Antioxidant Activities of Aqueous, Methanolic, Ethanolic and Ethyl Acetate Extracts of Himalayapotamon emphysetum Crab
Antioxidant Activities of Aqueous, Methanolic, Ethanolic and Ethyl Acetate Extracts of Himalayapotamon emphysetum Crab
Waqas Ahmad Shams1*, Gauhar Rehman2, Muhammad Ismail3, Rahmul Kabir4 and Abdul Qahar1
1Department of Zoology, Government Degree College, Daggar, Buner, Pakistan
2Department of Zoology, Abdul Wali Khan University, Mardan, Pakistan
3Department of Zoology, Islamia College University, Peshawar, Pakistan
4Department of Chemistry, University of Swat, Pakistan
Abstract | Antioxidants play a crucial role in protecting cells from oxidative damage, which is implicated in various diseases such as diabetes, cancer, and inflammation. In this study, we investigated the antioxidant potential of extracts obtained from Crab Himalayapotamon emphysetum using various in vitro assays. Different solvents of varying polarities including ethanol, methanol, ethyl acetate, and water were employed for extraction. The results demonstrated significant antioxidant activity across different extracts in different in vitro assays. Particularly, the aqueous extract exhibited potent 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity compared to other extracts. Similarly, the aqueous extract displayed high anti-OH* activity in the H2O2 assay. In contrast, the ethanolic extract showed superior antioxidant potential in the ferric cyanide (Fe3+) reducing assay compared to ethyl acetate and aqueous extracts. Furthermore, all extracts exhibited antioxidant properties in the nitric oxide (NO) scavenging assay, with the ethanolic extract showing significant dose-dependent activity. Overall, our findings suggest that both aqueous and ethanolic extracts possess substantial antioxidant potential, warranting further validation through in vivo experiments and clinical trials. This study underscores the promising therapeutic applications of Crab Himalayapotamon emphysetum extracts as natural antioxidants in combating oxidative stress-related diseases.
Novelty Statement | The findings highlight the potential of these natural extracts as sources of antioxidants, which could have implications for pharmaceutical and nutraceutical applications. This is the first comprehensive assessment of antioxidant properties in this species, adding valuable data to the field of marine-derived bioactive compounds.
Article History
Received: January 23, 2024
Revised: April 03, 2024
Accepted: April 12, 2024
Published: August 06, 2024
Authors’ Contributions
WAS conceived and presented the idea. GR develpoed the theory and perfromed computation. MI verified the analytical methods. RK conducted the expermients. AQ wrote the manuscript with the help of other authors.
Keywords
Himalayapotamon emphysetum, Crab, Radical scavenging, DPPH, Antioxidant activities
Copyright 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding Author: Waqas Ahmad Shams
wshams89@gmail.com
To cite this article: Shams, W.A., Rehman, G., Ismail, M., Kabir, R. and Qaharm, A., 2024. Antioxidant activities of aqueous, methanolic, ethanolic and ethyl acetate extracts of Himalayapotamon emphysetum crab. Punjab Univ. J. Zool., 39(2): 135-140. https://dx.doi.org/10.17582/journal.pujz/2024/39.2.135.140
Introduction
Free radicals are highly reactive molecules that contain one or more unpaired electrons. They are formed as natural byproducts of metabolism, but can also be generated by external factors such as pollution, cigarette smoke, radiation, and certain chemicals. Although some free radicals play essential roles in cellular signaling and immune function, excessive levels can cause damage to cells, proteins, and DNA, leading to oxidative stress (Mallick et al., 2007).
Oxidative stress occurs when there’s an imbalance between the production of free radicals and the body’s ability to neutralize them with antioxidants (Sentkowska et al., 2023) Prolonged oxidative stress has been linked to various diseases, including cancer, cardiovascular diseases, neurodegenerative disorders like Alzheimer’s and Parkinson’s, and aging (Chaves et al., 2020).
Antioxidants are molecules that can neutralize free radicals by donating an electron without becoming destabilized themselves. They are found in many fruits, vegetables, nuts, and seeds, as well as in supplements. Consuming a diet rich in antioxidants can help counteract oxidative stress and reduce the risk of associated diseases (Abdellatif et al., 2023).
