Advances in Animal and Veterinary Sciences
Research Article
Pharmacokinetics of Tetracycline and Tetracycline Loaded Nanoemulsion Formula in Rabbits
Aziza M.M. Amer1*, Shymaa A. El Badawy1, Mohamed S. Saber2, Omar A. Ahmed- Farid3, Wessam H. Abd-Elsalam4, Mohamed M. Amer5
1Deptartment of Pharmacology, Faculty Veterinary Medicine, Cairo University, Giza, Egypt; 2PhD Student in Pharmacology, Veterinary Service division, Ministry of Deviance, Egypt; 3Deptartment of Physiology NODCAR, Giza, Egypt; 4Department of Pharmaceutics and Industrial pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt; 5Department. of Poultry Diseases, Faculty Veterinary Medicine, Cairo University, Giza, Egypt.
Abstract | Nano-sized drug delivery systems used to improve drug pharmacokinetics especially bioavailability. Different tetracycline loaded nanoemulsions were formulated and evaluated for thermodynamic stability, morphology, droplet size and zeta potential measurements. Pharmacokinetic of TC-NE (10% Mig, 50% S/CoS and 40% water with drug concentration of 5%, w/w) was investigated in rabbits following a single oral and IV doses (50 mg/kg bwt) and compared to tetracycline HCl powder (TC- Powder) at the same dose. Tetracycline concentrations were determined in plasma samples using standard high performance liquid chromatography (HPLC) procedure. Following IV injection higher AUC0-inf (83.3 ± 4.2 and 74.8 ± 2.9 μg/ml.h) and volume of distribution (Vdss) (0.78 ± 0.06 L/kg and 0.71 ± 0.10 L/kg) reported for TC-NE compared to TC-Powder, respectively. Furthermore, after oral administration, TC-NE was slowly absorbed and eliminated than TC-Powder with longer t1/2ka (0.518 ± 0.091 h and 0.253 ± 0.024 h) and t1/2β (4.22 ± 1.67 h and 3.33 ± 0.68 h), respectively. Moreover, the time at which maximum tetracycline plasma concentration achieved (Tmax) was 0.869 ± 0.059 h for TC--NE and 0.397 ± 0.033 h for TC-Powder. Significantly higher area under curve AUC0-t 20.4 ± 1.5 μg/ml.h and 11.1 ± 0.6 μg/ml.h and consequently higher bioavailability 29.2 ± 2.3% and 13.9 ± 0.8% was recorded for TC-NE than TC-Powder, respectively. Following oral admistiration TC-NE formula exhibited prolonged T> MIC of 10.36 ± 0.64 h compared to 7.1±0.32 h in TC-powder. In conclusion, the prepared Tetracycline loaded nanoemulsion formulation has improved oral bioavailability and prolonged the blood concentration time than TC-Powder. Further clinical studies are required to justify dosage that supports clinical efficiency.
Keywords | Pharmacokinetics, Tetracycline, Tetracycline-loaded nanoemulsions, HPLC, Rabbits
Received | Novemebr 04, 2019; Accepted | December 26, 2019; Published | January 20, 2020
*Correspondence | Aziza M.M. Amer, Deptartment of Pharmacology, Faculty Veterinary Medicine, Cairo University, Giza, Egypt; Email: aziza.mahrous@gmail.com
Citation | Amer AMM, El Badawy SA, Saber MS, Farid OAA, Abd-Elsalam WH, Amer MM (2020). Pharmacokinetics of tetracycline and tetracycline loaded nanoemulsion formula in rabbits. Adv. Anim. Vet. Sci. 8(2): 130-139.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.2.130.139
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Amer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Tetracycline (TC) is a polymeride antibiotic produced by the Streptomyces genus of Actinobacteria. Tetracycline hydrochloride formula is C22H24N2O8 with CAS Number 64-75-5 (European Pharmacopoeia (EP) Reference Standard with relative molecular weight of 515.3). Tetracycline has low stability in strong acid or basic media (Oka, 1995). TC is an amphoteric molecule with three pKa values (3.3, 7.7 and 9.7). Thus, TC exists as a cationic, zwitterionic and anionic species under acidic, curcimneutral and alkaline conditions, respectively. Under acidic conditions, a reversible epimerization occurs at position of C4, forming 4-epi-TC, results in the relatively high solubility of TC (McCormick, 1957). Tetracycline is a time-dependent antimicrobial (Mestorino and Errecalde, 2012) that exerts a bacteriostatic action against various bacteria including mycoplasma, rickettsia, and chlamydia, in addition to some protozoa, Rickettsiae, and Ehrlichiae (Riviere and Spoo, 2001; Papich, 2016).
