Advances in Animal and Veterinary Sciences
Research Article
Assessment of Pesticide Residues in Edible and Unhatched Chicken Eggs in Sharkia Province, Egypt
Eman Nabil Abdelfatah1*, Ehsan Hashim Abu-Zeid2
1Food Control Department, Faculty of Veterinary Medicine, Zagazig Univeristy, Zagazig, Egypt; 2Forensic Medicine and Toxicology Department, Faculty of Veterinary Medicine, Zagazig Univeristy, Zagazig, 44511, Egypt.
Abstract | This work is proposed to investigate pesticide residues in chicken eggs in El-Sharkia Province, Egypt. Analysis of pesticide residues was performed on 120 egg samples included 80 random edible egg samples (40 farm and 40 home produced hen eggs) and other 40 unhatched eggs. Edible eggs were collected from different stores, places and supermarkets. unhatched incubated eggs were collected from different hatcheries during the winter season. Organochlorine pesticides (OCPs) and organophophorous insecticides (OPIs) residue analysis of samples was done using Agilent Gas chromatograph (GC) Model 6890 N. The results revealed presence of OPIs in higher concentration in home produced eggs than that in farm produced eggs, while OCPs present in farm produced egg in higher levels than that in home produced one. Unlike other studies we have not found higher levels of exposure for most pesticides in those consumers choose home produced eggs, and even farm produced eggs. Regarding unhatched eggs dimethioate recorded the highest concentration which exceeded Maximum Residual Limits (MRLs) of (0.05) mg/kg in all the examined samples. None of the other detected pesticide residues exceeded (MRLs). It was concluded that the levels of pesticide residues in edible eggs either home or farm produced eggs should not exceed Accepted Daily Intake (ADI) established by the World Health Organization (WHO) Joint Meeting on Pesticide Residues Report (JMPR). The study suggests the need for further investigations to determine toxic mechanisms by which dimethoate distress chick emberyos at different developmental stages and result in hatching failure.
Keywords | Organochlorine compounds, Organophosphorous cmpounds, Gas chromatography, Edible eggs, unhatched eggs
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | October 29, 2015; Revised | November 15, 2015; Accepted | November 17, 2015; Published | December 02, 2015
*Correspondence | Eman Nabil Abdelfatah, Zagazig Univeristy, Zagazig, Egypt; Email: [email protected]
Citation | Abdelfatah EN, Abu-Zeid EH (2016). Assessment of pesticide residues in edible and unhatched chicken eggs in Sharkia Province, Egypt. Adv. Anim. Vet. Sci. 4(1): 25-34.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016.4.1.25.34
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Abdelfatah aand Abu-Zeid. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
A pesticide is a general word which refers to chemicals used for controlling a wide variety of living organisms, but the use of pesticides contributes to accumulate persistent organic pollutants (POPs) in the final products which is considered as important (Renwick, 2002). Over the utmost 30 years a number of POPs have been highlighted as a reason for concern as these substances are toxic chemicals and resistant to degradation in the environment and biota (Boada et al., 2012). Consumption of contaminated food especially of animal origin contributes more than 90% of the full exposure and so food consumption is considered the most significant route of human to these contaminants (Almeida-Gonzalez et al., 2012).
Fresh eggs are considered one of the most important and nutritious food in the daily diet, as they are easily digested and can provide human body with a significant portion of the nutrients required daily for the proper growth and maintenance (Abdulkhaliq et al., 2012). Moreover, eggs are included in several food products for various functions (Leggli et al., 2010). Independently of the production method, the transfer of POPs into hen eggs, especially into yolk as their bound to egg fat content, has been widely documented for OCPs and other compounds as polychlorobiphenyls (Fournier et al., 2012). For this reason, securing the quality of hen eggs is an important issue for human food safety.
Chronic exposure to POPs was found to interfere with the activity of endogenous hormones in humans, including T4, T3 and TSH (Langer, 2010). Some evidence suggests that OCPs might contribute to increase the risk of exocrine pancreatic cancer (Soliman et al., 2006). It is also worth mentioning that chronic exposure to OPIs contribute to produce developmental neurotoxicity (Colborn, 2006).
