Antifertility Effects of Curcumin on Sperm Quality, Morphology of Testicular, and Seminal Vesicle in Rats (Rattus norvegicus)
Research Article
Antifertility Effects of Curcumin on Sperm Quality, Morphology of Testicular, and Seminal Vesicle in Rats (Rattus norvegicus)
Hilwah Nora1,2, Rajuddin2*, Hafizuddin3, Rachmad Suhanda4, M. Fathurrahman5
1Doctoral Program of Medicine Sciences, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; 2Departments of Obstetrics and Gynaecology, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; 3Laboratories of Reproduction, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh Indonesia; 4Departments of Public Health and Community Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; 5Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia.
Abstract | Several studies report that curcumin has antifertility effects and has the potential to regulate male fertility (contraception). However, determining the initial dose is preliminary studies based on spermatozoa quality parameters, morphology, and morphometry of testes and seminal vesicles that have never been reported. The main objective of this research is to examine the effects of curcumin on several aspects of reproduction and determine the optimal dose for using curcumin as an antifertility agent. The test animals in this study were Wistar rats (Rattus norvegicus) three-month-old males weighing 300 g. Rats were acclimatized for 7 days and given standard feed and water ad libitum. During the research, rats were given standard 552SP feed. A total of six rats were used in this study, then divided into two treatment groups, namely C1 (given standard feed and distilled water orally (without curcumin)); and C2 (given standard feed and oral curcumin 150 mg /kg body weight). The length of treatment followed the duration (cycle) of spermatogenesis in rats, namely 52 days. Data were analysed using the T-test independent. The results indicate a rise in variables related to spermatozoa abnormality and a reduction in spermatozoa concentration, showing no statistically significant differences (P>0.05). Similarly, there were no significant variations (P>0.05) observed in all variables related to testis and seminal vesicle morphology. The administration of curcumin did not yield a statistically significant effect (P>0.05) on decreasing spermatozoa quality concerning testicular morphology, morphometry, and seminal vesicle weight. However, a trend was observed indicating an increase in spermatozoa abnormalities and a decrease in spermatozoa concentration.
Keywords | Antifertility, Curcumin, Male contraception, Reproduction, Mouse, Turmeric
Received | December 01, 2023; Accepted | January 12, 2024; Published | February 09, 2024
*Correspondence | Rajuddin, Doctoral Program of Medicine Sciences, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Email: rajuddin@usk.ac.id
Citation | Nora H, Rajuddin, Hafizuddin, Suhanda R, Fathurrahman M (2024). Antifertility effects of curcumin on sperm quality, morphology of testicular, and seminal vesicle in rats (Rattus norvegicus). Adv. Anim. Vet. Sci., 12(3):532-538.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.3.532.538
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Indonesia and other Southeast Asian countries possess abundant natural resources with significant economic value on the global market, notably an extensive array of medicinal plants and spices. A substantial portion of the world’s medicinal plants is situated in Indonesia (Jadid et al., 2020; Navia et al., 2022). Among these, turmeric, particularly its rhizome, stands out as a medicinal plant with a rich history of traditional use spanning generations.
Turmeric (Curcuma longa) is a spice or medicinal plant originating from Asia, especially Southeast Asia (Ide, 2013). This plant is widely cultivated in South Asia, especially in India, Nepal, Pakistan, Bangladesh, Indonesia, Malaysia, Thailand, and the Philippines (Ahmad et al., 2020; Dosoky et al., 2019; Fithriyya et al., 2021). In Indonesia, turmeric grows easily in almost all regions, including Sumatra, Java, Bali, Kalimantan, Sulawesi, Maluku, and Papua (Rahmat et al., 2021).
Turmeric is renowned as a kitchen spice with high demand both domestically and internationally. Beyond its culinary use, turmeric holds significant potential in the field of medicine (Das, 2016; Tanvir et al., 2017). The active compound within turmeric, curcumin, exhibits diverse biological effects, including anti-carcinogenic, anti-inflammatory, potent antioxidant, anti-angiogenic, and antifertility properties (Maiti et al., 2021).