Different members of tetracycline were investigated in rabbits. Chlortetracycline was administered orally in a dose of 50 mg/kg b. wt. every 24 hours (Carpenter and Christopher, 2018). While Doxycycline (Long-acting tetracycline) was given orally (2.5mg/kg twice daily), that was efficient against anaerobes and intracellular bacteria and safe in rabbits with renal problems as it is excreted via the intestine (Carpenter and Christopher, 2018). In addition, Oxytetracycline was administered in rabbits in the doses of 50 mg/kg twice daily per Os and 15 mg/kg/day SC or IM (Saunders and Davies, 2005). Moreover, Tetracycline was used in rabbits orally (50 mg/kg every 8-12 hours in drinking water) for treatment of Tyzzers disease, Pasteurellosis and Mycoplasmosis (Carpenter and Christopher, 2018).
Pharmacokinetics of tetracycline hydrochloride was studied in various animals including pigs (Kniffen et al., 1989), man (Agwuh and MacGowan, 2006), rabbits (Percy and Black, 1988) and chickens (Anadon et al., 1985). Following repeated oral administration of tetracycline (35 mg/kg b.wt. for 5 days) in rabbit, There were no TC residues detected in the muscular and hepatic tissues at (7 and 14) days post last dose, respectively (Abdel Aziz et al., 2017). The antibacterial activities of tetracycline hydrochloride (TC-powder) and tetracycline nanoemulsion (TC-NE) formulations were previously evaluated on standard and field bacterial strains (Saber et al., 2019a). Moreover, Saber et al. studied the biochemical and pathological effects of the former formulations following repeated oral administration for 7 days. Four days after the last dose, there were no detectable residues in the liver, kidney and muscle tissues (Saber et al., 2019b).
The commonly used route for TC is the oral route with low oral bioavailability (Ahmad et al., 2011). Absorption of hydrophobic drugs might be improved by their incorporation into inert lipid vehicles and surfactants (Ahmad et al., 2012; Gupta et al., 2013; Costanzo and Angelico, 2019). Nanoemulsions are biocompatible, biodegradable, physically stable and relatively easy to produce on a large scale. Therefore, Nanoemulsions might be considered as ideal delivery system for therapeutic agents (Li et al., 2008; Aboofazeli, 2010). Intravenous (IV) administration of nanoemulsions had proved advantageous particularly due to their droplet size (< 1μm) (Tamilvanan et al., 2005) that provides a large interfacing area for better drug absorption (Chime et al., 2014).
The incorporation of TC, a water soluble drug, in the nanoemulsion system is suspected to change its release behaviour and hence modify its pharmacokinetic parameters. Therefore, the aim of this study was to prepare and characterize a nanoemulsion formula of tetracycline hydrochloride (TC-NE). As well as to compare pharmacokinetics of tetracycline hydrochloride powder (TC-Powder) with formulated TC-NE following oral and IV administration in healthy white male New Zealand rabbits.
MATERIAL AND METHODS
Chemicals
Miglyol® 812 (Mig) (Gattefossé, St-Priest, France). Isopropyl myristate (IPM) (Sigma Aldrich Chemical Co., St. Louis, MO, USA). Cremophor RH40 (BASF, Ludwigshafen, Germany). Polyethylene glycol 400 (PEG 400), sodium benzoate (Sigma-Aldrich, Inc., cairo Egypt). Tetracycline hydrochloride pure powder 100% (El-Nasr pharmaceutical chemicals Co., Abu-Zaabal, Egypt). calcium gluconate (Cal-Nate, Armitage-Carroll, London, Ontario). All chemicals were of analytical grade and used as received.
Preparation and characterization of tetracycline hydrochloride nanoemulsion (TC-NE)
Construction of phase diagrams
The phase diagrams constructed using Tri-plot software (Ver. 4.1.2, D. Graham and N. Midgley, Loughborough University, Leicestershire, England) as described by El-Assal et al. (2018). Nanoemulsion systems consisting of oils (IPM or Mig), surfactant (Cremophor RH40) and a cosurfactant (PEG 400) formulated by the water titration method as described previously (Ammar et al., 2009). Briefly, transparent and homogeneous blends of oils and Surfactant/Cosurfactant (S/CoS) mixtures (1:1, 1:2 and 2:1, respectively) ranging from 9:1 to 1:9 (w/w) ready by constant stirring. After each addition of water, systems were settled for equilibration and observed for phase transparency and flowability.
Preparation of Tetracycline HCl-loaded nanoemulsions (TC-NE)
Certain systems; namely, F1, F2 and F3 selected and formulated by mixing specified weight ratios of oil (10, 15 and 20%, w/w), a fixed S/CoS concentration (50%, w/w) and water (40, 35 and 30%, w/w), respectively. TC-NE (5%, w/w) formulated and preserved with sodium benzoate (0.01%, w/w). In order to rule out any possible effects of the components of nanoemulsions on biological tissues, a nanoemulsion lacking the drug was prepared and tested along with drug loaded preparation. The formulae kept at room temperature (25 oC) till further use. Composition of the investigated formulae presented in (Table 1).