A variety of antioxidants used to combat oxidative stress and protect cells from damage caused by free radicals. These antioxidants, includes vitamins, flavonoids, glutathione, and selenium, which play crucial roles in maintaining cellular integrity and overall health (Lin et al., 2024).
Himalayapotamon emphysetum, commonly known as the crab, encompasses various species found in Pakistan and other countries like China. It has been traditionally utilized in rural areas for treating diabetes mellitus (Shams et al., 2019). Despite its widespread use, there has been no prior research to confirm its medicinal benefits. Therefore, our study aims to investigate the potential antioxidant properties of Himalayapotamon emphysetum.
Materials and Methods
Apart from the basic laboratory chemicals, some chemicals were bought from Merck and Sigma chemical companies.
Acheta domesticus collection
Damp and grasslands were searched for collection of Himalayapotamon emphysetum in district Buner of Khyber Pakhtunkhwa Pakistan. After their identification, they were preserved in the zoological museum, Department of Zoology, Governament College Daggar Buner Khyber Puktunkhwa Pakistan.
Preparation of extract
The collected crabs were anesthetized and were changed to talc. Then, the talc was powder in liquid nitrogen. In Methanol, ethanol, water and Ethyl acetate Himalayapotamon emphysetum were extracted in 8 h, using Soxhlet apparatus. It was rotated for 72 h. It was precipitated through evaporation rotator. Many in vitro tests were carried out to hunt for anti-diabetic ability in the extract.
Nitric oxide (NO) radical scavenging assay
The mechanism of this test is to ascertain the capability of aqueous solution of sodium nitroprusside to give nitric oxide (NO) at normal pH, which reacts with O2 to form HNO2. This measurement of ions was through Griess reagent. Agents that can compete with oxygen in getting NO will result in under production of (NO). The test was started by incubation of selected 10 miliMolar concentration of sodium nitroprusside with 500 µL doses and with sodium phosphate buffer (pH 7.4). The extracts concentration from 5-250 µg/mL of 500 µL were further incubated at normal room temperature for 120 min. Then, 500 µL of Griess reagent was mixed with it. The nitrite was diazotized with sulphanilamide and chromophore was obtained which was measured at 546nm. The amount of generated NO produced was measured when the absorbance value was compared with the control (Elgorashi et al., 2003).
All the tests were performed thrice. The scavenging ability in Himalayapotamon emphysetum extract in case of NO was measured using the given formula:
Scavenging activity % = 1− As/ Ac x 100
Here As represents sample absorbance and Ac represents control absorbance.
DPPH radical scavenging assay
In short, 1ml of 1mM solution of DPPH with ethanol was mixed with 3ml of Himalayapotamon emphysetum extract in the presence of ethanol (5-100 µg) and a control solution was taken having no Himalayapotamon emphysetum extract. Absorbance was calculated after an hour and its level was observed to be 517nm on spectrophotometer. Increase in DPPH radical scavenging property is related to decrease of amount of sample and standard absorbance.
Scavenging activity % = 1− As/ Ac x 100
Here As represents sample absorbance and Ac represents control absorbance (Tuba and Gulcin, 2008).
Hydrogen peroxide inhibitory assay
To check H2O2 inhibition by Himalayapotamon emphysetum extract, 1.0ml of varied concentrations of the extract were put into 2.0ml of 20mM of H2O2 solution with phosphate saline buffer (pH 7.4). The solution was left for 10 min and later the absorbance was observed to be 230nm.
Ferric cyanide (Fe3+) reducing assay (FCRA)
Oyaizu method with some changes used for calculation of FCRA. For this test, incubation of 1 milliliter of different selected extracts (5–250 µg/mL) were carried out in 1 milliliter of PBS (0.2 Molar and pH 6.6). Then 1 percent potassium ferricyanide kFe3+ at 50 centigrade for half hour was mixed. Then, 1 milliliter of 10 percent trichloro acetic acid added to reaction mixture. Now, 1 miliLiter of it was added to 1mL of pure H2O and 200 microliter of 0.1 percent Iron trichloride. Through spectrophotometer at 700nm absorbance was found. It is related to the reducing ability of the extract (Cheikhyoussef and Embashu, 2013). The data obtained is shown in percentage redcucing of samples and standard absorbance.
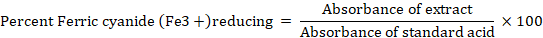
Statistical analysis
The study was done in three numbers. Then we selected the means and taken standard deviation. the data was analyzed through one way a nova.