In the male reproductive system, certain reports assert that curcumin administration is protective, potentially enhancing spermatozoa quality, including total sperm count, spermatozoa concentration, and spermatozoa (Alizadeh et al., 2018; Aparnak and Saberivand, 2019; Riahi et al., 2021). Conversely, several studies suggest that curcumin may inhibit (reduce) spermatozoa quality, affecting motility, spermatozoa viability, capacitation, acrosome reaction, and increasing spermatozoa abnormalities (Ashok and Meenakshi, 2004; Joshi et al., 2011; Naz, 2011, 2014; Putra, 2012). In in vitro studies, human and mouse spermatozoa incubated with curcumin exhibited decreased motility, capacitation, and acrosome reaction (Naz, 2014). The active compound curcumin is believed to exert its influence either directly by affecting spermatogenesis in the testicles or indirectly by inhibiting the secretion of gonadotropin hormones (follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (Şentürk and Sandıkçı, 2022).
Spermatogenesis is an essential process involving a series of high-level genetic and epigenetic events in germ cells that play an important role in converting spermatogonia into spermatozoa (Yuen et al., 2020). Antifertility effect of curcumin on spermatozoa caused a decrease in sperm count, sperm motility, sperm morphology and viability (Putra, 2012; Shah et al., 2008). This process is controlled by gonadotropin hormones, namely FSH and LH (Yuen et al., 2020). Inhibition of FSH secretion results in disruption of FSH and FSH-R binding which has an impact on the induction of a series of key regulatory molecules of spermatogenesis which will induce spermatogenesis disorders and a decrease in sperm quality which ultimately results in infertility. This phenomenon can be used as an opportunity to develop male contraceptive candidates (de la Iglesia et al., 2022; Schneider et al., 2020). However, in vivo in male Wistar rats (Rattus norvegicus) so far there is no information regarding its effects on reproductive function. This limited information also occurs in determining the correct dose, thus requiring a comprehensive study for the development of curcumin as an antifertility agent that can be used as a candidate for herbal contraception in men.
Based on existing studies, curcumin has the potential to regulate male fertility (herbal contraception) (Daniyal and Akram, 2015). According to Purwaningsih (2016) and (Nora et al., 2023), curcumin has the potential to control fertility, especially as an antifertility agent in men. Antifertility effect curcumin is reversible. In addition, the administration of curcumin to rats resulted in reversible inhibition of spermatogenic processes and fertility, thus suggesting the viability of this herb in male contraception (Mishra et al., 2018).
Based on these problems, it is necessary to study the antifertility effect of curcumin for determining the initial dose as a preliminary study based on spermatozoa quality parameters and testicular and morphometry of seminal vesicle. We hypothesized that increasing the existing dose would reduce the quality of spermatozoa, without disturbing the morphology of the testes and seminal vesicle.
MATERIALS AND METHODS
Animal samples
In this study, male white rats (Rattus norvegicus) served as the experimental animals. They underwent a 7-day acclimatization period and were provided with standard 552SP feed and ad libitum access to water. The male white rats weighed between 200-300 g, with an age range of 2-3 months. Selection of male white rats for treatment was done randomly based on the experimental design. Throughout the study, the male white rats received standard 552SP feed. Subsequently, they were divided into two treatment groups, each consisting of three experimental animals.
Treatment of curcumin
The curcumin utilized in this study is sourced from Curcuma longa (Turmeric) powder (Sigma-Aldrich®). The white rats were categorized into two treatment groups: C1, receiving standard feed and distilled water orally (without curcumin); and C2, receiving standard feed with oral curcumin at a dosage of 150 mg/kg body weight. The treatment duration aligns with the spermatogenesis cycle in rats, spanning 52 days (Opuwari and Monsees, 2020).
Body weight measurement
Brand digital analytical scale (taffware digipounds®) 12000 carried out every week during maintenance to determine the treatment dose.
Sampling and observation of rat testicular organs
Following the treatment period, euthanasia was conducted on male white rats using a cervical dislocation procedure. Subsequently, semen was collected from the epididymis, specifically from the cauda epididymis, to microscopically observe the condition of the spermatozoa post-treatment.
Macroscopic examination in anatomical pathology
Testicular length is assessed by aligning a tape with a measuring tool and calculating the length, while testicular diameter is measured using a caliper. Testicular volume is determined by placing the testicles in a measuring cup filled with water, and the volume is calculated based on the water displaced. Meanwhile, the weight of the seminal vesicle is measured using digital scales.
