Analysis of Growth, Nutrition and Economic Profitability of Gibel Carp (Carassius auratus gibelio ♀ × C. carpio ♂) Cultured in Zero-water Exchange System
Analysis of Growth, Nutrition and Economic Profitability of Gibel Carp (Carassius auratus gibelio ♀ × C. carpio ♂) Cultured in Zero-water Exchange System
Pingping Cang1, Mingming Zhang1, Guo Qiao1, Qirui Sun1, Dehai Xu3, Qiang Li1, Xinghua Yuan4 and Wenbin Liu2,*
1Yancheng Institute of Technology, Yancheng 224051, Jiangsu Province, China
2Key Lab of Aquatic Animal Nutrition and Feed Science and Technology, Nanjing Agricultural University, Nanjing 210095, China
3U.S. Department of Agriculture, Agricultural Research Service, Aquatic Animal Health Research Unit, 990 Wire Road, Auburn, AL 36832, USA
4Key Laboratory of Freshwater Fisheries, Freshwater Fishery Research Center, Chinese Academy of Fishery Sciences, Ministry of Agriculture, Wuxi 214081, Jiangsu Province, China
Pingping Cang and Mingming Zhang have contributed equally to this work and should be considered co-first authors.
ABSTRACT
Gibel carp (Carassius auratus gibelio ♀ × C. carpio ♂), a representative mudflat aquaculture species, was used to evaluate the effects of biofloc technology (BFT) on growth, nutrition and economic profitability in mudflat fish aquaculture. A 60-d experiment was conducted in zero-water exchange BFT system. The results demonstrated that weight gain (WG), specific growth (SG) and survival in BFT group were 133.94%, 1.20% day-1 and 90.0%, respectively. In control group with a 1/4-1/3 daily water exchange, WG, SG and survival were significantly lower than those in BFT group. There were no statistical differences in crude protein (CP), crude lipid (CL) and ash contents of gibel carp muscle between BFT and control group at the end of experiment (P >0.05). The essential amino acids, non-essential amino acids and total amino acids contents of gibel carp muscle in BFT group were higher than those in control group. Bioflocs were composed with 29.8% CP, 3.2% CL and 19.1% ash at day 60. CP content was appropriate, and LP was lower for gibel carp. Economic profitability analysis included analysis on gross revenue, break-even production, net present value, payback time, degree of operating leverage, and environmental costs under experimental conditions was conducted. Analysis results revealed that BFT system was more effective than the control system with water exchange in gibel carp culture, suggesting that BFT system could be successfully used in gibel carp aquaculture.
Article Information
Received 21 August 2018
Revised 19 November 2018
Accepted 14 December 2018
Available online 21 February 2019
Authors’ Contribution
PPC, WBL, QRS and GQ designed the study, performed experimental work and conducted the economic profitability analysis. QL and XHY conducted the market investigation and analyzed the data. MMZ, PC and GQ wrote the article. DHX revised the article.
Key words
Gibel carp, Biofloc technology, Growth performance, Nutrients, Profitability.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.2.619.630
* Corresponding authors: [email protected]
0030-9923/2019/0002-0619 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Aquaculture is one of the fastest growing food industries over last several decades to meet the global demand for sea foods. According to the Food and Agriculture Organization (FAO) of United Nations, the production of inland fish for human consumption from aquaculture has achieved 48850920 tons in 2015. Asia has contributed to 93.2% of the global inland water aquaculture products (FAO, 2015). However, intensive aquaculture leads to some acute toxic effects and long term environmental pollution by excess of organic materials, nutrients (Piedrahita, 2003), and drug residue from disease prevention or control during aquaculture. There are several solutions for water quality improvement, such as traditional method (Jackson et al., 2003) with increasing water exchange, recirculating aquaculture system (RAS) (Kolarevic et al., 2012) with waste treatment infrastructures and biofloc technology (BFT) with zero-water exchange (Avnimelech, 2006). Increasing water exchange leads to higher operating costs due to more water and energy consumption, and shorter retention time of nutrients within the culture system (Jackson et al., 2003; Crispim et al., 2007). Recently, BFT has become a hot topic in aquaculture since BFT can stabilize water quality with minimal or no water exchange, increase food production, recycle feed waste and effluents, enhance immunity and prevent disease outbreak by reducing pathogens transmission (Crab et al., 2010). BFT might be a promising solution for a sustainable aquaculture development in culturing both filter feeding and detritivorous species (Luo et al., 2014).
BFT can recycle nutrient by introducing additional carbon source to culture water to stimulate heterotrophic bacterial growth that converts ammonia into microbial biomass (Avnimelech and Kochba, 2009; Azim and Little, 2008; Zhao et al., 2014). Microbial biomass will further aggregate with other microorganisms and particles to form bioflocs. The bioflocs contain heterogeneous mixture of diatoms, macroalgae, food and fecal remnants, exoskeletons, bacteria, invertebrates and other microorganisms. Bioflocs can maintain good water quality, increase fish growth performance, reduce feed costs by recycling feed residues and fecal excrements, aid in the enzymatic activity, enhance innate immunity and disease resistance (Crab et al., 2012). BFT can minimize water exchange to save labor and environmental costs, water usage and wastes exhaust in aquaculture (Luo et al., 2014). In addition, aquatic drugs cannot be used for disease control in BFT system due to the key microorganism in BFT system. Thus, BFT is considered to be a more sustainable and environmental friendly alternative for intensive aquaculture species such as Nile tilapia (Oreochromis niloticus), Pacific white shrimp (Litopenaeus vannamei) and polyculture of silver carp (Hypophthalmichthys molitrix), common carp (Cyprinus carpio L.) and bighead carp (Aristichtys nobilis) (Avnimelech and Kochba, 2009; Azim and Little, 2008; Zhao et al., 2014).
The gibel carp (Carassius auratus gibelio ♀ × C. carpio ♂) was one of important farmed freshwater species in China and a representative species of mudflat fish (Podok et al., 2014; Zhang et al., 2018). Our previous study demonstrated that bioflocs can be uptaken by gibel carp which enhanced immune response (Zhang et al., 2018). This study would evaluate the growth, nutrition and economic profitability of gibel carp cultured in BFT zero-water exchange system. The results obtained will be beneficial for the mudflat fish aquaculture, helpful to protect environment by reducing water exchange and wastes exhaust, and increase aquaculture efficiency.