Results and Discussion
Effect on DPPH Radical Scavenging by Himalayapotamon emphysetum extracts
The Figure 1 shows the radical scavenging of DPPH by Himalayapotamon emphysetum extracts. The extract has shown good scavenging capability 78.81 % at 250 µg/ml. which looks numerically same when we see all other antioxidants in use and prominently higher (P<0.05) than chemicals obtained from other organisms. The IC50 values of Aqueous, methanolic, ethanolic and Ethyl acetate extracts is 1.87, 1.27, 1.23 and 1.63 µg/ml were calculated for crab extract respectively whereas 2.49 and 1.24 µg/ml for standards vitamin c (ascorbic acid) and trihydroxybenzoic acid (Gallic acid) respectively (Table 1).
Effect on H2O2 scavenging by Himalayapotamon emphysetum extracts
Data regarding hydroxyl radical scavenging ability of Himalayapotamon emphysetum extracts (Figure 2) proves that Aqueous extract obtained from it (IC50 1.13 µg/ml) has shown anti hydroxyl activity which is higher (P<0.05) than such quality in other methanolic, ethanolic and Ethyl acetate extracts. The extracts anti hydroxyl property is in relation to concentration i.e increasing the concentration of extracts increase percentages of hydroxyl scavenging.
Table 1: No scavenging by Himalayapotamon emphysetum extracts. All the results in mean and standard deviation of the triplicate.
|
Concentration (µg/ml) |
Aqueous |
Methanolic |
Ethanolic |
Ethyl acetate |
Ascorbic acid |
Gallic acid |
|
5µg |
3.07 |
2.44 |
9.3 |
1.16 |
20.49 |
1.61 |
|
10µg |
5.85 |
3.49 |
12.57 |
2.32 |
23.22 |
3.23 |
|
25µg |
11.42 |
6.51 |
16.47 |
4.39 |
26.33 |
6.24 |
|
50µg |
25.18 |
14.43 |
31.44 |
11.9 |
44.77 |
21.73 |
|
100µg |
45.68 |
32.71 |
45.66 |
23.67 |
70.18 |
25.43 |
|
150µg |
51.68 |
42.95 |
52.83 |
30.53 |
78.32 |
30.17 |
|
200µg |
58.88 |
53.31 |
61.5 |
39.19 |
86.15 |
34.56 |
|
250µg |
65.59 |
61.69 |
72.95 |
47.34 |
92.31 |
37.45 |
Effect on NO scavenging by Himalayapotamon emphysetum extracts
Table 1 tells that radical of Nitric oxide trap by extracts of Himalayapotamon emphysetum. A quantity independent NO inhibition impact of extract was seen, quantity-dependent NO inhibition action was seen for ethanolic extract greater than standard gallic acid.
Effect on Ferric cyanide (Fe3+) reducing by Himalayapotamon emphysetum extracts
Figure 3 shows the whole reduction ability of Himalayapotamon emphysetum extracts and standard ascorbic acid. From these values, the antioxidant potencies of all extracts are clear and mostly increase drastically with increase in concentration. But, Himalayapotamon emphysetum ethanolic extract showed a prominent (P<0.05) higher Fe3+ to Fe2+ reduction capability.
Himalayapotamon emphysetum, commonly known as the crab, boasts several species thriving in Pakistan. This versatile species has long been relied upon in rural areas for its purported ability to alleviate diabetes mellitus and other diseases. Surprisingly, despite its extensive traditional use, no scientific investigations have yet delved into its medicinal potential (Shams et al., 2019).
Various methods for in-vitro anti-oxidation in laboratory were followed as a single methodology may not provide a clear image of the anti-oxidation abilities of various products used in the study. It is suggested that the reduction power of animal products is related to anti oxidation capacity. Antioxidants are reducing agents that deactivate oxidants. Because of these chemicals Fe3+/ferric cyanide complex is changed to the Fe2+ which can be seen at 700nm on spectrophotometer (Chung et al., 2002). It’s ability to reduce increases with increase in concentration. This indicates that electron donation is dependent upon concentration. The high reduction capability of the ethanolic extract at different concentrations used tells that it has powerful reduction that may work as reductant, hydrant and dioxidene scavenger.