Evaluation of spermatozoa quality
Spermatozoa motility
Spermatozoa motility was assessed by placing the samples on a glass surface, followed by the addition of one drop of physiological NaCl. The observation was conducted through a microscope with a 40x magnification. The calculation of the number of motile sperm was based on their movement, categorized as fast progressive (A), slow progressive (B), circular (C), and vibratory (D) motions (Husnurrizal et al., 2023). The percentage was determined using the following formula:
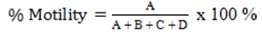
Spermatozoa viability
The examination of viability was performed by introducing one drop of spermatozoa on a glass slide, followed by the addition of one staining eosin-nigrosine drop. A smear preparation was made and fixed on a spirit lamp, then evaluated using a microscope of 40x magnification. The dead cells absorb a red pigmentation, while the live spermatozoa tend not to absorb anything colour, leading to a white appearance. The spermatozoa were then counted and divided by the total visible and presented as a percentage value (Hafizuddin et al., 2023).
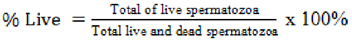
Spermatozoa morphology
This observation was performed by dripping spermatozoa and eosin-nigrosine on the object glass, fixed on a spirit lamp, and observing in a microscope with 40x magnification. The morphological examination identified deformities that are categorized as primary (small/large head size, double head or double tail, and abnormal head shape) and secondary abnormalities (head rupture, tail breaking at the neck or middle, and folded tail) (Hafizuddin et al., 2021). The minimum spermatozoa observed were 200 cells, and the calculations were conducted using the following formula:
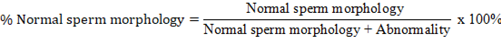
Spermatozoa concentration
The concentration of spermatozoa is determined by sucking the spermatozoa to a scale of 0.5 and adding 3% NaCl solution to the 101 marks. The mixture is homogenized for 2-3 minutes and a few drops of the semen solution are discarded. Counting is prepared by preparing the Neubauer counting chamber and covering it with a cover glass. On the inside of Neubauer’s counting chamber, semen solution was dripped, and then observed under a microscope (Olympus CX21). Sperm counts were carried out in five large boxes, with a magnification of 40x. The spermatozoa concentration was calculated using the formula N x 5 x 200 x 10,000 (N= number of spermatozoa in 5 boxes) (Syafruddin et al., 2020).
Data analysis
All data were presented as mean ± standard deviation. The comparison between treatment groups (C1 vs C2) was analysed using an independent-sample T test, p-value ≤ 0.05 considered significant (Ramadan et al., 2023).
RESULTS AND DISCUSSIONS
Evaluation of spermatozoa quality
The results of evaluating the quality of spermatozoa consist of Spermatozoa motility (%), spermatozoa viability (%), spermatozoa abnormalities (%), and spermatozoa concentration (x 106 cells / mL) are presented in Table 1.
Table 1: Characteristics of rat spermatozoa.
|
Parameter |
Curcumin Dosage |
|
|
C1 (without curcumin) |
C2 (150 mg /kg BW) |
|
|
Spermatozoa motility (%) |
66.92 a ± 9.79 |
69.50 a ±10.17 |
|
Spermatozoa viability (%) |
65.50 a ±13.43 |
86.67 a ±4.50 |
|
Spermatozoa abnormalities (%) |
4.25 a ± 3.18 |
8.50 a ± 2.64 |
|
Spermatozoa concentration (x 106 cells / mL) |
760 a ± 537.40 |
607 a ±240.06 |
athe same superscript indicates non-significant differences
Based on the T-test with a 95% confidence level, it shows that there is no significant difference (P>0.05). However, the variables abnormality of spermatozoa (%) and concentration of spermatozoa (x 106 cells / mL) showed that the decrease in the quality of spermatozoa was not significant.
Table 2: Morphometry of testes and seminal vesicle of rat.
|
Parameter |
Curcumin dosage |
|
|
C1 (without curcumin) |
C2 (150 mg/kg BW) |
|
|
Testicle length (cm) |
2.00a±0.00 |
1.93 a ±0.05 |
|
Testicle width (cm) |
0.90a±0.00 |
0.87 a ±0.05 |
|
Testicular diameter (cm) |
3.75a±0.35 |
4.00 a ± 0.00 |
|
Testicular volume (mL) |
3.00a± 0.00 |
2.67 a ± 0.57 |
|
Seminal vesicle weights (g) |
1.10a ±0.14 |
1.00 a ± 0.35 |
a the same superscript indicates non-significant differences.
