that conventional fruit drinks are often laden with excessive sugar, providing limited nutritional value (Chen et al., 2022). As a response to the growing concern over the adverse health effects associated with high sugar consumption, particularly in the context of diseases like diabetes, the food industry is increasingly focused on the formulation of low-calorie alternatives (Wang et al., 2020). These substitutes, although cost-effective, have raised concerns due to potential health risks with their regular consumption. Consequently, there is a growing emphasis on developing natural, health-friendly, low-calorie sweetened drinks (Saeedi et al., 2019).
In recent years, Stevia, generally recognized as safe by the FDA, has captured the attention of researchers (Jabeen et al., 2019). Derived from the Rebaudiana bertoni shrub, a member of the Asteraceae family, Stevia primary sweetening components include rebaudioside and stevioside. Both compounds are commercially produced through chemical and physical processes. Stevia has gained traction as a means to promote healthier food options, boasting a rich nutrient profile with proteins, potassium, and various bioactive compounds which have additional health benefits (Yildiz and Karhan, 2021). It can be integrated into dietary plans as a carbohydrate source as it contains carbohydrates ranging from 35.2-61.9% (Wang et al., 2020).
Stevia exceptional sweetness, at 300 times the level of white sugar, is a prominent feature, making it a compelling sugar substitute (Peteliuk et al., 2021). Additionally, it contains a wealth of phytochemicals, including stevioside, thiamine, rebaudi-oxide, dulcoside, steviol, riboflavin, niacin, beta-carotene, and Austroinulin (Wang et al., 2020). The leaves of the Stevia plant offer versatility, serving not only as sweeteners but also for enhancing the taste and color of beverages, salads, fruits, and coffee (Abou-Arab et al., 2010).
Recognizing its low-calorie profile and safety, several international health regulatory authorities, including CODEX, CAC, FAO, and WHO, have approved Stevia use in various food products. Nevertheless, recommended acceptable levels, such as the 4mg/kg body weight/day standard set by the European Food Safety Authority (EFSA), must be adhered to (Peteliuk et al., 2021; EFSA, 2010). The versatility of Stevia extends to its applications in diverse food products, encompassing beverages, soft drinks, bakery items, and home-cooked meals (Jabeen et al., 2019).
Building on prior research, the present study endeavors to create a low-calorie sweet orange drink through the incorporation of Stevia powder as a sugar replacement. In addition to physicochemical analysis, sensory characteristics were also evaluated to measure consumer acceptance of orange drinks containing different concentration of Stevia.
Materials and Methods
Orange drink preparation and storage
Ready to serve (RTS) orange drink (OD) was prepared with the addition of 15% orange juice, 13% sugar, water, and stevia in different concentrations as mentioned by. 0.1% Sodium benzoate was added as a preservative. The prepared orange fruit RTS drink was filled in properly labeled PET bottles (250ml). These bottles were kept in storage at 20±0.4oC for three months period. After every 15 days, the products were analyzed for sensory and physicochemical properties. Based on initial trial, it was observed that lowering stevia below 0.65% and above 0.75% negatively affect sensory characteristics of the drink, therefore, the following categories of drink was prepared to finalize consumer acceptable low-calorie drink and to compare this with sugar containing orange drink.
OD0 (15% juice + 13% sugar + 0.1% S.B + 72% water)
OD1 (15% juice + 0.065% stevia powder + 0.1% S.B + 84.83% water)
OD2 (15% juice + 0.070% stevia powder + 0.1% S.B + 84.83% water)
OD3 (15% juice + 0.075% stevia powder + 0.1% S.B + 84.82% water)
Physicochemical analyses
pH and ascorbic acid determination: To determine pH of the drink, the meter was turned on and the probe was placed in the beaker containing orange drink. After the digits got stabilized reading was recorded (Jabeen et al., 2019). Ascorbic acid in low calorie orange drink was determined according to (Rodrigues et al., 2022). In this method 20mL of orange juice was added with 3mL of Sulfuric acid (12 M) and 3mL (0.5% m/v) of starch. Iodine solution (0.01 M) was titrated against the mixture after homogenization till the appearance of dark color. This mixture was again titrated with sodium thiosulphate (0.01 M) till dark color disappeared. The mixture was again titrated with iodine solution till dark color reappear. The given formula was used to calculate AA content.