Unhatched eggs are a common phenomenon in domestic birds. Up to date, several studies have attempted to determine the cause of hatching failure in domestic birds (Bakst et al., 1998; Christensen, 2001; Sellier et al., 2005). Indeed, some recent studies (Geiger et al., 2010; Mineau and Whiteside, 2013; Hallmann et al., 2014; Gibbons et al., 2015) signifying that pesticide use is increasingly recognized as a key factor for explaining population declines of farmland animals, besides it would be more implicated in this ongoing reduction than formerly reported (European Common Bird Monitoring Schemes, 2014). Pesticide exposure of females during egg formation, especially yolk formation, may result in the contamination of eggs, as a maternal effect (Sauveur and de Reviers, 1988). Feeding habits of birds seem to take on an important role in the occurrence of pesticides in their eggs (Fasola et al., 1987). The presence of pesticides in the developing avian egg has been shown to result in decreased hatchability (Indyk, 1999), increased mortality (Pourmirza, 2000) and various congenital anomalies (Sahu and Ghatak, 2002). Accordingly, we aimed in this study to investigate the extent of edible egg contamination with different pesticide residues in our area, and to establish whether relative differences in these pesticide residue concentrations occurred within the different forms (farm and home produced) of egg production or not, taking in considerations the level of human exposure to these contaminants through consumption of eggs from both types that were chosen. Additionally, it is known that hatching success are measures of reproductive performance in many species, so samples of unhatched eggs are utilized to define the relationship between different pesticide residues concentration and hatchability as well on the success of the poultry industry.
MATERIALS AND METHODS
Collection of Samples
A total of 120 egg samples included 80 random edible egg samples (40 farm produced hen eggs and 40 home produced hen eggs) were gathered from different stores, places and supermarkets and additionally 40 unhatched incubated eggs were gathered from different hatcheries. Collection of samples occurred during the winter season (from October, 2014 till February, 2015) and then samples were transported to the laboratory as soon as possible, the contents were broken up and poured in chemical cleaned glass containers then kept frozen at -20 ˚C until analysis.
Preparation of Samples
Samples of eggs weighing about 10g were placed in a centrifuge tube then 2g of NaCl, 19.3 ml of acetonitrile (MeCN) were added to them, samples were blended for 1 min. using a probe blender and centrifuged for 10 min (Lehotay et al., 2001), 10µL (5 mg of egg equivalent) of the extract were subjected to analysis.
Gas Chromatographic Analysis
The OCPs residues were determined by analysis of samples using Agilent Gas chromatograph (GC) Model 6890 N equipped with an Ni63 -electron capture detector. GC conditions: PAS 5 capillary column (30m length x 0.32 mm internal diameter (i.d) x 0.25µm film thickness), carrier gas: N2 at a flow rate of 4 mL/min; injector and detector temperature were 300°C and 320°C, respectively. The initial column temperature was initial oven temperature, 160°C for 2 min, raised at 3°C/min. and then held at 260°C for 15 min.
OPIs residues were determined by analysis of samples using Agilent GC Model 6890 N equipped with a flame photometric detector (FDP) with phosphorous filter. A fused silica capillary PADS-1701), column containing 14% cyanopropilsyloxane as stationary phase (30m long x 0.25µm film thickness), was used for the separation in the GC. GC operating conditions were as follow: injector and detector temperature were 240°C and 250°C, initial oven temperature, 160°C for 2min, raised at 5°C/min. and then held at 240°C for 2min. The carrier gas was nitrogen at 3ml/min. and hydrogen and air were used for the combustion at 75 and 100 mL/min, respectively.
Statistical Analysis
All the data were analyzed using the Statistical Package for Social Sciences version 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS AND DISCUSSION
In Egypt, the use of OCPs has been officially prohibited from use by the Ministry of Agriculture for about 30 years. Despite limitations and prohibitions on the use of many OCPs, they continue to remain in the environment (Abd Al-Rahman, 2010).
Regarding to the edible eggs, OCPs represented by numerous compounds beginning with heptachlor, it was detected in 75% of farm produced eggs, while in home produced eggs it was present in only 50% (Table 1). Heptachlor was used as an insecticide to control household insects and pests and is utilized as a soil treatment for controlling ants, wireworms (Worthing, 1991). Heptachlor epoxide is not commercially available, but is an oxidation product of heptachlor (IARC, 1979). Ahmed et al. (2010) recorded it with a higher percentage at 5% of examining samples, while Luzardo et al. (2013) did not find it at all. Oral exposure to heptachlor has resulted in some neurological effects, including convulsions, muscle tremors, salivation, dizziness and irritability (ATSDR, 1993). Heptachlor was classified as a Group B2 carcinogen to human (EPA, 1999).