This study found that the quality of spermatozoa was not significantly different (P>0.05). This probably happened because the dose given was still the minimum dose (Belhan et al., 2020). Results of research Santonastaso et al. (2021) with a dose of 20 μM curcumin in freezing spermatozoa medium caused a progressive increase in motility, a significant decrease in intracellular ROS, and a decrease in DNA fragmentation of frozen sperm cells. Other research conducted by Roshankhah et al. (2017) also found this. The results of the study showed that curcumin at minimum doses (10, 30, and 60 mg/kg), significantly increased the average percentage of sperm motility, number, weight of testicles, and serum testosterone levels compared to the control group. However, research results in mice with high doses (600 mg/kg) can significantly reduce the quality of spermatozoa (Mishra and Singh, 2009). Other studies also show the same thing, that high doses of curcumin can reduce the quality of spermatozoa and reduce fertility (Hembrom et al., 2015; Naz, 2011). Based on this research, in future research, it is necessary to try giving high doses of curcumin, but not up to a lethal dose of 50, by looking for references again.
In this study, although sperm quality was not significantly different, there was a tendency for spermatozoa quality to decrease in variables of spermatozoa abnormalities, and spermatozoa concentration. This can be an indicator that there is an impact antifertility of curcumin by suppressing spermatogenesis. The suppressive effect of curcumin on the spermatogenesis stage will result in more sperm abnormalities. Likewise, it will cause the concentration of spermatozoa to decrease in number (Karakus et al., 2021; Rithaporn et al., 2003). Previous research has also reported that the effect of curcumin can improve spermatogenesis defects in dexamethasone-treated rats (Khorsandi et al., 2013).
Research by Naz (2011) found that curcumin inhibits sperm function, fertilisation and fertility. Curcumin blocks conception, reduces sperm motility, sperm capacitation and acrosome reaction. At high concentrations, its ability to block sperm motility and function lasts for 5-15 minutes. Curcumin can be developed as an ideal contraceptive in the future. The antifertility effect of curcumin is reversible, and at the time it was the first study to report the inhibitory effect of curcumin on sperm function, fertilisation and fertility.
In another study, it was reported that the administration of low concentrations of curcumin (30g/mL) decreased motility without decreasing viability and the highest concentration of 300g/mL decreased total sperm motility after 60 minutes. Curcumin inhibits human sperm protein kinase C which plays a role in sperm flagellum movement (Shah et al., 2008).
Although curcumin has the potential to reduce spermatozoa quality, more research is still needed to determine the optimal dose and duration of use in the development of male contraception.
Morphology of testes and seminal vesicle
Average morphometric measurements of testes and seminal vesicle white rat strain Wistar in both treatment groups are shown in Table 2.
All morphometric parameters measured (testicular length, testicular width, testicular diameter, testicular volume) and weight of seminal vesicle showed no significant difference (P>0.05). This shows that the curcumin given did not harm the morphology of the testes and seminal vesicle.
This study is in line with research by Roshankhah et al. (2017), who stated that curcumin had a positive effect on testicular morphometry. Curcumin has been shown to also have a positive effect on testicular morphology and morphometry. This is based on research by Taba et al. (2019), supplementation with curcumin can improve testicular structure and weight. This mechanism occurs with the effect of curcumin which can protect the testicles from oxidative stress damage.
The cause of seminal vesicle did not experience significant changes (P>0.05) because in certain doses curcumin is protective. This is in accordance with the statement Li et al. (2019), that curcumin treatment can reduce oxidative stress in seminal vesicles by reducing the expression of NOX1, NOX2 and NOX4, thus improving apoptosis and atrophy of seminal vesicles.
The results of other studies indicate that Curcumin has a strong protective effect against testicular toxicity and may be clinically useful. This mechanism occurs through its ability to increase the expression of BCL-2 protein, an important anti (Khorsandi et al., 2013). Recent research also reported that curcumin not only demonstrated superior protective effects as a protective measure but also opened new avenues to tie the benefits of curcumin in the face of reproductive toxicity and related health challenges (Mohamed et al., 2023).
According to Mohebbati et al. (2017) turmeric and curcumin have protective effects on reproductive organ activities such as, anti-inflammatory, anti-apoptotic, and antioxidant effects in normal cells but show pro-apoptotic effects in malignant cells. Therefore, the different effects of turmeric and curcumin depend on the dose and type of cells used in the various models studied.
Conclusions and Recommendations
The administration of curcumin at a dose of 150 mg/kg BW did not have a significant effect (P>0.05) on several aspects of male rat reproduction. Therefore, it is necessary to treat with various higher doses for further research development as an antifertility agent.