Materials and methods
Gibel carp maintenance
Gibel carp (Carassius auratus gibelio ♀ × C. carpio ♂) were obtained from a fish farm at Yancheng City, Jiangsu Province and acclimated in a tank of static water with temperature 26-28°C and pH 7.8-8.2 for 2 weeks prior to the experiments. The tank was provided with aeration and water was exchanged 1/4-1/3 daily before experiment. Dissolved oxygen (DO) was higher than 5 mg L-1. Gibel carp were fed with commercial diets (Tongwei Feeding Company, China) three times daily at 3.0% of their body weight under a 12 h light/12 h dark cycle. The diets contained 32.25% crude protein, 5.90% crude lipid, 1.18% calcium and 1.23% total phosphorus.
Experimental design
The experiment was conducted in 6 indoor concrete tanks (3.0 m×1.0 m×0.8 m), including biofloc technology (BFT) culture group and control group. Each group had triplicated tanks filled with freshwater at the volume of 1.80 m3 and 250 fish (19.9 ± 1.6 g) per tank. Water was circulated with pump to suspend bioflocs and increase DO level in each tank. In BFT group, no water was exchanged and molasses was added into tank two hours after feeding at a C/N ratio of 15. The water was only added weekly to the initial level to supplement water loss due to evaporation or water sampling. In control group, aerated freshwater was exchanged 1/4 to 1/3 daily to maintain water quality. Gibel carp in both groups were fed with commercial diets three times daily at 3.0% of their body weight during 60 day experimental period.
Water quality parameters
Water temperature, DO and pH were measured daily using a multiple function DO meter (Lovibond Senso Direct 150, German). Dissolved inorganic nitrogen [total ammonium nitrogen, and nitrite-N (NO2--N)] was determined every three days following the procedures in the Standard Methods for the Examination of the Water and Wastewater (APHA, 1998). Total suspended solid (TSS) concentration and flocs volume (FV) were monitored following the protocols (Strickland and Parsons, 1972). Turbidity was measured using turbidity meter (Lovibond SGZ-B, German). The aeration was provided in each aquarium using air blower to make bioflocs suspend in water and keep DO higher than 5 mg L-1.
Proximate composition of bioflocs
Bioflocs were sampled in situ using Imhoff cone (Hargreaves, 2013). At day 14, 21, 28, 45 and 60, culture water was sampled by withdrawing the water from the middle of tank into Imhoff cone. The bioflocs were collected from the turn-knob at the bottom tip of the Imhoff cone and dried in an oven at 105oC to constant weight or freeze-dried to analyze proximate composition. The crude protein of bioflocs was determined according to AOAC (1995). The ammonia acid composition was determined using an automatic analyzer (Biochrom 30+) (Boonyoung et al., 2013). For total amino acids, samples were digested at 110oC for 22 h with 4 M methanosulphonic acid (Sigma-Adlrich, St. Louis, MO, USA), filtered by 0.45 μm membrane and then injected into the automatic analyzer. Amino acid contents in N (mg/g) were calculated as: [essential amino acid contents (%) × 1000]/16 (Lin et al., 2006; Huang et al., 1999). The crude lipid content was determined by ether extraction with a Soxhlet extractor (AOAC, 1995). The crucible was placed in a muffle furnace and heated at 550oC to 600oC for 6 h until the sample reduced to ash. The ash together with the crucible was cooled down in a desiccator and weighed. The ash content was calculated using the formula: % ash = (weight of ash/ weight of dried samples) × 100. Poly-β-hydroxybutyrate (PHB) was extracted in chloroform at 1:40 (dry weight of bioflocs: volume of chloroform) at 40oC for 11 h. PHB content was determined according to standard curve by ultraviolet spectrophotometric method using standard PHB (Sigma-Aldrich).
Growth performance and nutrition of gibel carp muscle
At the end of 60-day experiment, fish were starved for 24 h and anesthetized with 200 mg L-1 MS222 before being handled. The survival was expressed as the percentage of live fish at the end of experiment relative to the total initially stocked fish. Fish from each tank were used to calculate weight gain (WG), specific growth (SG), condition factor (CF), viscerosomatic ration (VR) and hepatosomatic index (HSI) using following equations to compare fish growth and body indices between treatments (Huisman, 1987):

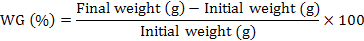
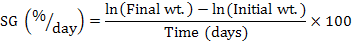
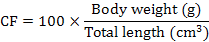

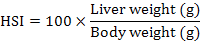
Muscle was sampled from 5 fish per group, finely grounded and analyzed in triplicate following the standard procedures for chemical analysis (AOAC, 2005). Crude protein, lipid, ash content, ammonia acid composition, and nutritional evaluation were analyzed for gibel carp muscle as described above.
Economic profitability analysis of gibel carp culture in BFT system
Since the current study on BFT system was designed on a laboratory scale and economic profit of control group was based on the farm investigation, the amplified costs of BFT system would increase to the same scale with outdoor farm. The market prices of gibel carp was investigated weekly from agriculture by-product wholesale market (Fig. 1).
The indicators of profitability used in the present study were gross revenue, cost price, break-even production and operational profit using following equations (Bezerra et al., 2016; Yuan et al., 2017):
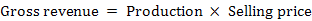
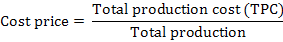

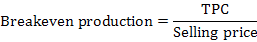
In addition, the net present value (NPV) and payback time were estimated for the analysis of investment. The NPV compares the initial investment with future returns. An activity is considered economically feasible when the NPV is positive at the end of a given period. NPV was calculated using following equations (Patterson et al., 1990; Peng, 2014):

Where, NCF is net cash flow, i is initial investment, and C is discount rate.
Moreover, degree of operating leverage (DOL) was measured to assess the risk of breeding operation. DOL was calculated using following equations (Wang and Tang, 2013):
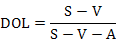
Where, S is gross revenue, V is total variable cost, and A is total fixed cost are.
Furthermore, environmental pollution cost as an external cost was evaluated according to material balance method (Yang et al., 2012; Lin et al., 2016) and shadow price method (Coggin and Swinton, 1996; Wu et al., 2014; Xiao et al., 2014). The material balance method calculates the pollution load based on the content of nitrogen and phosphorus in feed, bioflocs and organism. The equation is P = S – W, where P is environmental load of nitrogen and phosphorus in the water environment, S is contents of nitrogen and phosphorus in feed and bioflocs, and W the contents of nitrogen and phosphorus in cultured organism, respectively.