Characterization of tetracycline-loaded formulae
Thermodynamic stability studies
The thermodynamic stability of TC-NE assessed according to the protocol designed by Shafiq et al. (2007). Firstly, nanoemulsions exposed to six heat (45 oC) - cool (4 oC) cycles. This was achieved through storage at each temperature for 48 h. Secondly; nanoemulsions subjected to centrifugation at 3500 rpm for 30 min. Finally nanoemulsions went through three freezes (−21 oC) – thaw (25 oC) cycles and this was accomplished by storage at each temperature for 48 h.
Nanoemulsion droplet size analysis
The mean droplet size (MDS) of nanoemulsions and the polydispersity index (PDI) determined by Photon Correlation Spectroscopy (PCS) using a Zetasizer Nano ZS (Ver.6.20, Malvern Instruments Ltd., Worcestershire, England). The technique explores the oscillations in light scattering caused by the Brownian motion of particles and thus assumes the average droplet size. The zeta potential (ZP) values of nanoemulsions recorded by the electrophoretic light scattering (ELS) technology using a Laser Doppler Anemometer coupled with the same equipment. The method elucidates the electrophoretic mobility of globules under an electric field. All measurements were carried out, in triplicate, at room temperature (25 oC).
Transmission electron microscopy (TEM)
The morphological features of the selected TC-NE visualized via TEM. One drop of the formula loaded onto a copper grid, and the excess was absorbed with filter paper. The grid air-dried for 10 min. Examination of the grid was achieved via a transmission electron microscope (Jeol JEM 1230, Tokyo, Japan).
Pharmacokinetic study
Animals
The protocol was approved by Institutional Animal Care and Use Committee of Cairo University (IACUC). Sixteen white male New Zealand rabbits weighing (3.4- 3.6) kg were obtained from local farms, housed indoor at laboratory animal facilities of faculty of veterinary medicine, Cairo University and fed commercial pellets and fresh hay ad libitum with free access to drinking water.
Drug preparation and route of administration
For IV administration, crystalline tetracycline hydrochloride (TC-Powder) was dissolved in a 23% solution of sterile calcium gluconate at concentration of 50 mg of tetracycline HCl per mL of solution. At a dose of 1ml/kg.b.wt, the prepared solution was administered intravenously via IV catheter in the ear vein or orally through a mouth gag using 3 mL syringe according to group allocation. Tetracycline HCl nanoemulsion (TC-NE) prepared in a concentration of 50 mg/ml and used for oral or IV administration (1ml/kg b wt) as described above. For sequential blood sampling, ear vein catheters were applied as described by (Echols and Pollock, 2013).
Experimental design
Pharmacokinetics of tetracycline HCl was investigated in rabbits following oral and IV administration of tetracycline HCl Loaded nanoemulsion (TC-NE) and tetracycline HCl powder (TC-Powder). Formulations were used at the same dose, for both routes to investigate the bioavailability and sustainability of the new formula. Following one week of accommodation, rabbits were randomly allocated into two groups of eight animals each. Animals of first group received TC-NE in single IV dose 50 mg/kg b wt. via intravenous catheter. Fourteen days were allowed to make sure complete clearance of TC from their bodies, then the animals of the 1st group received TC-NE at the same dose (50 mg/kg b wt) orally through a mouth gag. The second group received single dose of TC-Powder 50 mg/kg b.wt, via intravenous catheter. Fourteen days later the second group received single oral dose of TC-Powder (50 mg/kg b wt.).
Blood samples were collected through ear vein catheter at the following time intervals; 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12 and 24 hours after administration. Blood samples were collected into clean sterile heparinized centrifuge tubes, then centrifuged at 3000×g for 15 min. A 2 ml of the plasma were harvested from each sample (Parasuraman, et al., 2010; Baby et al., 2017) and stored at -70 ◦C until analyzed within 1 month for determination of Tetracycline HCl concentration using HPLC.
HPLC determination of tetracycline in plasma samples
Sample preparation
The plasma samples were prepared guided by Chang et al. (1992) and Shalaby et al. (2011). The extraction was conducted using citrate buffer (pH4) plus trichloroacetic acid (80: 20%), followed by centrifugation 1000 g for 10 minutes then elution using 0.01 M methanolic oxalic acid.
Chromatographic conditions
Agilent HPLC 1260 series (5301 Stevens Creek Blvd Santa Clara, CA 95051 United States). Separation done using Agilent ZORBAX StableBond SB-C18 80Å 3.5µm, 4.6 x 75mm column. Injection was accomplished using a 20 µL loop injector and blunt-needle glass syringe (Hamilton Co., USA). Mobile phase consisted of A: 0.025 MKH2PO4 in H2O with pH 3 and B: CAN, gradient at 5% B to 60% B in 10 min then 60% B to 5% B in 2min, wavelength was 350 nm, flow rate was 1ml/min that performed at Temperate of 25oC.
Standard solutions and caliberation curves
The HPLC method was validated using ICH guidelines. Standards run before and after the samples and blank samples were included with each set. Six calibration curves constructed on six separate days to check the linearity of each calibration curve.