Acknowledgments
This research was funded by the Directorate of Research, Technology and Community Service, Directorate General of Higher Education, Research and Technology, Ministry of Education, Culture, Research and Technology under the Implementation Contract for the Operational Assistance Program for State Universities, Research Program for Fiscal Year 2023, Number: 168 /E5/PG.02.00.PL/2023 June 19, 2023. Special appreciation gratefully acknowledges to Drh. Fauzan Fajri, M.Si. (Universitas Syiah Kuala, Banda Aceh, Indonesia) for supervision and treatment in animal laboratory, and to Drh. Husnurrizal, M.Si. for spermatozoa evaluation assistance.
Novelty Statement
This study can provide new information about the antifertility effects of curcumin on spermatozoa quality, morphology, and morphometry of testes and seminal vesicles of male rats.
AUTHOR’S CONTRIBUTION
HN and MF: Participated in performing, selecting samples, sample collection, and writing the initial manuscript. HH: Performed manuscript revision and data analysis. RS: Conducted the research and performed practical experiments. RR: developed the original idea and protocol and revised the final manuscript.
Animals ethics
This study was approved by the Veterinary Ethics Committee, Faculty of Veterinary Medicine Universitas Syiah Kuala Ref: 217/KEPH/V/2023.
Conflict of interests
The authors have declared no conflict of interest.
REFERENCES
Ahmad RS, Hussain MB, Sultan MT, Arshad MS, Waheed M, Shariati MA, Plygun S, Hashempur MH (2020). Biochemistry, safety, pharmacological activities, and clinical applications of turmeric: A mechanistic review. Evid. Based Complement. Altern. Med., pp. 7656919. https://doi.org/10.1155/2020/7656919
Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK (2018). Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother. Res., 32: 514-521. https://doi.org/10.1002/ptr.5998
Aparnak P, Saberivand A (2019). Effects of curcumin on canine semen parameters and expression of NOX5 gene in cryopreserved spermatozoa. Vet. Res. Forum, pp. 221.
Ashok P, Meenakshi B (2004). Contraceptive effect of Curcuma longa (L.) in male albino rat. Asian J. Androl., 6: 71-74.
Belhan S, Yıldırım S, Huyut Z, Özdek U, Oto G, Algül S (2020). Effects of curcumin on sperm quality, lipid profile, antioxidant activity and histopathological changes in streptozotocin-induced diabetes in rats. Andrologia, 52: e13584. https://doi.org/10.1111/and.13584
Daniyal M, Akram M (2015). Antifertility activity of medicinal plants. J. Chinese Med. Assoc., 78: 382-388. https://doi.org/10.1016/j.jcma.2015.03.008
Das, K., 2016. Turmeric (Curcuma longa) oils, In: Essential oils in food preservation, flavor and safety. Elsevier, pp. 835-841. https://doi.org/10.1016/B978-0-12-416641-7.00095-X
de la Iglesia A, Jodar M, Oliva R, Castillo J (2022). Insights into the sperm chromatin and implications for male infertility from a protein perspective. WIREs Mech. Dis., pp. e1588. https://doi.org/10.1002/wsbm.1588
Dosoky NS, Satyal P, Setzer WN (2019). Variations in the volatile compositions of Curcuma species. Foods, 8: 53. https://doi.org/10.3390/foods8020053
Fithriyya NR, Syafa HN, Financia DC, Cahyaningrum NE, Wijayanti HR, Nabila HL, Kayana SK, Putri GHS, Tanjung NH, Widyaningsih PN (2021). Prediction of the role of curcuminoids in Curcuma longa as GSK-3 inhibitors on tertier dentin growth. Med. Health J., 1: 1-10. https://doi.org/10.20884/1.mhj.2021.1.1.4642
Hafizuddin H, Helmi TZ, Riady G, Husnurrizal H, Akmal M, Gholib H, Santosa SF, Yusmadi Y, Muhtar M, Jauhari J, Munzir M, Azmi Z, Subekti D (2023). Protein profiles of seminal plasma and their correlation with semen quality in Aceh bull. Vet. Med. Zoot, 81: 51-55.