Statistical analysis
All data except economic profit were expressed as mean ± SD (standard deviation) and subjected to one-way analysis of variance using the statistical software program SPSS version 17.0 (SPSS Inc., IL, USA). Daily water quality from two groups was analyzed by Linear Mixed Models. Significant differences between two groups were determined using Dunnett’s t-test. Significant difference among different culture period was analyzed by Tukey’s range test. A significant difference between treatments was considered when P <0.05.
Results and discussion
Water quality
No significant difference on water temperature, DO and pH was observed between groups BFT and control during the experimental period (P > 0.05). Water temperature was maintained at 24.2-29.8oC, DO at 5.4-7.9 mg L-1, and pH at 7.0-8.4 for all tanks. The water temperature, DO and pH were within optimal range for gibel carp culture (Wang et al., 2012).
NO2--N increased gradually and reached the highest value (0.15 mg L-1) at day 12, and then decreased to undetectable level with the development of bioflocs in BFT group (Fig. 2). NO2--N concentration in control group was significantly higher than that in BFT group after day 24 (P <0.05). NO2--N is an important factor to determine aquaculture success. Wang et al. (2012) demonstrated that NO2--N changed from 0.01 mg L-1 to 0.06 mg L-1 and no toxic effect was observed during an annual investigation in gibel carp outdoor ponds. In the present study, NO2--N in BFT group was nearly undetectable at the end of experiment. It was much lower than that in BFT system cultured Nile tilapia and African catfish (Clarias gariepinus) (Azim and Little, 2008; Ekasari et al., 2016).
Total suspended solid is related with bioflocs formation in BFT group (Fig. 3). In the current study, TSS increased with the development of bioflocs in BFT system and was much higher than that in control group due to water exchange in control (P <0.05). TSS increased gradually from 19.5 mg L-1 to 795 mg L-1 within 60-day experiment and reached optimal for gibel carp growth. TSS concentration of 400-600 mg L-1 was considered suitable for super-intensive culture of L. vannamei (mean of body weight of 6.8 g) (Gaona et al., 2017). When TSS was lower than 100 mg L-1, water quality was difficult to maintain well or nitrification rate was low. However, high TSS (≥ 800 mg L-1) might become a stressor to respiration of the shrimp since gills were clogged with suspended solids (Schveitzer et al., 2013). In another study, Gaona et al. (2017) found that the growth of L. vannamei was not affected by TSS at 4000 mg L-1 when dissolved oxygen was high. Our other study also demonstrated that TSS of 600-800 mg L-1 was more suitable for gibel carp culture in BFT system (Qiao et al., 2018). Bioflocs contain various types of organisms such as microalgae, protozoan, rotifers, zooplankton and organic matter (Schryve et al., 2008). Many of these organisms contributed to generate dissolved oxygen in the pond which was utilized by cultured species and other heterotrophic microorganisms in the ecosystem (Ekasari et al., 2016). In addition, microalgae could produce beneficial metabolites and absorb excreted organic matters. The degrading and nutrient bio-incorporating activity of microorganisms in bioflocs can improve water quality (Ekasari et al., 2016).
Table I.- Proximate composition (% dry matter) of bioflocs.
|
Proximate composition |
Culture period (d) |
||||
|
14 |
21 |
28 |
45 |
60 |
|
|
Crude protein |
35.2±2.7ab |
39.0±1.0b |
39.8±0.2b |
34.0±0.9ab |
29.8±3.6a |
|
Crude lipid |
2.4±1.0a |
5.2±0.9b |
3.1±1.0ab |
4.0±0.3ab |
3.2±0.6ab |
|
Ash |
15.3±0.1a |
16.9±0.2b |
17.9±0.2c |
17.6±0.5c |
19.1±0.4d |
The data (mean ± SD) were derived from three independent experiments and analyzed by Dunnett’s t-test. Values within the same row marked with a different superscript are significantly different among different culture period (P < 0.05).
Table II.- Total amino acid composition (as a percentage of dry matter, n = 3) in bioflocs and muscle of gibel carp cultured at two different systems at day 60.
|
Total amino acid composition |
Bioflocs |
Fish muscle of gibel carp in two groups |
||
|
Control |
BFT |
|||
|
ArginineA,C |
0.44 ± 0.10 |
0.68 ± 0.10a |
0.91 ± 0.15ab |
|
|
HistidineA, C |
0.40 ± 0.03 |
0.44 ± 0.03a |
0.87 ± 0.06b |
|
|
IsoleucineA |
0.51 ± 0.09 |
0.87 ± 0.16 |
1.08 ± 0.18 |
|
|
LeucineA |
1.17 ± 0.22 |
1.88 ± 0.31a |
2.48 ± 0.25ab |
|
|
LysineA |
1.04 ± 0.17 |
1.09 ± 0.13a |
2.26 ± 0.22b |
|
|
MethionineA |
0.29 ± 0.05 |
0.40 ± 0.15 |
0.65 ± 0.12 |
|
|
PhenylalanineA |
0.99 ± 0.32 |
1.26 ± 0.24a |
2.28 ± 0.41b |
|
|
ThreonineA |
0.79 ± 0.21 |
1.15 ± 0.22a |
1.47 ± 0.31ab |
|
|
ValineA |
0.02 ± 0.00 |
0.04 ± 0.00 |
0.06 ± 0.01 |
|
|
AlanineB |
1.02± 0.35 |
1.65 ± 0.35a |
2.28 ± 0.46b |
|
|
Aspartic acidB |
1.34 ± 0.21 |
1.91 ± 0.18 |
2.45 ± 0.24 |
|
|
CysteineB, C |
1.70 ± 0.19 |
3.00 ± 0.32 |
3.87 ± 0.22 |
|
|
Glutamic acidB |
2.04 ± 0.38 |
3.10 ± 0.13 |
4.03 ± 0.20 |
|
|
GlycineB |
1.85 ± 0.12 |
2.98 ± 0.26a |
4.09 ± 0.18ab |
|
|
SerineB |
0.83 ± 0.14 |
1.20 ± 0.15a |
1.62 ± 0.22ab |
|
|
TyrosineB |
0.53 ± 0.09 |
0.87 ± 0.16 |
1.19 ± 0.14 |
|
|
EAAsA |
5.65 |
7.81 |
12.06 |
|
|
SEAAsC |
2.54 |
4.12 |
5.65 |
|
|
NEAAsB |
9.31 |
14.71 |
19.53 |
|
|
TAAs |
14.96 |
22.52 |
31.59 |
|
|
EAAs ration in TAAs |
37.77 |
34.68 |
38.18 |
|
Values are shown as mean ± SD. Means with the different superscript in each row are significantly different (P < 0.05) between two groups by Dunnett’s t-test. AEAAs, essential amino acids; BNEAAs, non-essential amino acids; CSEAAs, semi essential amino acids; TAAs, total amino acids. BFT, biofloc technology group; Control, control group with 1/4-1/3 daily water exchange.