Standards and quality control QC stock solutions of tetracycline were prepared in phosphate buffer solution pH3: ACN (50:50%) to produce working stock solutions of 500, 100, 10, 1, 0.01 µg/mL. Then, stock solutions were stored at about 5°C and used to prepare plasma calibration standards. Samples for recovery, precision and accuracy were prepared by spiking control rabbits plasma in bulk at proper concentrations (0.01, 1, 10 and 15 µg/mL) and 100 µL volumes were aliquot into different tubes and stored as samples at −80°C until analysis. Calibration curves were obtained by plotting the peakarea ratio of tetracycline against the nominal concentration of calibration standards.
The Tetracycline concentrations used were 500, 100, 10, 1, 0.01 µg/mL. The results fitted to linear regression analysis without the introduction of weighing factors. Limit of detection (LOD) and limit of quantification (LOQ) calculated as mentioned by El Badawy et al. (2015) and to be 10 and 30 ng/ml, respectively.
Precision and accuracy
The intra-assay precision and accuracy estimated by analyzing three replicates containing tetracycline at three different QC levels i.e. 1, 10 and 15 µg/mL. The inter-assay precision determined by analyzing the three levels QC samples on three different runs. The criteria for acceptability of the data included accuracy NMT 2% RSD.
Extraction recovery
The recovery determined by comparing peak areas of spiked plasma extracts with those of un-extracted prepared in phosphate buffer: ACN (50:50%). The recovery value calculated at the various concentrations of tetracycline.
Pharmacokinetic modeling
Pharmacokinetic values calculated using PK solver program (Zhang et al., 2010) for each rabbit. Bioavailability (F) of tetracycline after oral injection calculated as a percentage using following standard equation: F%= (AUC0-inf.oral/ AUC0-inf.iv) X 100 (Toutain and Bousquet-Melou, 2004). The minimal effective concentration considered based on the in-vitro MIC for Staphylococcus aureus 6538 was 0.14 µg/mL in our earlier work on the same formulations (Saber et al., 2019a). The time that plasma tetracycline concentration exceeded 0.14 µg/mL (T> MIC) determined for each rabbit from the concentration-time relationship curve.
Statistical analysis
Data presented as mean ± SD. Selected pharmacokinetic values compared following IV and oral administration of both tetracycline formulations (TC-NE and TC- Powder) using repeated measure ANOVA, using procedure and an autoregressive (1) covariance matrix (SAS 9.3, SAS Inc, Cary, NC). A P value <0.05 was considered significant (*) while, P value <0.05 was considered highly significant (**).
RESULTS
Preparation of tetracycline nanoemulsion
Pseudoternary phase diagrams
The phase diagrams of 2 oils (IPM- and Mig) based systems showed the clear zones at the investigated three Cremophor RH40/PEG 400 (1:1, 1:2 and 2:1) mass ratios were graphically presented in (Figure 1). As noticed from the phase diagrams, there is a direct relation between the S/CoS ratio and the clear area. The largest clear zones accomplished with systems prepared using S/CoS mixture ratios of 1:1. Therefore, three formulae were chosen from this phase diagram and their compositions are listed in (Table 1). To ensure the safety of the ready formulae, the least amount (50%) of S/CoS mixture. Three formulae (F1, F2, and F3) selected containing Mig at concentrations of 10, 15 and 20%, 50% of S/CoS (1:1) and water at 40, 35 and 30 %.
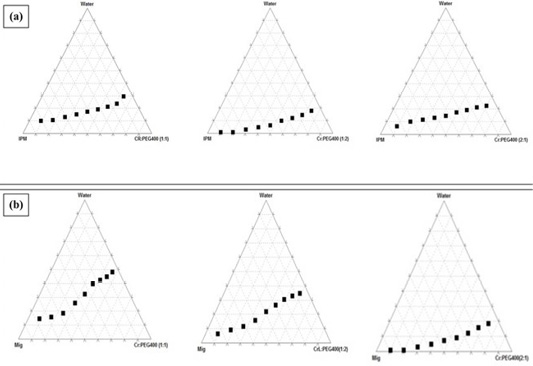
Figure 1: Pseudoternary phase diagrams of (a) IPM and (b) Mig based systems showing clear zones using Cremophor RH40/PEG 400 at indicated mass ratios.
Characterization of tetracycline-loaded nanoemulsion (TC-NE)
Thermodynamic stability studies
The selected systems were assessed through three stages; cycles, centrifugation, and finally cycle. The systems remained clear through the alternate cycles. Throughout the second stage; centrifugation, signs of creaming and phase separation was observed with F3. Upon freezing the systems at -21◦C, turbidity was detected, but then the systems became clear again on thawing.