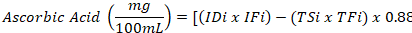
Where, IDi is the amount of iodine solution used in titration and IFi is the correction factor. Similarly, TSi is the amount of sodium thiosulphate used and TFi is the correction factor.
TSS and TA determination: To determine total soluble solids in orange drink, refractometer was cleaned using distilled water. After drying the glass part, few drops of the drink were poured onto the surface and the lid was closed. The results were expressed in % (Jabeen et al., 2019). The method described by Rodrigues et al. (2022) was employed for the determination of titratable acidity. Two drops of phenolphthalein indicator were added in 10mL juice homogenized with 50mL distilled water. The prepared mixture was titrated with Sodium hydroxide (0.1 M) till the appearance of pink color. Using mL of NaOH utilized in the titration, molarity of NaOH and amount of juice, TA was calculated as follows.
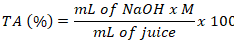
Sensory analyses of orange drink
Sensory analysis was carried out by 30 skilled judges, employing 9-point hedonic scale. Sensory analyses in terms of color, taste, aroma, and overall acceptability was done at 15 days interval during storage period for 3 months (Jabeen et al., 2019).
Statistical analysis
To analyze the whole data, Complete Randomized Design (CRD) with 2 factors was utilized along with the separation of means with the least significance difference testing as per the directions of Steel and Torrie (1960).
Results and Discussion
pH and ascorbic acid content of orange drink
The pH values of the orange drink, prepared with varying concentrations of sugar and stevia, are depicted in Figure 1A. Over a 90-day storage period, a consistent decline in pH was observed. Initially, the pH of the orange drinks fell within the range of 3.68, 3.69, 3.68, and 3.67. However, over time, these values decreased to 3.15, 3.27, 3.35, and 3.32 for the groups designated as OD0, OD1, OD2, and OD3, respectively. Notably, after the storage period, OD2 exhibited the highest pH at 3.35, which was statistically significant (P≤0.05) when compared to the other concentrations of stevia and sugar-containing drinks, which had a pH of 3.15. Similarly, there was a decline in the ascorbic acid content of the orange drink samples throughout the 90-day storage duration, as illustrated in Figure 1B. The results of this study clearly demonstrate that replacing sugar with stevia powder did not have a significant impact on the ascorbic acid content of the drinks. The initial ascorbic acid values for OD0, OD1, OD2, and OD3 were 8.58, 8.88, 8.34, and 7.56 mg/100mL, respectively. As the storage duration increased, the ascorbic acid content in all samples continuously decreased. However, OD2 maintained a higher ascorbic acid content of 6.64 mg/100mL after 90 days, significantly higher (P≤0.05) compared to the sugar-containing drink (4.63 mg/100mL) which is supported by the previous study of (Raghavan et al., 2023).
It is important to note that pH is considered one of the critical factors that affect the shelf life of ready to serve drinks (Sarvarian et al., 2022). On the other hand, ascorbic acid acts as an antioxidant, playing a role in preserving the shelf life of the drinks (Tö, 2020). The continuous decline observed in both pH and ascorbic acid content during the storage period can be attributed to the ongoing increase in acidity Figure 2B. As acidity increases, the pH of the samples tends to decrease, as there is an inverse relationship between pH and acidity (Jabeen et al., 2019). The fermentation of sugars in the juice over time leads to the production of more organic acids, further contributing to the lowering of pH (Rodrigues et al., 2022). Citric, malic, ascorbic, galacturonic, oxalic, and quinic acids are among the organic acids found in citrus juice that are responsible for the reduction in pH (Asencio et al., 2018). Additionally, during storage, the degradation of ascorbic acid into dehydroascorbic acid occurs, which is a common chemical reaction in various types of juices (Rodrigues et al., 2022). The formation of reductones or ethylglyoxal as a final product from the degradation of ascorbic acid leads to their reaction with amino acids, resulting in the formation of brown pigments that can affect the color of the juice (Jabeen et al., 2019).