Aldrin was present in 25% of both home produced and farm produced eggs with a nearly equal mean (Table 1). Ahmed et al. (2010) and Luzardo et al. (2013) did not find it in any of their examined eggs. Aldrin is a synthetic OCPs, that was used as broad-spectrum soil insecticide to control several insect vectors of disease (EPA, 2003). It is worth mentioning that it cause severe health problems through ingestion or inhalation as nervous system effects, including irritability, convulsions and nausea in many people with extreme exposure to this insecticides (ATSDR, 2002a).
Methoxychlor was detected in farm produced eggs, while it wasn’t detected in home produce eggs (Table 1). Luzardo et al. (2013) did not get it in both types of examining eggs. It was practiced as an insecticide effective against a broad range of pests (ATSDR, 1994). Exposure to it above the maximum contaminant level cause damage to liver, kidney, and heart, diarrhea, central nervous depression. It is not classifiable as a carcinogen (EPA, 2006).
Deltamethrin was not detected in home produced eggs, but present in only 25% of farm produced eggs (Table 1). It is a pyrethroid insecticide that was used to control numerous insect pests of field crops (Thomson, 1992). Oral ingestion of this chemical caused vomiting, epigastric pain, nausea and coarse muscular fasciculations. Coma was occurred within 15-20 minutes when exposed to higher doses of 100-250 mg/kg. Deltamethrin has no teratogenic activity nor mutagenic one (Hayes, 1990).
Concerning DDT and its metabolites, Table 1 made these clear that ṕṕ-DDD and ṕṕ-DDT the metabolites of DDTs were found in our examined samples. ṕṕ-DDD was found in higher percentages than ṕṕ-DDT in both home and farm produced eggs as it was present in 50% of both types while ṕṕ-DDT was present in only 25% of both types also. Ahmed et al. (2010) did not find ṕṕ-DDD in the examined egg samples while ṕṕ-DDT was found with higher % (0.142 mg/kg) than that recorded in our results. On the other hand Luzardo et al. (2013) failed to detect ṕṕ-DDD in home produced eggs and found it in 8.33% of farm one by lower concentration, while he found ṕṕ-DDT in 25% of both home produced and farm produced eggs with a lower concentration than our study, while Tao et al. (2009) found both ṕṕ-DDT and ṕṕ-DDD with higher concentration as they recorded 0.394±0.271 µg /gm. and 0.711±0.658 µg /gm for each respectively.
DDT is one of OCPs which was used to control mosquito-borne malaria, but it has been replaced by other insecticides which are less persistent (Royal Society of Chemistry, 1991). It is believed to be a human carcinogen, although the majority of studies suggest it is not directly genotoxic. It got clear that ṕṕ-DDT has no estrogenic or androgenic activity (Cohn et al., 2007). It has harmful effects on reproduction and on the adrenal gland after short term exposure to DDT and metabolites in food (ATSDR, 2002b).
As regards to herbicides Metrobuzin, it was detected in only 25% farm produced eggs, while it failed to be detected in home produce eggs (Table 1). It is a herbicide used both pre and post emergence in crops and has been set up to contaminate groundwater (Undabeytia et al., 2011). It is slightly to moderately toxic to humans by oral, skin or inhalation routes of exposure (EPA, 1988).
Pertaining to OPIs, they were used worldwide in agriculture and urban areas to control a wide range of insects due to high efficacy and rapid environmental degradation (Mirajkar, 2005), but they are reported to adversely affect reproductive functions in males particularly semen quality and the hormone balance (Salazar-Arredondo et al., 2008). Any effect on development and growth during the early life stage, threatens the health of the population (González-Doncel, 2004). Diazinon and dimethioate only the two compounds that were detected in our study subject.
For diazinon, it was detected in 50% of both home produced and farm produced eggs with nearly equal mean (Table 1). Because of its alkylating features such compound is potential mutagens (Erturk, 1990). These alkylating agents can alkylate all the bases in DNA causing mutations (Velazquez et al., 1990).