Mean droplet size (MDS) and zeta potential (ZP) measurements
The mean droplet sizes (MDS) of the selected formulae were shown in (Table 1). The MDS of the selected formulae ranged from 32.33 ± 3.81 to 101.5 ± 9.86 nm. The PDI of all TC-NE were in the range of 0.11 ± 0.01: 0.41 ± 0.07 revealing narrow size distributions and good homogeneity (Table 1). The formulated TC-NE possessed ZP values ranged from -25.45±3.43 to -33.47±2.11 mV (Table 1).
Transmission electron microscopy
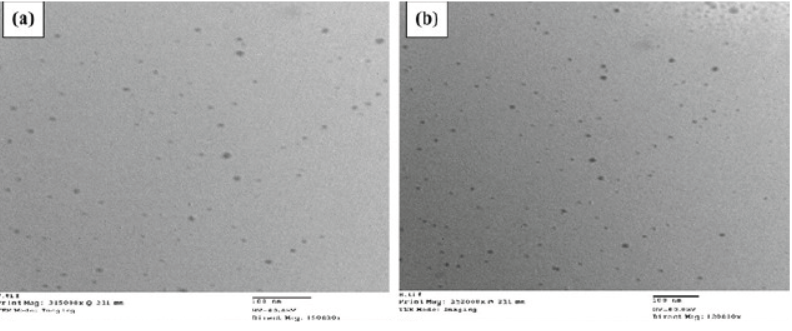
TEM micrography showed spherical globules with uniform droplet size distribution. Representative micrography of F1 and F2 was shown in Figure 2.
The globules of F1 (Figure 2a) and F2 (Figure 2b) seemed to have a comparable size to that calculated by photon correlation spectroscopy.
Pharmacokinetic analysis
In this study, there was no apparent clinical sign noticed in rabbits received a single dose (50 mg/kg) of TC-NE formula or TC-Powder in both IV and oral routes. Graphs of the tetracycline-time relationship in plasma after a single IV and oral administration of TC-NE and TC- Powder were presented in (Figures 3 and 4). The highest plasma tetracycline concentration was after 0.5 and 1 hour from administration and persisted for at least 7 hours and 11 hours after oral administration in TC-Powder and TC-NE groups, respectively. The prolonged persistence of tetracycline in rabbits plasma after IV and oral administration of TC-NE enhanced the time dependent activity of tetracycline. The sustained release suggestion of the TC-NE formula is indicated by the prolonged absorption half-life time t1/2ka (0.518 ± 0.091 h.) and distribution half-life time t1/2α (0.550 ± 0.090 h.) than that in TC- powder (t1/2ka 0.253 ± 0.023 h. and t1/2α 0.253 ± 0.023h.). In addition, the MRT was significantly prolonged (3.93 ± 0.47 hrs.) for the TC-NE formula than for TC- Powder (2.77 ± 0.29 hrs.). The prolonged t1/2ka, t1/2α and tmax for the TC-NE formula indicated the persistence of Tetracycline for prolonged time with higher AUC, which is reflected by the higher bioavailability of TC-NE (29.2 ± 2.3 %) than that for TC-powder (13.9 ± 0.8%) after oral administration.
The pharmacokinetics variables that describe the two compartment disposition of tetracycline following a single oral and IV administration of TC-Powder and TC-NE formulas presented in (Table 2).
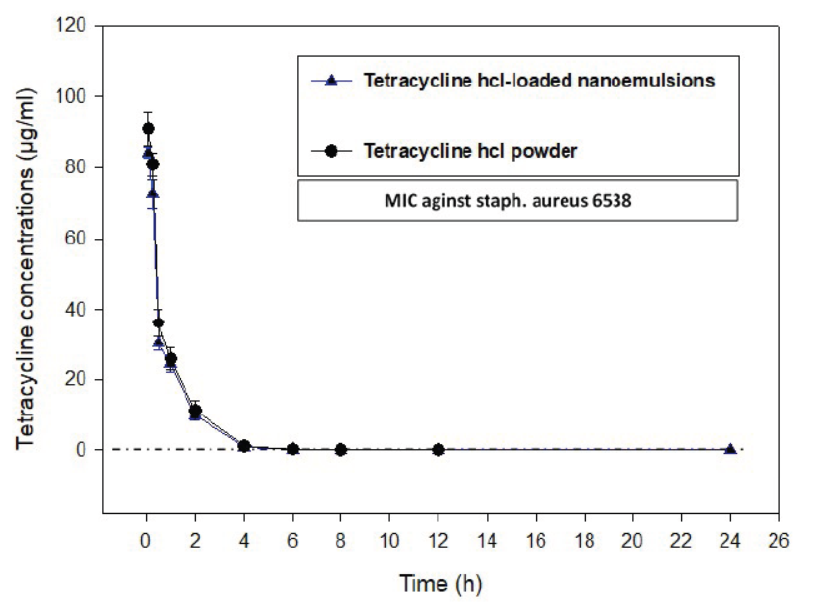
Figure 3: The time concentration relationship of Tetracycline hydrochloride (TC-Powder) or Tetracycline loaded nanoemulsion (TC-NE) after IV administration (50 mg/kg b.wt) in white new zealand rabbits. (Mean ± SD, N=8).