Proximate composition of bioflocs
The nutritional quality of biolfocs was listed in Tables I and II, including proximate composition and total amino acid composition. In the present study, CP and ash content of bioflocs was 29.8-39.8% and less than 19.13%, respectively. The proximate composition of bioflocs was appropriate for gibel carp growth. Previous study demonstrated that diets were suitable for gibel carp, with 28-39% crude protein (CP), 11.6% crude lipid (CL) and less than 16% ash (Wang et al., 2015). At the initial stage of bioflocs formation, the CP content was higher than 30.0% reported by Luo et al. (2014) and Ekasari et al. (2016). After day 60, CP content decreased to 29.8%, which was similar with Luo et al. (2014) and Ekasari et al. (2016). During the whole experimental period, ash content was much lower than 31.3% (Ekasari et al., 2016). The CL content of bioflocs was 2.4-5.2% which was lower than commercial feed or diets required by gibel carp (Table I). Other studies also reported low CL content in bioflocs, such as 1.27 ± 0.61% LP of bioflocs in tilapia BFT system (Luo et al., 2014), 4.2 ± 0.1% in shrimp BFT system (Rajkumar et al., 2016).
Sixteen amino acids were identified in bioflocs at day 60 (Table II). The essential amino acids (EAAs), non-essential amino acids (NEAAs) and total amino acids (TAA) contents of bioflocs were 5.65%, 9.31% and 14.96%, respectively. For EAAs, contents of leucine were highest, followed by lysine and phenylalanine. For NEAAs, the glutamic acid was highest, followed by glycine, cysteine and aspartic acid. The total amino acids of contents were 14.96%. These results demonstrated that bioflocs contained adequate amount of nutrient for gibel carp culture. In other studies, bioflocs have been demonstrated to be used as food by freshwater and saltwater aquaculture species (Azim and Little, 2008; Schveitzer et al., 2013).
PHB content in bioflocs was 212.4 mg g-1 [PHB/dry matter of variable suspended solids (VSS)], which is slightly higher than 150-200 mg g-1 (PHB/VSS) under a C/N ratio of 15-18 (Ruan et al., 2011). PHB content in fish meal is approximately 2.25-4% (Ruan et al., 2011; de Schryve et al., 2010), and PHB has positive effects on growth and innate immunity (Yamazak et al., 2016; Franke et al., 2017; Hung et al., 2015). PHB content in bioflocs could reach the PHB requirement for fish, and it might replace ingredient for prebiotics in aquaculture. The native bacterial isolates such as Bacillus megaterium associated with PHB production in bioflocs can be considered as effective probiotics in future zero-water exchange system (unpublished). It will reduce the PHB production costs and simplify PHB addition process. However, the suitable bioflocs PHB level for different species should be further studied for optimal usage in aquaculture.
Growth performance
WG and SG were significantly higher in BFT group than those in control group at day 7, 28, 45 and 60 (Table III). Condition factor at day 60 in BFT group was significantly higher than that in control group (P <0.05). The survival in BFT group was 90.0%, which was higher than 77.8% in control group (P <0.05). There was no difference on viscerosomatic ration and hepatosomatic index between two groups (P >0.05). BFT has been reported to increase the growth of shrimp (Xu and Pan, 2013; Schveitzer et al., 2013), common carp, and Nile tilapia (Azim and Little, 2008; Mansour and Esteban, 2017).
Proximate composition of gibel carp muscle
Muscle composition of gibel carp cultured at two different systems is shown in Table IV. There were no statistical differences in crude protein (CP), crude lipid (CL) and ash contents for gibel carp muscle between BFT and control group at the end of experiment (P >0.05). The CP, CL and ash contents of muscle were 78.3%, 9.1% and 5.9% (% dry matter) in BFT group at day 60, respectively. These values were 79.5%, 9.5% and 5.9% (% dry matter) in control group, respectively (Table IV).
Table III.- Growth performance and body indices of gibel carp cultured at two different systems.
|
Items |
Groups |
Culture period (d) |
||||
|
0 |
7 |
28 |
45 |
60 |
||
|
Initial body weight (g) |
BFT |
19.09±1.50a |
||||
|
Control |
20.65±1.78a |
|||||
|
Final body weight (g) |
BFT |
21.78±0.52a |
38.48±2.18a |
42.21±1.35a |
44.67±0.57a |
|
|
Control |
22.64±1.18a |
33.94±1.64b |
39.05±1.36b |
40.56±0.51b |
||
|
Weight gain (%) |
BFT |
140.91±2.71a |
101.53±11.40a |
121.06±7.07a |
133.94±2.95a |
|
|
Control |
96.37±5.69b |
64.39±7.92b |
89.10±6.58b |
96.43±2.46b |
||
|
Special growth (% day-1) |
BFT |
4.48±0.27b |
2.50±0.20a |
1.72±0.07a |
1.20±0.15a |
|
|
Control |
3.04±0.61a |
1.77±0.17b |
1.38±0.08b |
0.95±0.02b |
||
|
Condition factor (%) |
BFT |
2.13±0.38a |
2.38±0.17a |
2.59±0.27a |
2.37±0.47a |
1.79±0.10a |
|
Control |
2.07±0.34a |
2.58±1.00a |
2.66±0.31a |
2.45±0.23a |
1.45±0.10b |
|
|
Visceros-omatic ration (%) |
BFT |
7.68±1.40a |
11.45±2.32a |
11.36±0.65b |
14.56±2.54a |
13.83±3.11a |
|
Control |
9.55±2.03a |
13.12±4.81a |
13.97±0.17a |
14.94±3.56a |
14.98±3.25a |
|
|
Hepatos-omatic index (%) |
BFT |
0.91±0.42a |
2.14±0.39a |
2.49±0.21a |
3.40±0.77a |
3.35±0.47a |
|
Control |
1.06±1.00a |
3.30±2.14a |
2.89±0.76a |
2.90±0.47a |
3.32±0.53a |
|
|
Survival (%) |
BFT |
90.0±3.8a |
||||
|
Control |
77.8±2.0b |
|||||
Values with the different superscript within the same column (day) were significantly different between BFT and control group (P < 0.05). BFT, biofloc technology system; Control, common recirculating aquaculture system with 1/4-1/3 water exchange each day.