Reducing of DPPH radicals is well known assay to find antioxidant potencies of different extracts. It can absorb a hydrogen ion or electron to form easy magnetic attachment. Therefore, this assay is therefore commonly in use to analyze the radical scavenging action in anti-oxidative extracts (Mohamed et al., 2014). Through this technique, the aqueous crab Himalayapotamon emphysetum extract also showed very good antioxidant effect having. A lot of studies have been done on Animals parts, products and families but such an IC50 value is very less observed as the aqueous extracts of Himalayapotamon emphysetum showed. Which tells us that this extract have good radical scavenging action.it will decrease the oxidative pressure and as result it solve many disease due to oxidative pressure like diabetes mellitus (Kalaivani and Mathew, 2004).
The OH radical is a highly potent free radical. Commonly known as destructive species that can denature bio-molecules. The OH radical is calculated as the % inhibition of these radicals due to the Fenton’s reaction mixture. There is a competition among de-oxyribose and extracts obtained from natural source (Wu et al., 2010). All extracts from Himalayapotamon emphysetum must have some zoo-chemicals with OH radical scavenging action. However, the aqueous extract observed highly active in OH scavenging, than the other Extracts. This may be due to Hormesis phenomenon present in these extracts. Hormesis is a biphasic phenomenon which means that the extracts show stimulation in lowers concentration but when the concentration increase at starts inhibition as the methanolic extracts showed (Kendig et al., 2010). Therefore, Himalayapotamon emphysetum extract has optimal points for active scavenging of OH radical (Calabrese et al., 2023).
NO is not a stable species that is studied as an agent in the pathology of cancer, Diabetes 2 and many other ailments (Hinnerburg et al., 2006). Ethanolic extracts of crab show better scavenging against NO radicals than the ethyle acetate, Methanolic and aqueous extract. This is also a proof of antioxidant ability of Ethanolic extract. Taking into account all the antioxidant assays in this study, it is it is assumed that the variation between the antioxidant potentialities is due to different concentration and different bioactive compounds (Pirgon et al., 2013).
Therefore, it was concluded that the Himalayapotamon emphysetum. extracts has prominent antioxidant action which can be confirmed by clinical trials and in vivo experiments in in future.
Conclusions and Recommendations
Different invitro assays like H2O2 scavenging, DPPH Assay, Ferric cyanide (Fe3+) reducing assay and NO radical scavenging assay confirmed the antioxidant potencies of different extracts of Himalayapotamon emphysetum. After confirmation by in vivo experiment and clinical trials, it will be use to cure different diseases like cancer DM etc.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdellatif, A.A.H., El-Hack, M.E.A., El-Saadony, M.T., Gharib, H.S., Taha, A.E., Nader, M.M. and Salem, H.M., 2023. Antioxidants and amino acids exert protective roles against oxidative stress in poultry production: A comprehensive review. Antioxidants, 12: 80.
Calabrese, E.J., Osakabe, N., di Paola, R., Siracusa, R., Fusco, R., D’Amico, R. and Calabrese, V., 2023. Hormesis defines the limits of lifespan. Ageing Res. Rev., pp. 102074.
Chaves, N., Santiago, A. and Alías, J.C., 2020. Quantification of the antioxidant activity of plant extracts: Analysis of sensitivity and hierarchization based on the method used. Antioxidants, 9: 76. https://doi.org/10.3390/antiox9010076
Cheikhyoussef, A. and Embashu, W., 2013. Antioxidant and antiradical activities of Strychnos spinosa Lam. (Loganiaceae) root extracts and their total phenolics content. Asian Pac. J. Trop. Med., 6: 509-515.
Chung, H.S., Shin, E.J., Hwang, H.R., Choi, H.K. and Jeong, J.H., 2002. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytother. Res., 16: 580-583.