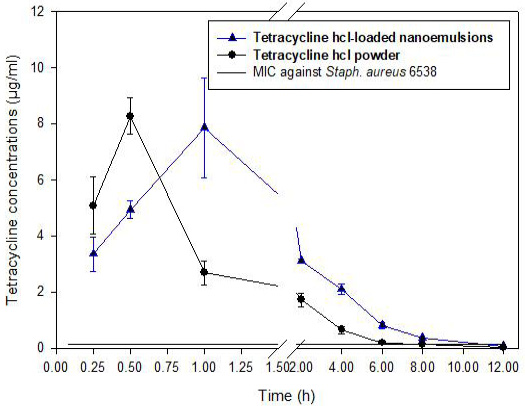
Figure 4: The time concentration relationship of Tetracycline hydrochloride (TC-Powder) or Tetracycline loaded nanoemulsion (TC-NE) after oral administration (50 mg/kg b.wt) in white new zealand rabbits. (Mean ± SD, N=8).
DISCUSSION
Six systems composed of one of the two oils; IPM (ester of a long-chain fatty acid) or MIG (triglycerides of medium-chain fatty acids), surfactants; Cremophor RH40 (non-ionic surfactant, HLB=14-16) and cosurfactant; PEG 400 by the water titration method, Oil/water (o/w) (Kommuru et al., 2001). PEG 400 as being a cosurfactant; reduced the critical packing values (CPP). Oil/water (o/w) nanoemulsions ready owing to the high HLB value of the surfactant of the surfactants (Shinoda et al., 1991).
Table 1: Mean droplet size (MDS), zeta potential (ZP) measurements and polydispersity index (PDI) of selected formulae.
| Formula | Composition | Mean droplet size (MDS) nm |
Zeta potential (ZP) mV |
Polydispersity index (PDI) | ||
| Miglyol | Cremophor RH40/PEG400 (1:1) | Water | ||||
| F1 | 10 | 50 | 40 | 32.33 ± 3.81 | -33.47 ± 2.11 | 0.11 ± 0.01 |
| F2 | 15 | 50 | 35 | 52.79 ± 4.38 | -28.90 ± 4.22 | 0.21 ± 0.00 |
| F3 | 20 | 50 | 30 | 101.5 ± 9.86 | -25.45 ± 3.43 | 0.41 ± 0.07 |
| Drug-free Nanoemulsion (NE) | 10 | 50 | 40 | 25.52 ± 3.37 | -31.56 ± 3.92 | 0.10 ± 0.00 |
Table 2: Tetracycline pharmacokinetic parameters in plasma after oral and IV administration of tetracycline HCl powder (TC-Powder) and tetracycline Hcl loaded nanoemulsion (TC-NE) (50 mg/kg b.wt) in male white new zealand rabbits. Tetracycline concentration was assessed using High Performance Liquid Chromatography (Mean ± SD, n=8). P value <0.05 was considered significant (*) while, P value <0.005 was considered highly significant (**).
| Parameter | Unit | Oral administration | IV administration | ||
| Tetracycline HCl powder (TC-Powder) |
Tetracycline loaded nanoemulsion (TC-NE) |
Tetracycline HCl powder (TC-Powder) |
Tetracycline loaded nanoemulsion (TC-NE) |
||
| k10 | 1/h | 1.49 ± 0.11 | 0.700 ± 0.102** | ||
| k12 | 1/h | 0.969 ± 0.208 | 0.379 ± 0.104** | 0.608 ± 0.241 | 0.795 ± 0.165 |
| k21 | 1/h | 0.391 ± 0.135 | 0.406 ± 0.335 | 1.09 ± 0.29 | 1.26 ± 0.16 |
|
t1/2α |
h | 0.265 ± 0.025 | 0.550 ± 0.090 ** | 0.289 ± 0.0446 | 0.247 ± 0.0289 |
|
t1/2β |
h | 3.33 ± 0.68 | 4.22 ± 1.66 | 1.20 ± 0.26 | 1.12 ± 0.09 |
|
t1/2ka |
h | 0.253 ± 0.023 | 0.518 ± 0.091** | ||
| CL | L.h/kg | 4.350 ± 0.2522 | 0.800 ± 0.1398** | 0.602 ± 0.0304 | 0.67 ± 0.026 |
|
Tmax |
h | 0.397 ± 0.033 | 0.869 ± 0.059** | ||
|
Cmax |
μg/ml | 6.78 ± 0.66 | 6.38 ± 0.91 | ||
|
AUC0-inf |
μg/ml.h | 11.5 ± 0.6 | 21.8 ± 2.08** | 74.8 ± 2.9 | 83.3 ± 4.2** |
| AUMC |
μg/ml.h2 |
32.0 ± 4.4 | 86.3 ± 17.4** | 99.3 ± 20.2 | 87.1 ± 4.2 |
| MRT | h | 2.77 ± 0.29 | 3.93 ± 0.47** | 1.19 ± 0.20 | 1.17 ± 0.06 |
| T> MIC | h | 7.1 ± 0.32 | 10.36 ± 0.64** | ||
| F | % | 13.9 ± 0.8 | 29.2 ± 2.3** | ||
| C0 | μg/ml | 111.7 ± 8.5 | 104.8 ± 2.7* | ||
|
Vdss |
L/kg | 0.71 ± 0.10 | 0.78 ± 0.06* | ||
K10: rate constant for absorption; k12: rate constant for compartment 1 to 2; k21: rate constant for compartment 2 to 1; t1/2α: apparent distribution half-life ; T1/2β: the apparent elimination half-life; t1/2ka: absorption half -life; tmax: time of occurrence of Cmax; Cmax: maximal plasma concentration; AUC0-: area under the plasma concentration–time curve from time 0 to last sample time; AUC0-inf : area under the plasma concentration–time curve from time 0 to infinity; AUMC: area under the moment curve; MRT: mean residence time from time 0 to infinity; F: bioavailability; C0: plasma concentration at time 0; Vdss volume of distribution at steady state.