Table IV.- Proximate composition (% dry matter) of gibel carp (Carassius auratus gibelio ♀ × C. carpio ♂) muscle cultured at two different systems.
|
Proximate composition |
Groups |
Culture period (d) |
|||||
|
0 |
14 |
21 |
28 |
45 |
60 |
||
|
Crude protein |
Control |
73.8±0.7aA |
73.8±1.0aA |
74.0±2.0aA |
81.4±0.5bA |
81.7±0.6bB |
79.5±0.9bA |
|
BFT |
73.3±0.4aA |
74.2±0.9aA |
77.1±0.5bB |
80.3±0.3cA |
80.5±0.1cA |
78.3±0.6bA |
|
|
Crude lipid |
Control |
6.3±0.5aA |
6.5±0.4aA |
7.1±0.1aA |
7.8±0.2aA |
9.9±0.5bA |
9.5±0.4bA |
|
BFT |
6.1±0.3aA |
6.6±0.7aA |
6.7±0.5aA |
7.8±0.4aA |
9.6±0.4bA |
9.1±0.5bA |
|
|
Ash |
Control |
6.0±0.7aA |
6.0±1.3aA |
7.3±0.3aA |
6.9±0.7aA |
6.4±0.3aA |
6.5±0.1aA |
|
BFT |
6.2±0.3abA |
6.6±0.5abA |
6.5±0.2abA |
7.4±0.4bA |
6.9±0.5abA |
5.9±0.2aA |
|
Values within the same row marked with a different lowercase superscript are significantly different among different culture period for the same group (P < 0.05). Values within the same column marked with a different capital superscript are significantly different between two groups for the same culture period (P < 0.05). BFT, biofloc technology group without water exchange and with carbon addition; Control, control group with 1/4-1/3 daily water exchange.
Table V.- Comparative analysis of partially economic profitability of biofloc technology system applied in gibel carp culture.
|
Items |
Groups |
|
|
BFT |
Control |
|
|
Stocking (fish mu-1 yield) (1 mu = 667 m2) |
10000 |
4000 |
|
Mean weight (g) |
19.09 |
20.65 |
|
Survival (%) |
90 |
77.8 |
|
Fry (RMB, ¥) |
3000 |
1200 |
|
Cost of carbon sources (RMB, ¥) |
1800 |
0 |
|
Exchange water (m3) |
0 |
24012 |
|
Cost of electricity (RMB, ¥) |
3600 |
780 |
|
Cost of feed (RMB, ¥) |
18000 |
7391 |
|
Medicine use (RMB, ¥) |
0 |
480 |
|
Disinfectant (RMB, ¥) |
214.5 |
214.5 |
|
Variable cost (RMB, ¥) |
26614.50 |
10065.50 |
|
Cost of labor (RMB, ¥) |
240 |
240 |
|
Land rent (RMB, ¥) |
400 |
400 |
|
Depreciation (RMB, ¥) |
468.9 |
135 |
|
Fixed cost (RMB, ¥) |
1108.9 |
775 |
|
Total cost (RMB, ¥) |
27723.4 |
10840.5 |
|
Feeding conversation rate |
1.6 |
1.9 |
|
Degree of operating leverage |
1.13 |
1.48 |
|
Water consumption (m3 kg-1) |
0.30 |
31.7 |
|
Total nitrogen (kg) |
0 |
43.97 |
|
Total phosphorus (kg) |
0 |
1.09 |
|
Annual production (kg) |
2250 |
778 |
|
Selling price (¥ kg-1) |
16 |
16 |
|
Gross revenue1 (RMB, ¥) |
36000 |
12448 |
|
Cost price2 (¥ kg-1) |
12.32 |
13.93 |
|
Break-even production3 (kg) |
1732.70 |
677.53 |
|
Operational profit4 (RMB, ¥) |
8276.6 |
1607.5 |
|
Net present value |
45352.08 |
1484.85 |
|
Payback time (year) |
1.17 |
4.44 |
1Gross revenue = production × selling price; 2Total production cost (TPC)/total production; 3TPC/selling price; 4Operational profit = Gross revenue – TPC. BFT, biofloc technology system without any water exchange; Control, common recirculating aquaculture system with 1/4-1/3 water exchange each day.
When compared with the baseline (day 0), the CP and CL contents of muscle increased significantly (P <0.01) at day 60 in both BFT and control groups (Table IV). The types and contents of essential amino acids (EAAs) are considered important factors to evaluate the food nutrition. In our study, EAAs, non-essential amino acids (NEAAs), and total amino acids (TAA) contents were higher in gibel carp muscle in BFT group than those in control group. For EAAs, contents of histidine, lysine and phenylalanine were significantly higher in BFT group. For NEAAs, the alanine and glycine contents were significantly higher in BFT group. TAAs in BFT and control groups were 31.59% and 22.52%, respectively (Table IV). It suggests that BFT does not reduce fish nutrition and can provide green food for human since fish are not used any aquatic drugs. Drug residues are considered a very important food safety issue. The effect of BFT on fish growth might be related with the bioflocs uptaken by fish and maintenance of good water quality.
Economic profitability analysis of gibel carp cultured in BFT system
The results of economic profitability analysis are presented in Table V. For BFT system, the annual gross revenue would exceed ¥36000 based on an annual production of approximately 2250 kg and the selling price of ¥16 kg-1 gibel carp. If the cost price was estimated at ¥12.32 kg-1, the break-even production would be approximately 1732.70 kg, and the operational profit would equal ¥8276.60. For the control group, the annual gross revenue was ¥12448 according to the annual production of approximately 778 kg. The break-even production would be 677.53 kg. The cost price was estimated at ¥13.93 kg-1. Considering a time line of 5 years, positive NPV would be generated in both BFT (¥45352.08) and control group (¥1484.85), and the payback times would be 1.17 and 4.44 years, respectively. In the present study, a minimum attractive rate of return (MARR) of 10% was set for BFT and control groups, which is consistent with other similar studies (Kam and Leung, 2008). The payback time represents the time required for the recovery of the initial investment through the sum of discounted net cash inflows. The payback time in gibel carp BFT culture system was significantly lower than that in control group.