Elbandy, M., Rho, J.R. and Afifi, R., 2014. Analysis of saponins as bioactive zoochemicals from the marine functional food sea cucumber Bohadschia cousteaui. Eur. Fd. Res. Technol., 238: 937-955. https://doi.org/10.1007/s00217-014-2171-6
Elgorashi, E.E., Taylor, J.L.S., Maes, A., van Staden, J., DeKimpe, N. and Verschaeve, L., 2003. Screening of medicinal plants used in South African traditional medicine for genotoxic effects. Toxicol. Lett., 143: 195-207. https://doi.org/10.1016/S0378-4274(03)00176-0
Fang, Y.Z., Yang, S. and Wu, G., 2002. Free radicals, antioxidants and nutrition. Nutrition, 18: 872-879. https://doi.org/10.1016/S0899-9007(02)00916-4
Gille, L., Schott-Ohly, P., Fresen, N., Schulte, I.M., Walde, S., Udilova, N., Nowl, H. and Gleichmann, H., 2002. Generation of hydroxyl radicals mediated by streptozotocin in pancreatic islets of mice in vitro. Pharmacol. Toxicol., 90: 317–326. https://doi.org/10.1034/j.1600-0773.2002.900605.x
Hinnerburg, I., Damien, H.J. and Hiltumen, R., 2006. Antioxidant activities of extracts from selected culinary herbs and spices. Fd. Chem., 97: 122-129. https://doi.org/10.1016/j.foodchem.2005.03.028
Kalaivani, T. and Mathew, L., 2004. Free radical scavenging activity from leaves of Acacia nilotica (L.) Wild. ex Delile, an Indian medicinal tree. Fd. Chem., 87: 17-20.
Kaneto, H., Katakami, N., Kawamori, D., Miyatsuka, T., Sakamoto, K., Matsuoka, T.A., Matsuhisa, M. and Yamasaki, Y., 2007. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid. Redox Signal., 9: 355–366. https://doi.org/10.1089/ars.2006.1465
Kendig, E.L., Le, H.H. and Belcher, S.M., 2010. Defining hormesis: Evaluation of a complex concentration response phenomenon. Int. J. Toxicol., 29: 235-246. https://doi.org/10.1177/1091581810363012
Lin, J., Wu, Y., Huang, Q., Liu, Z., Xu, J., Ji, R. and Zhou, L., 2024. Optimization of antioxidant activity of compounds generated during ginseng extract fermentation supplemented with Lactobacillus. Molecules, 29: 1265. https://doi.org/10.3390/molecules29061265
Mallick, C., Chatterjee, K., Guhabiswas, M. and Ghosh, D., 2007. Antihyperglycaemic effect of separate and composite extract of roots of Musa paradisiaca and leaf of coccinia in streptozotocin-induced diabetic male albino rats. Afr. J. Trad. Complement. Altern. Med., 4: 362-371. https://doi.org/10.4314/ajtcam.v4i3.31230
Mohamed, E.A., Siddig, I.I. and Yagi, S., 2014. Antioxidant and free radical-scavenging activity of S. libanotis growing in Sudan. J. Appl. Pharma. Sci., 4: 66-70.
Pirgon, O., Bilgin, H., Kurku, H., Cekmez, F. and Dundar, B.N., 2013. Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. J. Clin. Res. Pediat. Endocrinol., 5: 825.
Quilliot, D., Walter, E., Bonte, J.P., Fruchart, J.C., Duriez, P. and Ziegler, O., 2005. Diabetes mellitus worsens antioxidant status in patients with chronic pancreatitis. Am. J. Clin. Nutr., 81: 1111-1117. https://doi.org/10.1093/ajcn/81.5.1117
Sentkowska, A., Bres, L.M., Woznicka, O., Kopytko, M. and Michalak, M., 2023. The effect of dietary antioxidants on oxidative stress in patients with type 2 diabetes mellitus. J. Clin. Med., 12: 563.
Shams, W.A., Rehman, G., Onoja, S.O., Ali, A., Khan, K. and Niaz, S., 2019. In vitro antidiabetic, anti-inflammatory and antioxidant potential of the ethanol extract of Uromastyx hardwickii skin. Trop. J. Pharma. Res., 18: 2109-2115. https://doi.org/10.4314/tjpr.v18i10.16
Sofowora, A., Ogunbodede, E. and Onayade, A., 2013. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Trad. Complement. Altern. Med., 10: 210-229. https://doi.org/10.4314/ajtcam.v10i5.2
Tuba, A.K. and Gulcin, I., 2008. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact., 174: 27-37. https://doi.org/10.1016/j.cbi.2008.05.003
Wu, W., Liu, Y. and Zhu, D., 2010. π-conjugated molecules with fused rings for organic field-effect transistors: design, synthesis and applications. Chem. Soc. Rev., 39: 1489-1502.
To share on other social networks, click on any share button. What are these?