TSS and TA of orange drink
The total soluble solids (TSS) of the sugar-containing drink and drinks containing different concentrations of stevia are depicted in Figure 2A. During storage, a slight increase in TSS was observed in all samples. Upon analyzing the results, it becomes evident that replacing sugar with stevia significantly (P≤0.05) reduced the TSS of the samples. Throughout the storage period, the highest TSS was observed in OD0, measuring 13.42%, 13.45%, 13.48%, 13.55%, 13.62%, 13.67%, and 13.72% on day 0, 15, 30, 45, 60, 75, and 90, respectively. Among the stevia-containing samples, a slight difference in TSS was observed, which was statistically non-significant. Both sugar and stevia, as well as storage duration, significantly affected the TSS of the orange drinks. Similarly, the titratable acidity (TA) of all the samples increased continuously during the 90-day storage period Figure 2B. Initially, TA values for OD0, OD1, OD2, and OD3 were 0.21%, 0.19%, 0.23%, and 0.22%, respectively, which increased to 0.31%, 0.26%, 0.29%, and 0.28%, respectively, after 90 days. The TA of sugar-containing samples and those containing different concentrations of stevia were non-significantly different from each other; however, the effect of storage duration on TA was significant.
Drinks made with sugar exhibited higher TSS compared to those prepared with stevia (Jabeen et al., 2019). Sucrose, a disaccharide sugar, clearly increased the TSS content of the drink (Byanna and Gowda, 2012). In contrast, stevia, a low-caloric sweetening agent, imparted sweetness to the drink without elevating the TSS levels of the samples (Salar et al., 2020). The slight increase in TSS during storage can be attributed to the hydrolysis of complex carbohydrates into simple oligosaccharides and monosaccharides (Byanna and Gowda, 2012). Regarding TA, it also increased during the storage of orange drinks. Typically, the sugar present in the juice ferments, resulting in the production of organic acids, leading to increased acidity (Kaddumukasa et al., 2017). Jabeen et al. (2019) also reported that free sugars present in the drink degrade into carboxyl acids, resulting in increased acidity. Thus, autolysis reactions occurring in the juice release acids, which might be the reason for the increased acidity. Similar results were also reported by (Jothi et al., 2014; Singh and Sharma, 2017).
Sensory analysis of orange drink
The color of orange drink samples, which contained various concentrations of stevia powder in addition to sugar, is illustrated in Figure 3A. A decreasing trend in color intensity was observed during the storage period. Initially, the color scores for the drink samples OD0, OD1, OD2, and OD3 were within the range of 8.8, 8.7, 8.7, and 8.6, respectively. After 90 days of
storage, the highest color score (P≤0.05) was noted for sample OD2, which was 7.6, in contrast to the sugar-containing sample OD0, which scored 5.8, indicating fair acceptability. Figure 3B presents the organoleptic scores for taste of the sugar and low-calorie stevia-containing orange drink samples. On day 0, the panel of judges rated OD0, OD1, OD2, and OD3 as 8.8, 8.8, 8.6, and 8.6, respectively. Over the 90-day storage period, the taste scores for all samples declined at each evaluation interval. Nevertheless, after the initial day, OD2 consistently maintained significantly higher taste scores. On day 90, OD2 received a score of 7.4, surpassing both the sugar-containing sample (5.6) and the other stevia-containing samples. Similar to the organoleptic scores for color and taste, the scores for aroma displayed a declining trend throughout the three-month storage period, as shown in Figure 3C. On day 0, the aroma scores for OD0, OD1, OD2, and OD3 were 8.8, 8.8, 8.6, and 8.6, respectively. A consistent decline was observed, and the difference between OD0 and the other stevia-containing samples remained statistically significant (P≤0.05) at each evaluation interval during storage. OD2 consistently received higher ratings for aroma, starting from day 0, and emerged as the preferred sample in terms of aroma by consumers. The combination of aesthetically appealing color, taste, and aroma can enhance the overall acceptability of products, as depicted in Figure 3D. Throughout the storage period, with the exception of day 0, OD2 received higher ratings from the panel of judges in terms of color, taste, and aroma compared to both the sugar-containing drink and the drinks containing different concentrations of stevia. These results clearly indicate that OD2 was highly rated and this preference was reflected in the overall acceptability rating.