For the BFT system with a gross revenue ¥36000, variable cost ¥26614.50 and fixed cost ¥1108.90, DOL was equal to 1.13 (Table V). For the control group, DOL was 1.48. These results illustrated that operation risk of BFT was lower than control group. Meanwhile, break-even production was also estimated to assess the operation risk, and the similar result of lower operation risk of BFT system was obtained with DOL.
Regardless of the production system, feed was one of major operational costs, which was in line with several other reports on marine fish production (Bombeo-Tuburan et al., 2001; Miao and Tang, 2002). Cost of gibel carp feed varied with culture system. Feed cost took up to 65% of the operational cost and feed conversion rate (FCR) was 1.6 in BFT system. In control group, feed cost spent 68% of the operational cost with a 1.9 FCR. Feed cost was ¥8.0 kg-1 gibel carp in BFT system, and ¥9.5 kg-1 gibel carp in control group, which was lower in BFT system than that in control group. Therefore, effective feed management is crucial for the economic feasibility of gibel carp culture. Any improvement on feeding rate and frequency, use of automatic or semiautomatic feeding systems and underwater recording systems to monitor actual feed intake would be beneficial to improve feed management and decrease the FCR as demonstrated in salmon farming (Sarker et al., 2013).
In addition, environmental costs should be considered to save natural resource and support sustainable aquaculture developments in future. This cost on environmental effect is mainly being undertaken by government, not by farmers to date. It might be undertaken by farmers themselves in future to develop sustainable aquaculture and protect environment. In past, environmental costs had often been ignored in aquaculture practice due to priceless resources, low raw materials and high commodity price. The environmental costs include water resource cost and environment pollution cost. The present study was conducted in Jiangsu Province, and the water resources cost is free there so far. There is no water exchange in BFT system so water resource costs had not been taken into consideration in the present study.
Material balance method and shadow price method were used in this study to evaluate environment pollution cost. The shadow price is introduced by the Dutch mathematical economist econometrics founder, T. Jain, and the former Soviet Union mathematician economist, Nobel Prize winner in 1975, L.V. Kantorovich. This price refers to the prices at which various economic resources should be obtained under optimal allocation in production. The marginal productivity of resource shadow prices is one of the common methods for pricing natural resources. Scarce resources will command higher prices than more abundant resources. In this study, no water was exchanged in BFT system so no wastes emitted to the environment. BFT was a friendly-environmental system and no environmental cost was produced. In control group with water exchange, nitrogen and phosphorus contents of gibel carp were 12.53% and 0.175%, respectively. According to material balance method and shadow price, one kilogram gibel carp can produce 0.057 kg nitrogen and 0.0014 kg phosphorus pollutant with a pollution cost of ¥1.04 kg-1 as reported by Huang (2011). Huang (2011) estimated nitrogen and phosphorus pollution of olive flounder (Paralichthys olivaceus) industrial farming and showed shadow price of nitrogen ¥18023.8 ton-1 and phosphorus ¥14786.5 ton-1. Thus, if pollution cost has been considered, the operation cost is ¥14.97 kg-1 in control group, while operation cost is ¥12.32 kg-1 in BFT system.
Conclusions
The current study evaluated growth, nutrition and economic profitability of gibel carp cultured in zero-water exchange BFT system. This research demonstrated that BFT system can be applied in gibel carp culture with increasing fish growth and survival, and culture economic profitability. WG, SG and survival of gibel carp in BFT group were significantly higher than those in control group. EAAs ration in TAAs in BFT group was slightly higher than that in the control group. Meanwhile, the crude protein content in bioflocs met the nutritional requirements of gibel carp, but the crude lipid content was low. The operation risk, payback time and environmental cost were lower in BFT system.
Acknowledgement
This research was financially supported by the National Nature Science Foundation of China (Grant No. 31602179) and ‘the Nature Science Foundation of Jiangsu Province (Grant No. BK20150426) and partially by the “Talent Introduction Program” at Yancheng Institute of Technology (Grant No. XJ201513), and the open fund offered by the Department of Agriculture Key Laboratory of Freshwater Fishery and Genetic Resources (KF201503). MMZ was financially supported by the Postdoctoral Project of Jiangsu Province (1501101C).
Statement of conflict of interest
All authors declare that there is no conflict of interests regarding the publication of the manuscript.
References
AOAC, 1995. Official methods of analysis, 16th ed. Patricia Cunniff (editora), Association of Official Analytical Chemists, Washington, DC.
AOAC, 2005. Official methods of analysis. Association of Official Analytical Chemists, Washington, DC.
APHA, 1998. Standard methods for the examination of the water and wastewater. 22nd ed. Washington, United States: American Public Health Association.
Avnimelech, Y., 2006. Bio-filters: The need for a new comprehensive approach. Aquacult. Engin., 34: 172-178. https://doi.org/10.1016/j.aquaeng.2005.04.001
Avnimelech, Y. and Kochba, M., 2009. Evaluation of nitrogen uptake and excretion by tilapia in biofloc tanks, using 15N tracing. Aquaculture, 287: 163-168. https://doi.org/10.1016/j.aquaculture.2008.10.009
Azim, M.E. and Little, D.C., 2008. The biofloc technology (BFT) in indoor tanks: water quality, biofloc composition, growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture, 83: 29-35. https://doi.org/10.1016/j.aquaculture.2008.06.036
Boonyoung, S., Haga, Y. and Satoh, S., 2013. Preliminary study on effects of methionine hydroxy analog and taurine supplementation in a soy protein concentrate-based diet on the biological performance and amino acid composition of rainbow trout (Oncorhynchus mykiss) (Walbaum). Aquacult. Res., 44: 1339-1347. https://doi.org/10.1111/j.1365-2109.2012.03138.x
Bezerra, T.R.Q.D., Domingues, E.C., Filho, L.F.A.M., Rombenso, A.N., Hamilton, S. and Ronaldo, O., 2016. Cavalli economic analysis of cobia (Rachycentron canadum) cage culture in large- and small-scale production systems in Brazil. Aquacult. Int., 24: 609-622. https://doi.org/10.1007/s10499-015-9951-2
Bombeo-Tuburan, I., Coniza, E.B., Rodriguez, E.M. and Agbayani, R.F., 2001. Culture and economics of wild grouper (Epinephelus coioides) using three feed types in ponds. Aquaculture, 201: 229-240. https://doi.org/10.1016/S0044-8486(01)00744-X
Crispim, M.C., Vieira, A.C.B., Coelho, S.F.M. and Medeiros, A.M.A., 2007. Nutrient uptake efficiency by macrophyte and biofilm: practical strategies for small-scale fish farming. Limnology, 21: 387-391.