There was direct relation between the S/CoS ratio and the clear zones showed in phase diagrams. The largest clear zones accomplished with Mig-based systems prepared using S/CoS mixture ratios of 1:1. This could be explained in light of better micelles formation in presence of higher S/CoS mixture ratios, which consequences in enhancing the solubilization capacity of the nanoemulsion (Kawakami et al., 2002). Widest clear zone were achieved with Mig-based system, Cremophor RH40/PEG 400 in ratio 1:1. To ensure the safety of the ready formulae, the least amount (50%) of S/CoS mixture was chosen (Khalil et al., 2015). Three formulae (F1, F2, and F3) were taken containing Mig at concentrations of 10, 15 and 20%. As mentioned by (Lawrence and Rees, 2000) nanoemulsion for IV administration, diluted with water without causing precipitation of the drug during use in clinic.
The observed signs of creaming and phase separation with F3 in the second stage of thermodynamic stability studies could be attributed to its high oil content where the surfactants failed to emulsin this amount of oil under further stress caused by the centrifugation process. Reported turbidity upon freezing at -21 ◦C, which disappeared on thawing is in line with those reported by Ammar et al. (2009) that attributed to coagulation of the internal phase at such low temperature. The small size of nanoemulsions could be justified by the cosurfactant molecules into the surfactants film that lowers the fluidity and surface viscosity of the interfacing film and decreases the radius of curvature of the droplets.Those forming transparent systems (Tenjarla, 1999). Regarding the size of the selected formulae, it was obvious that adding a higher amount of oil resulted in increasing the MDS. This is due to oil droplet of the nanoemulsion by increasing the oil amount (Yuan et al., 2006). Regarding PDI, the 0 value specify monodispersed particle population, while a value of 1 suggests highly polydispersed vesicles (Zeisig et al., 1996). The narrow PDI values of all TC-NE revealed narrow size distributions and good homogeneity.
A system considered stable when ZP possesses a value about ± 30mV; where the value ensures the electric repulsion between particles (Du et al., 2009). The formulated TC-NE ZP values ranged from -25.45 to -33.47 mV (Table 1) indicating that they have enough charges that would inhibit their aggregation (Silva et al., 2015; FDA, 2014).
The pharmacokinetics of tetracycline hydrochloride released from TC- NE (sustained release formula) was studied after a single IV and oral administration (50 mg/kg b. wt.) and compared to that of TC- Powder. Following IV administration, detected tetracycline concentration in plasma at 0.083 h was 91.03 ± 4.71 and 84.20 ± 1.60 µg/ml and persisted exceeding MIC for at least 12 hours in all rabbits treated with TC-Powder and 24 hours for TC-NE, respectively. These results supported by the results in rabbits (Saber et al., 2019a) whom reported persistence of TC-NE in rabbits plasma than TC-powder. The prolonged persistence of tetracycline in rabbits plasma after IV and oral administration of TC-NE enhanced the time dependent activity of tetracycline. Consistently, Neuschl (1991) reported CTC serum levels averaged 2.3 mg/l 3 h after a single oral dose of 20 mg/kg b.wt. in californian rabbits and declined to a mean value of 0.09 mg/l by 12 h, and to 0.08 mg/l by 24 h. Peak plasma concentrations of 3.6 mg/l were observed in rat 2 h after treatment, declining to 0.5 mg/l after 6 h. (Berté and Vandoni, 1962).