During the three-month storage period, we observed that the intervals of storage and the replacement of sugar with stevia had a significant impact on the organoleptic characteristics of orange drinks, including color, taste, aroma, and overall acceptability. After the 90-day storage period, we found that samples containing stevia, particularly OD2, maintained a more acceptable color compared to other concentrations of stevia or sugar-containing samples Figure 3A. The decline in acceptable color can be attributed to the loss of pigments resulting from oxidative catabolic reactions and polymerization reactions between proteins and phenolic compounds (Murata, 2021). Jabeen et al. (2019) also reported that during storage, non-enzymatic oxidative decomposition of nutrients such as phenolics leads to the formation of dark-colored compounds, contributing to the development of undesirable color in ready-to-serve drinks (Hariharan and Mahendran, 2016; Fennema, 2008). Similar to the color scores, the organoleptic scores for taste were higher in samples containing stevia, particularly OD2, compared to OD0 Figure 3B. The taste score for OD2 and OD3 was slightly lower than that of OD0 and OD1, which may be due to the bitter aftertaste of stevia (Jabeen et al., 2019). However, after 15 days of storage, the taste score for OD2 was significantly higher compared to other samples (Voorpostel et al., 2014; Hariharan and Mahendran, 2016). Furthermore, the taste of the orange juice was affected by factors such as changes in pH, oxidation, enzymatic reactions, and microbial activities (Pan et al., 2023). The odor of orange ready-to-serve drinks is generally influenced by several factors, including temperature, oxygen availability, light exposure, and contamination by microorganisms (Pan et al., 2023). These factors contribute to the degradation of various volatile components of orange juice, leading to the loss of its characteristic aroma (Li et al., 2018; Wibowo et al., 2015). Moreover, the presence of different aromatic compounds in stevia contributes to the characteristic pungent aroma (Jabeen et al., 2019), and the loss of different essential oils during storage can be attributed to changes in the aroma of the drinks, as reported by Radi et al. (2018). In our study, we found that a product exhibiting acceptable color, taste, and aroma, leads to enhanced overall acceptability (Figure 3). This trend was consistent with our findings, as OD2, which received higher ratings for color, taste, and aroma, also received higher ratings for overall acceptability compared to other concentrations of stevia and sugar. Similar results were also reported in several other studies (Jabeen et al., 2019).
Conclusions and Recommendations
The research concluded that the formulation OD2, comprising 15% juice, 0.070% stevia powder, 0.1% S.B, and 84.83% water, exhibited optimal properties for a low-calorie citrus beverage. Over a 90-day storage duration, it consistently maintained the pH, ascorbic acid content, TSS, and TA within permissible limits. Moreover, sensory evaluations by consumers favored its color, taste, aroma, and overall acceptability. These results indicate that OD2 holds potential as a viable recipe for creating a stable and popular low-calorie orange beverage which can be used as a substitute to sugar-based products. The mentioned formulation not only meets dietary requirements of consumers generally and diabetes patient particularly but also meet organoleptic preferences just like sugar containing products. Nonetheless, it is crucial to acknowledge that further investigations may be necessary to evaluate other aspects, such as shelf life and microbial safety, prior to its commercial launch.
Acknowledgments
This work was carried out as a requirement of M.Sc. (H) degree of the first author (MU) in the University of Agriculture Peshawar, Pakistan. The manuscript has not been published or submitted to other journals previously. All authors have contributed significantly and are in agreement with the content of the manuscript. All authors agree to the conditions outlined in the copyright assignment form.
Novelty Statement
This research paper presents development of low-calorie orange ready-to-serve drink using stevia powder. It also represents exact formulation for preparing consumer acceptable orange drink, which is the novelty of this study.
Author’s Contribution
Muhammad Usman: Designed, investigated, analyzed and interpreted the data.
Majid S. Hashmi: Designed and supervised this study.