Hafizuddin H, Karja NWK, Praharani L, Setiadi MA (2021). Breed and age effects on concentration of adiponectin and reproductive performance in Anglo Nubian, Etawah grade and its crossbred bucks. Biodiversitas, 22: 1112-1119. https://doi.org/10.13057/biodiv/d220305
Hembrom AR, Verma A, Singh V (2015). Antifertility effects of rhizome of Curcuma longa on seminal parameters of Swiss Albino male mice. Res. J. Pharm. Technol., 8: 404-406. https://doi.org/10.5958/0974-360X.2015.00068.2
Husnurrizal H, Siregar TN, Eriani K, Wahyuni S, Hafizuddin H, Ramadhan MR, Azmi Z, Anwar A, Febretrisiana A (2023). Comparison of in vivo and in vitro PGF2α administrations on the sperm quality of Gembrong goats. IOP Conf. Ser. Earth Environ. Sci., 1174: 012023. https://doi.org/10.1088/1755-1315/1174/1/012023
Ide P (2013). Health Secret of Turmeric (Kunyit). Elex Media Komputindo.
Jadid N, Kurniawan E, Himayani CES, Andriyani, Prasetyowati I, Purwani KI, Muslihatin W, Hidayati D, Tjahjaningrum ITD (2020). An ethnobotanical study of medicinal plants used by the Tengger tribe in Ngadisari village, Indonesia. PLoS One, 15: e0235886. https://doi.org/10.1371/journal.pone.0235886
Joshi SC, Sharma A, Chaturvedi M (2011). Antifertility potential of some medicinal plants in males: An overview. Int. J. Pharm. Pharm. Sci., 3: 204-217.
Karakus FN, Kuran SB, Solakoglu S (2021). Effect of curcumin on sperm parameters after the cryopreservation. Eur. J. Obst. Gynecol. Reprod. Biol., 267: 161-166. https://doi.org/10.1016/j.ejogrb.2021.10.027
Khorsandi L, Mirhoseini M, Mohamadpour M, Orazizadeh M, Khaghani S (2013). Effect of curcumin on dexamethasone-induced testicular toxicity in mice. Pharma. Biol., 51: 206-212. https://doi.org/10.3109/13880209.2012.716854
Li R, Li H, Rao K, Liu K, Zhang Y, Liu X, Wang T, Wang S, Liu Z, Liu J (2019). Curcumin ameliorates atrophy of seminal vesicle via reduction of oxidative stress in castrated mice. PeerJ, 7: e7192. https://doi.org/10.7717/peerj.7192
Maiti R, Roy U, Das S, Das A (2021). Antifertility effect of curcumin, an indigenous medicine in rats. Int. J. Basic Clin. Pharmacol., 10: 167. https://doi.org/10.18203/2319-2003.ijbcp20210185
Mishra RK, Singh S, Singh SK (2018). Natural products in regulation of male fertility. Indian J. Med. Res., 148: S107.
Mishra RK, Singh SK (2009). Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception, 79: 479-487. https://doi.org/10.1016/j.contraception.2009.01.001
Mohamed AAR, Behairy A, Abd El-Hakim YM, Metwally MM, Khamis T, Abuzahrah SS, Abdelhamid AE, Alqahtani LS, Essawi WM, Alotaibi BS (2023). Comparable bio-evaluation of curcumin and chitosan-encapsulated curcumin nanoparticles against the reprotoxic potential of fenpropathrin pyrethroid in rats: Genomic and morphometric prospectives. Food Chem. Toxicol., 179: 113977. https://doi.org/10.1016/j.fct.2023.113977
Mohebbati R, Anaeigoudari A, Khazdair M (2017). The effects of Curcuma longa and curcumin on reproductive systems. Endocrine Regul., 51: 220-228. https://doi.org/10.1515/enr-2017-0024
Navia ZI, Adnan A, Harmawan T, Suwardi AB (2022). Ethnobotanical study of wild medicinal plants in Serbajadi protected forest of East Aceh District, Indonesia. Biodiversitas, pp. 23. https://doi.org/10.13057/biodiv/d231001
Naz RK (2011). Can curcumin provide an ideal contraceptive? Mol. Reprod. Dev., 78: 116-123. https://doi.org/10.1002/mrd.21276
Naz RK (2014). The effect of curcumin on intracellular pH (pHi), membrane hyperpolarization and sperm motility. J. Reprod. Infert., 15: 62.