Crab, R., Chielens, B., Wille, M., Bossier, P. and Verstraete, W., 2010. The effect of different carbon sources on the nutritional value of bioflocs, a feed for Macrobrachium rosenbergii postlarvae. Aquacult. Res., 41: 559–567. https://doi.org/10.1111/j.1365-2109.2009.02353.x
Coggin, J.S. and Swinton, J.R., 1996. The price of pollution: a dual approach to valuing SO2 allowances. J. environ. Manage., 30: 58-72. https://doi.org/10.1006/jeem.1996.0005
Ekasari, J., Suprayudi M.A., Wiyoto, W., Hazanah, R.F., Lenggara, G.S., Sulistiani, R., Alkahfia, M. and ZairinJra, M., 2016. Biofloc technology application in African catfish fingerling production: the effects on the reproductive performance of broodstock and the quality of eggs and larvae. Aquaculture, 464: 349-356.
Franke, A., Clemmesen, C., Schryver, P.D., Garcia-Gonzalez, L., Miest, J.J. and Roth, O., 2017. Immunostimulatory effects of dietary poly-β-hydroxybutyrate in European sea bass postlarvae. Aquacult. Res., 48: 5707-5717. https://doi.org/10.1111/are.13393
FAO, 2015. The state of world fisheries and aquaculture. FAO Fisheries and Aquaculture Department, Food Agriculture Organization of the United Nations, Rome.
Gaona, C.A.P., Almeida, M.S.D., Viau, V., Poersch, L.H. and Wasielesky, Jr. W., 2017. Effect of different total suspended solids levels on a Litopenaeus vannamei (Boone, 1931) BFT culture system during biofloc formation. Aquacult. Res., 48: 1070-1079. https://doi.org/10.1111/are.12949
Hargreaves, J.A., 2013. Bioflocs production system for aquaculture. SRAC Publication No. 4503.
Huang, F., Yan, A.S. and Xiong, C.X., 1999. Evaluation of the nutrition and the rate of flesh in the whole body of Pelteobagrus fulvidraco. Freshw. Fish., 29: 3-6.
Huisman, E.A., 1987. The principles of fish culture production. Ph.D. thesis, Wageningen University, Wageningen City, The Netherlands.
Hung, N.V., DeSchryver, P., Thanh, T., Garcia-Gonzalez, L., Bossier, P. and Nevejan, N., 2015. Application of poly-β-hydroxybutyrate (PHB) in mussel larviculture. Aquaculture, 446: 318-324. https://doi.org/10.1016/j.aquaculture.2015.04.036
Huang, S.P., 2011. Economical efficiency and environmental impacts of bastard halibut culture in China. Master Degree Thesis. Shanghai Ocean University.
Jackson, C.N., Preston, P.J. and Thompson, M., 2003. Nitrogen budget and effluent nitrogen components at an intensive shrimp farm. Aquaculture, 218: 397-411. https://doi.org/10.1016/S0044-8486(03)00014-0
Kolarevic, J., Selset, R., Felip, O., Good, C., Snekvik, K., Takle, H., Ytteborg, E., Bæverfjord, G., Åsgård, T. and Terjesen, B.F., 2012. Influence of long term ammonia exposure on Atlantic salmon (Salmo salar L.) parr growth and welfare. Aquacult. Res., 44: 1649-1664. https://doi.org/10.1111/j.1365-2109.2012.03170.x
Kam, L.E. and Leung, P.S., 2008. Financial risk analysis in aquaculture. In: Understanding and applying risk analysis in aquaculture (eds. M.G. Bondad-Reantaso, J.R. Arthur and R.P. Subasinghe). FAO Fisheries and Aquaculture Technical Paper No. 519, FAO, Rome.
Luo, G., Gao, Q., Wang, C., Liu, W., Sun, D., Li, L. and Tan, H.X., 2014. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Creochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture, 422-423: 1-7. https://doi.org/10.1016/j.aquaculture.2013.11.023
Lin, L.M., Wang, Q.Y., Wang, Z.Y., Zhang, Y.Z., Liu, J.F. and Xie, F.J., 2006. Comparison of biochemical compositions of muscle among three stocks and wild-caught large yellow croaker (Pseudosciaena crocea). J. Fish. Sci. China, 13: 286-291.
Lin, S.H., Zhou, X., Zhou, T.T., Jing, Z.Q. and Song, Y.H., 2016. Performance assessment of large-scale recirculation aquaculture system on nitrogen and phosphorous pollution control. Chinese J. environ. Engin., 10: 5535-5541.
Miao, S. and Tang, H.C., 2002. Bioeconomic analysis of improving management productivity regarding grouper Epinephelus malabaricus farming in Taiwan. Aquaculture, 211: 151-169. https://doi.org/10.1016/S0044-8486(02)00190-4
Mansour, A.T. and Esteban, M.Á., 2017. Effects of carbon sources and plant protein levels in a biofloc system on growth performance, and the immune and antioxidant status of Nile tilapia (Oreochromis niloticus). Fish Shellf. Immunol., 64: 202-209. https://doi.org/10.1016/j.fsi.2017.03.025
Piedrahita, R.H., 2003. Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture, 226: 35-44. https://doi.org/10.1016/S0044-8486(03)00465-4
Podok, P., Xu, L.J., Xu, D. and Lu, L.Q., 2014. Different expression profiles of interleukin 11 (IL-11), intelectin (ITLN) and purine nucleoside phosphorylase 5α (PNP 5α) in crucian carp (Carassius auratus gibelio) in response to Cyprinid herpesvirus 2 and Aeromonas hydrophila. Fish Shellf. Immunol., 38: 65-73.