Ayaz Ahmad, Zahid Alam and Fawad Ahmad: Reviewed and edited the manuscript.
All authors read and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Abou-Arab, A.E., A.A. Abou-Arab and M.F. Abu-Salem. 2010. Physico-chemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana Bertoni plant. Afr. J. Food. Sci., 4(5): 269-281.
Ambreen, S., M.U. Arshad, A. Imran, M. Afzaal and F.K. Madilo. 2023. A comparative study of high-pressure processing and thermal processing techniques on characteristics and microbial evaluation of orange juice. Int. J. Food Prop., 26(2): 3214-3225. https://doi.org/10.1080/10942912.2023.2271678
Asencio, A.D., M. Serrano, S. García-Martínez and M.T. Pretel. 2018. Organic acids, sugars, antioxidant activity, sensorial and other fruit characteristics of nine traditional Spanish Citrus fruits. Eur. Food Res. Technol., 244: 1497-1508. https://doi.org/10.1007/s00217-018-3064-x
Byanna, C.N. and I.N.D. Gowda. 2012. Standardisation of recipe for the preparation of nectar from sweet orange (Citrus sinensis Osbeck) var. sathgudi using sugar substitutes and its storage. Int. J. Process. Postharvest. Technol., 3(1): 101-106.
Chen, L., W. Wu, N. Zhang, K.H. Bak, Y. Zhang and Y. Fu. 2022. Sugar reduction in beverages: Current trends and new perspectives from sensory and health viewpoints. Food. Res. Inter., 162: 112076. https://doi.org/10.1016/j.foodres.2022.112076
EFSA, A., 2010. Scientific opinion on safety of steviol glycosides for the proposed uses as a food additive. EFSA J., 8(4): 1537. https://doi.org/10.2903/j.efsa.2010.1537
Fennema, O.R., 2008. Fennema’s food chemistry. S. Damodaran, and K.L. Parkin (Eds.). Boca Raton: CRC press.
Food and Agriculture Org. 2021. Citrus Fruit Fresh and Processed Statistical Bulletin 2020. Rome, Italy (https://www.fao.org/markets-and-trade/commodities/citrus/en/).
Hariharan, G. and T. Mahendran. 2016. Physico-chemical, sensory and microbial evaluation of ginger-lime ready-to-serve (RTS) functional beverage, sweetened by Palmyra sugar candy. Imperial J. Interdiscip. Res., 2(5): 1545-1552.
Jabeen, F., S. Wahab, M.S. Hashmi, Z. Mehmood, A. Riaz, M. Ayub and M. Muneeb. 2019. Liquid stevia extract as a substitute of sucrose in the preparation of guava drink. Fresenius. Environ. Bull., 28: 233-243.
Jothi, J.S., P. Karmoker and K. Sarower. 2014. Quality assessment of mized fruit squash: Physico-chemical analysis, senory evaluation and storage studies. J. B. Agric. Univ., 12: 195-201. https://doi.org/10.3329/jbau.v12i1.21412
Kaddumukasa, P.P., S.M. Imathiu, J.M. Mathara and J.L. Nakavuma. 2017. Influence of physicochemical parameters on storage stability: Microbiological quality of fresh unpasteurized fruit juices. Food Sci. Nutr., 5(6): 1098-1105. https://doi.org/10.1002/fsn3.500
Li, X., J.N. Ren, G. Fan and S.Y. Pan. 2018. Changes of aroma compounds and qualities of freshly-squeezed orange juice during storage. J. Food Sci. Technol., 55: 4530-4543. https://doi.org/10.1007/s13197-018-3389-2
Murata, M., 2021. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J., 38: 283-292. https://doi.org/10.1007/s10719-020-09943-x
Pan, X., S. Bi, F. Lao and J. Wu. 2023. Factors affecting aroma compounds in orange juice and their sensory perception: A review. Food. Res. Int., 169: 112835. https://doi.org/10.1016/j.foodres.2023.112835
Peteliuk, V., L. Rybchuk, M. Bayliak, K.B. Storey and O. Lushchak. 2021. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI. J., 20: 1412.