Following IV administration tetracycline had higher volume of distribution Vdss (0.78 ± 0.06 L/kg) in TC-NE than in TC- Powder (0.71 ± 0.10 L/kg) and clearance rate, CL (0.67 ± 0.026 L.h/kg) in TC-NE than in TC-Powder (0.602 ± 0.0304 L.h/kg). The former results showed enhancement of TC delivery by nanoemulsion system (Aboofazeli, 2010). Consistent results obtained by Saber et al. (2019a) and Percy and Black (1988) in male and female rabbits after IV administration 10 mg/kg. In contrast to results reported by Nielsen and Gyrd‐Hansen (1996) who measured disposition of TC in pigs received a dose of 10 mg/kg intravenously, TC was present in plasma up to 30 hours and volume of distribution was 1.2 L/kg body weight. The later variation may be attributed to species difference.
The higher volume of distribution and prolonged elimination supported by the significant higher AUC 0-inf (83.3 ± 4.2 μg/ml.h for TC-NE and 74.8 ± 2.9 μg/ml.h for TC-Powder. These results supported by the results obtained by Saber et al. (2019a) in rabbits; Percy and Black (1988) in male and female rabbits and disagree with Kniffen et al. (1989) and Agwuh and MacGowan (2006) in pigs.
After oral administration of TC-NE and TC-Powder (50 mg/kg b. wt) tetracycline detected at 0.25 h. and the lowest detected concentration was at least for 8 h for TC-Powder and about 11 hours TC-NE. Our results supported by results in rabbits (Saber et al. (2019a)), in rats (Berte and Vandoni, 1962), in female rats and male guinea-pigs (Eisner et al., 1953). Tetracycline is time dependent, this suggested that sustained release is critical for its efficacy. Following oral admistiration TC-NE formula had prolonged t> MIC of 10.36 ± 0.64 compared to 7.1 ± 0.32 h in TC-powder which is suspected with delayed distribution half-life time t1/2α of 0.550 ± 0.090 h than that in TC- powder 0.253 ± 0.023 h as well as the significantly prolonged MRT (3.93 ± 0.47 hrs.) for the TC-NE formula than for TC-Powder (2.77 ± 0.29 hrs.). These results supported the time dependent activity of tetracycline, increased bioavailability and might be positively reflected on the clinical activity of TC-NE in treatment of tetracycline sensitive bacteria.
Following oral administration, tetracycline was rapidly absorbed after administration of TC-Powder and TC-NE with significant longer absorption half-life t1/2ka for 0.518 ± 0.091 h. in TC-NE and t1/2ka 0.253 ± 0.023 h for TC-Powder. These results were in agreement with (Agwuh and MacGowan, 2006). Generally, after oral administration the reported significant short absorption half-life in TC-Powder than TC-NE t1/2α 0.550 ± 0.0902 h and t1/2β 4.215± 1.6613 h that indicated rapid absorption of the drug in both tested forms and routes were shorter than those reported in rabbits (2.0 h) (Percy and Black, 1988), chickens (2.8 h) (Anadon et al. 1985) and pigs (2.8 h) (Kniffen et al., 1989). Consequently, significant higher bioavailability reported for TC-NE 29.182 ± 2.2784 % than TC-Powder 13.858 ± 0.7505% in treated rabbits.
The significant higher AUC0-inf (21.837 ± 2.0784 μg/ml.h) and prolonged MRT (3.926 ± 0.4712 h.) for TC-NE than for TC-Powder; AUC0-inf (11.526 ± 0.6415 μg/ml.h) and MRT (2.771 ± 0.2932 h.), which reflected by the higher bioavailability of TC-NE (29.2 ± 2.3 %) than that for TC-powder (13.9 ± 0.8%) after oral administration that supported the sustained release hypothesis and time dependent activity of tetracycline. These results were in accordance with; Tamilvanan (2004); Tamilvanan et al. (2005); Li et al. (2008) and Aboofazeli (2010). Nano-carriers improves the pharmacokinetics activity of the carried drug (Shafiq et al., 2007; Han et al., 2009; Kotta et al., 2013; Sharma et al., 2014; Yen et al., 2018). Moreover, the recorded time above MIC of 0.14µg/ml against Staphylococcus aureus 6538 (Saber et al., 2019a), were Longer in TC-NE than TC-Powder group following both oral and IV administration.
CONCLUSIONS
The formulated Tetracycline loaded nanoemulsion formulation had improved oral bioavailability and prolonged the blood concentration time than TC-Powder. Further clinical studies are required to prove dosage that supports clinical efficiency.
ACKNOWLEDGMENTS
TC-NE was prepared in Dept. of Pharmaceutical, faculty of Pharmacy, Cairo University. The experimental work of the research was facilitated and completed in department of pharmacology faculty of veterinary medicine, Cairo University.
ETHICAL APPROVAL
The research plan was approved from Cairo University institutional animal care and use committee (CU-IACUC) with approval number CU-II-F-99-18.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interests to declare regarding the publication of this paper. Also, the authors declare that the work was self-funded.
AUTHOR CONTRIBUTION
AMA, SAE and MMA designed and planned this study. WHA prepared and characterized TC-NE. SAE, MMA, MSS and OAF performed experimental work, samples collection and all laboratory tests. All authors shared manuscript writing, drafted, revised the manuscript and approved the final manuscript.
REFERENCES