Patterson, J.H., Talbot, F.B., Slowinski, R. and Weglarz, J., 1990. Computation experience with a backtracking algorithm for solving a general class of precedence and resource-constrained scheduling problems. Eur. J. Oper. Res., 49: 68-79. https://doi.org/10.1016/0377-2217(90)90121-Q
Peng, W.Z., 2014. A case study on Zhejiang Province: Ore and energy resources values evaluation based on NPV. Resour. Indust., 2: 56-63.
Qiao, G., Zhang, M.M., Li, Y., Xu, C., Xu, D.H., Zhao, Z.G., Zhang, J.L. and Li, Q., 2018. Biofloc technology (BFT): An alternative aquaculture system for prevention of Cyprinid herpesvirus 2 infection in gibel carp (Carassius auratus gibelio). Fish Shell. Immunol., 83: 140-147. https://doi.org/10.1016/j.fsi.2018.09.015
Ruan, Y.J., Zhu, L. and Xu, X.Y., 2011. Study on the flocs poly-β-hydroxybutyrate production and process optimization in the bio-flocs technology system. Bioresour. Technol., 102: 75-99. https://doi.org/10.1016/j.biortech.2011.05.028
Rajkumar, M., Pandey, P.K., Aravind, R., Vennila, A., Bharti, V. and Purushothaman, C.S., 2016. Effect of different biofloc system on water quality, biofloc composition and growth performance in Litopenaeus vannamei (boone, 1931). Aquacul. Res., 47: 3432-3444. http://doi.org/10.1111/ are.12792
Sarker, P.K., Bureau, D.P., Hua, K., Drew, M.D., Forster, I., Were, K., Hicks, B. and Vandenberg, G.W., 2013. Sustainability issues related to feeding salmonids: A Canadian perspective. Rev. Aquacult., 5: 1-21. https://doi.org/10.1111/raq.12013
Schryve, P.D., Sinha, A.K., Kunwar, P.S., Baruah, K., Verstraete, W., Boon, N.D., Boeck, G. and Bossier, P., 2010. Poly-β-hydroxybutyrate (PHB) increases growth performance and intestinal bacterial range-weighted richness in juvenile European sea bass (Dicentrarchus labrax). Appl. Microbiol. Biotechnol., 86: 1535-1541. https://doi.org/10.1007/s00253-009-2414-9
Schveitzer, R., Arantes, R,. Costódio, P.F.S., do Espírito Santo, C.M., Arana, L.V., Seiffert, W.Q. and Andreatta, E.R., 2013. Effect of different biofloc levels on microbial activity, water quality and performance of Litopenaeus vannamei in a tank system operated with no water exchange. Aquacult. Engin., 56: 59-70. http://doi.org/10.1016/j.aquaeng.2013.04.006
Strickland, J.D.H., and Parsons, T.R., 1972. A practical handbook of seawater analysis. Fisheries Research Board of Canada. 2ed. Ottawa. Bulletin. pp. 167.
Wang, Y.Z. and Tang, B.Y., 2013. Risk analysis on listed shipping enterprise based on operating leverage. Commer. Account., 2: 75-77.
Wang, A.M, Yang, W.P., Shen, Y.L., Han, G.M., Lv, F., Yu, Y.B., Huang, J.T. and Zhang, J.L., 2015. Effects of dietary lipid levels on growth performance, whole body composition and fatty acid composition of juvenile gibel carp (Carassius auratus gibelio). Aquacul. Res., 46: 2819-2828. http://doi.org/ 10.1111/are.12571
Wu, X.R., Zhang, J.B., Zhu, Y. and Tian, Y., 2014. Evaluation of provincial low-carbon agriculture performance and estimation of marginal abatement costs in China. China Popul. Resour. Environ., 10: 57-63.
Wang, Z.S., Zhao, W.H., Lv, F., Zhang, H. and Huang, J.T., 2012. Study on annual nitrogen and phosphorus nutrients changes in gibel carp (Carassius auratus gibelio) culture. Jiangsu Agric. Sci., 40: 252-254.
Xiao, X.C., He, B.H., Ni, J.P. and Xie, D.T., 2014. Study on emission efficiency, shadow price and motivation factors of agricultural non-point source pollution in ecological barrier zone of three gorges reservoir area. China Popul. Resour. Environ., 11: 60-68.
Xu, W.J. and Pan, L.Q., 2013. Enhancement of immune response and antioxidant status of Litopenaeus vannamei juvenile in biofloc-based culture tanks manipulating high C/N ratio of feed input. Aquaculture, 412-413: 117-124. https://doi.org/10.1016/j.aquaculture.2013.07.017
Yuan, Y., Yuan, Y.M., Dai, Y.Y. and Gong, Y.C., 2017. Economic profitability of tilapia farming in China. Aquacult. Int., 25: 1253-1264. https://doi.org/10.1007/s10499-017-0111-8
Yang, H.Y., Tang, K.Y., Fan, X.Y. and Yang, Z.Y., 2012. Approaches to assessment of environmental costs of eutrophication of aquaculture ponds- A case study of a conventional fish pond in Qingpu, Shanghai. J. Ecol. Rural Environ., 1: 26-31.
Yamazak, Y., Meirelles, P.M., Mino, S., Suda, W., Oshima, K., Hattori, M., Thompson, F.L., Sakai, Y., Sawabe, T. and Sawabe, T., 2016. Individual Apostichopus japonicus fecal microbiome reveals a link with polyhydroxybutyrate producers in host growth gaps. Scient. Rep., 6: 21631. https://doi.org/10.1038/srep21631
Zhang, M.M., Li, Y., Xu, D.H., Qiao, G., Zhang, J.L., Qi, Z.T. and Li, Q., 2018. Effect of different water biofloc contents on the growth and immune response of gibel carp cultured in zero water exchange and no feed addition system. Aquacult. Res., 49: 1647-1656. https://doi.org/10.1111/are.13620
Zhao, Z.G., Xu, Q.Y., Luo, L., Wang, C.A., Li, J.N. and Wang, L.S., 2014. Effect of feed C/N ratio promoted bioflocs on water quality and production performance of bottom and filter feeder carp in minimum-water exchanged pond polyculture system. Aquaculture, 434: 442-448. https://doi.org/10.1016/j.aquaculture.2014.09.006
To share on other social networks, click on any share button. What are these?