Radi, M., S. Akhavan-Darabi, H.R. Akhavan and S. Amiri. 2018. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food. Process. Preserv., 42(2): 13441. https://doi.org/10.1111/jfpp.13441
Raghavan, G., A. Bapna, A., Mehta, A., Shah and T. Vyas. 2023. Effect of sugar replacement with stevia-based tabletop sweetener on weight and cardiometabolic health among Indian adults. Nutrients, 15(7): 1744. https://doi.org/10.3390/nu15071744
Rathinasamy, M., S. Ayyasamy, S. Velusamy and A. Suresh. 2021. Natural fruits based ready to serve (RTS) beverages: A review. J. Food. Sci. Technol., 59: 4563–4569. https://doi.org/10.1007/s13197-021-05275-2
Rodrigues, P.V., D.M. Vieira, P.C. Martins, V.G. Martins, M.C.R. Castro and A.V. Machado. 2022. Evaluation of active LDPE films for packaging of fresh orange juice. Polymers., 15(1): 50. https://doi.org/10.3390/polym15010050
Saeedi, P., I. Petersohn, P. Salpea, B. Malanda, S. Karuranga, N. Unwin, S. Colagiuri, L. Guariguata, A.A. Motala and K. Ogurtsova. 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas. Diabetes. Res. Clin. Pract., 157: 107843. https://doi.org/10.1016/j.diabres.2019.107843
Salar, F.J., V. Agulló, C. García-Viguera and R. Domínguez-Perles. 2020. Stevia vs. Sucrose: Influence on the phytochemical content of a citrus–maqui beverage. A shelf life study. Foods, 9(2): 219. https://doi.org/10.3390/foods9020219
Sarvarian, M., A. Jafarpour, C.G. Awuchi, A.O. Adeleye and C.O.R. Okpala. 2022. Changes in physicochemical, free radical activity, total phenolic and sensory properties of orange (Citrus sinensis L.) juice fortified with different oleaster (Elaeagnus angustifolia L.) extracts. Molecules, 27(5): 1530. https://doi.org/10.3390/molecules27051530
Singh, S.K. and M. Sharma. 2017. Review on biochemical changes associated with storage of fruit juice. Int. J. Curr. Microbiol. Appl. Sci., 6(8): 236-245. https://doi.org/10.20546/ijcmas.2017.608.032
Steel, R.G.D. and J.H. Torrie. 1960. Principles and procedures of statistics. Principles and procedures of statistics. McGraw Hill Publishing Company Inc. New York.
Tö, Ş., 2020. evaluation of ascorbic acid content and total antioxidant status of fresh-squeezed orange juices. Carpathian. J. Food Sci. Technol., 12(2): 17-25. https://doi.org/10.34302/crpjfst/2020.12.2.2
Voorpostel, C.R., M.B.D.L. Dutra and H.M.A. Bolini. 2014. Sensory profile and drivers of liking for grape nectar among smoker and nonsmoker consumers. Food. Sci. Technol., 34: 164-173. https://doi.org/10.1590/S0101-20612014000100024
Wang, J., H. Zhao, Y. Wang, H. Lau, W. Zhou, C. Chen and S. Tan. 2020. A review of stevia as a potential healthcare product: Up-to-date functional characteristics, administrative standards and engineering techniques. Trends. Food Sci. Technol., 103: 264-281. https://doi.org/10.1016/j.tifs.2020.07.023
Wibowo, S., T. Grauwet, B.T. Kebede, M. Hendrickx and A. Van Loey. 2015. Study of chemical changes in pasteurised orange juice during shelf-life: A fingerprinting-kinetics evaluation of the volatile fraction. Food. Res. Int., 75: 295-304. https://doi.org/10.1016/j.foodres.2015.06.020
Yildiz, M. and M. Karhan. 2021. Characteristics of some beverages adjusted with stevia extract, and persistence of steviol glycosides in the mouth after consumption. Int. J. Gastron. Food Sci., 24: 100326. https://doi.org/10.1016/j.ijgfs.2021.100326
To share on other social networks, click on any share button. What are these?