Nora H, Suhanda R, Indirayani I (2023). Curcumin, a potential oral herbal male contraceptive: A review article. Bali Med. J., 12: 82-86. https://doi.org/10.15562/bmj.v12i1.3937
Opuwari C, Monsees T (2020). Green tea consumption increases sperm concentration and viability in male rats and is safe for reproductive, liver and kidney health. Sci. Rep., 10: 15269. https://doi.org/10.1038/s41598-020-72319-6
Purwaningsih E (2016). Potential effect of curcumin as anti fertility agent. J. Kedokteran Yarsi, 24: 203-211.
Putra GP (2012). Pengaruh Ekstrak Etanol Rimpang Kunyit (Curcuma longa L) terhadap Kuantitas Dan Kualitas Spermatozoa Mencit (Mus musculus) Jantan Galur BALB-C, Universitas Jember.
Rahmat, E., Lee, J., Kang, Y. (2021). Javanese turmeric (Curcuma xanthorrhiza Roxb.): Ethnobotany, phytochemistry, biotechnology, and pharmacological activities. Evid Based Complement. Altern. Med., 2021. https://doi.org/10.1155/2021/9960813
Ramadan ES, Ali ME, Elkhiat MA (2023). MicroRNA-122 expression versus ALT for liver injury detection involved in feline infectious peritonitis. Adv. Anim. Vet. Sci., 11: 189-354. https://doi.org/10.17582/journal.aavs/2023/11.2.288.294
Riahi MM, Behnam B, Henney NC, Jamialahmadi T, Sahebkar A (2021). Protective effects of curcumin in the reproductive system: Anti-toxic, semen cryopreservative, and contraceptive actions. Nat. Prod. Hum. Dis. Pharmacol. Mol. Targets Therapeut. Benefits, 2021: 223-242. https://doi.org/10.1007/978-3-030-73234-9_15
Rithaporn T, Monga M, Rajasekaran M (2003). Curcumin: A potential vaginal contraceptive. Contraception, 68: 219-223. https://doi.org/10.1016/S0010-7824(03)00163-X
Roshankhah S, Salahshoor M, Aryanfar S, Jalili F, Sohrabil M, Jalili C (2017). Effects of curcumin on sperm parameters abnormalities induced by morphine in rat. J. Med. Biomed. Sci., 6: 1-10. https://doi.org/10.4314/jmbs.v6i2.1
Santonastaso M, Mottola F, Iovine C, Colacurci N, Rocco L (2021). Protective effects of curcumin on the outcome of cryopreservation in human sperm. Reprod. Sci., 28: 2895-2905. https://doi.org/10.1007/s43032-021-00572-9
Schneider S, Shakeri F, Trötschel C, Arévalo L, Kruse A, Buness A, Poetsch A, Steger K, Schorle H (2020). Protamine-2 deficiency initiates a reactive oxygen species (ROS)-mediated destruction cascade during epididymal sperm maturation in mice. Cells, 9: 1789. https://doi.org/10.3390/cells9081789
Şentürk Ş, Sandıkçı M (2022). Effects of curcumin on changes in spermatogenetic cells of rats treated with cisplatin. Med. Lab. Technol. J., https://doi.org/10.31964/mltj.v8i1.483
Shah HC, Tatke P, Singh KK (2008). Spermicidal agents. Drug Discov. Therapeut., 2: 200-210.
Syafruddin S, Iryandi F, Rahmi RAS, Husnurrizal H, Armansyah T, Panjaitan B, Sayuti A, Sutriana A, Aliza D, Hafizuddin H, Siregar TN (2020). The effect of gonadotropin-releasing hormone (GnRH) on semen quality and testosterone level of Nubian goats. Vet. Med. Zoot., 77: 16-21.
Taba MY, Mohammadi S, Jalali M, Beheshti F, Attari SS (2019). Effects of different doses of curcumin on testicular histopathology, apoptosis, and reproductive organs weight index in mice D-galactose-induced aging model. Comp. Clin. Pathol., 28: 997-1002. https://doi.org/10.1007/s00580-018-2870-7
Tanvir E, Hossen MS, Hossain MF, Afroz R, Gan SH, Khalil MI, Karim N (2017). Antioxidant properties of popular turmeric (Curcuma longa) varieties from Bangladesh. J. Food Qual., 2017: 8471785. https://doi.org/10.1155/2017/8471785
Yuen F, Nguyen BT, Swerdloff RS, Wang C (2020). Continuing the search for a hormonal male contraceptive. Best Pract. Res. Clin. Obst. Gynaecol., 66: 83-94. https://doi.org/10.1016/j.bpobgyn.2020.02.003
To share on other social networks, click on any share button. What are these?