For dimethioate residues, results shown in Table 1, cleared that it was detected in 50% of both home produced and farm produced eggs, but it was detected by higher value in home produced eggs than that farm produced eggs. It is moderately toxic by many routes like ingestion, dermal absorption and inhalation (Gallo and Lawryk, 1991). It is a possibly human teratogen, mutagen and carcinogen (Hallenbeck, 1985).
In the existing study, the obtained results revealed that OPIs present in higher concentration in home produced eggs than that in farm produced eggs, while OCPs present in farm produced egg in higher percentages than that in home produced one. Van Overmeire et al. (2006) and Windal et al. (2009) have reported that the eggs that are
Table 1: Levels of pesticide residues detected in edible eggs (from two different production methods) and non-hatched eggs samples
|
Samples |
|||||||||
|
Component |
Home produced egg (n* = 40) |
Farm produced egg (n = 40) |
Non-hatched egg (n = 40) |
||||||
|
Frequency (%) |
Range (µg/gm) |
Mean±SE (µg/gm) |
Frequency (%) |
Range (µg/gm) |
Mean±SE (µg/gm) |
Frequency (%) |
Range (µg/gm) |
Mean±SE**(µg/gm) |
|
|
Ethoprophs |
ND*** |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Phorate |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Diazinon |
20(50%) |
0.19-0.26 |
0.224± 0.006 |
20(50%) |
0.19-0.25 |
0.22± 0.005 |
20 (50%) |
0.13-0.14 |
0.136± 0.001 |
|
Dimethioate |
20(50%) |
0.39-0.69 |
0.542± 0.028 |
20(50%) |
0.30-0.48 |
0.395±0.013 |
30 (75%) |
0.43-1 |
0.637±0.0383 |
|
Pirimiphos-methyl |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Chlorpyriphos |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Fenitrothion |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Quinelphos |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Profenophos |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Fenamiphos |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Ethion |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Triazophos |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
α-BHC |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
HCB |
ND |
ND |
ND |
ND |
ND |
ND |
10 (25 %) |
0.0001-0.0005 |
0.0003±0.00006 |
|
γ – BHC |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
β – BHC |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Heptachlor |
ND |
ND |
ND |
ND |
ND |
ND |
10 (25 %) |
0.0001-0.001 |
0.0006±0.000011 |
|
Aldrin |
10(25%) |
0.0001-0.0005 |
0.0003±0.00005 |
10(25%) |
0.0001-0.0004 |
0.0003±0.00004 |
10 (25%) |
0.0001-0.0008 |
0.0005±0.00009 |
|
Heptachlore epoxide |
20(50%) |
0.0004-0.004 |
0.002±0.00039 |
30(75%) |
0.0007-0.0008 |
0.0008±0.00001 |
30 (75%) |
0.001-0.005 |
0.0027±0.00025 |
|
γ – chlordane |
ND |
ND |
ND |
ND |
10 (25%) |
0.0001-0.001 |
0.004±0.00047 |
||
|
ṕṕ -DDE |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Endrin |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
ṕṕ- DDD |
20(50%) |
0.020-0.040 |
0.0318±0.0014 |
20(50%) |
0.008-0.09 |
0.0366±0.0069 |
20 (50%) |
0.006-0.009 |
0.0074±0.00022 |
|
ṕṕ -DDT |
10(25%) |
0.0001-0.002 |
0.0008±0.00004 |
10(25%) |
0.0007-0.002 |
0.0011±0.00016 |
10 (25%) |
0.002-0.006 |
0.004±0.00047 |
|
Methoxychlor |
ND |
ND |
ND |
10(25%) |
0.001-0.003 |
0.0021±0.00028 |
ND |
ND |
ND |
|
Meothrin |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Fenpropathrin |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Lambdacyhalothrin |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Permithrin |
ND |
ND |
ND |
ND |
ND |
ND |
10 (25 %) |
0.004-0.02 |
0.0088±0.00205 |
|
Cypermithrin |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Es-fenvelarate |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Lufenuron |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
Metrobuzin |
ND |
ND |
ND |
10(25%) |
0.002-0.04 |
0.0117±0.00476 |
ND |
ND |
ND |
|
Deltamethrin |
ND |
ND |
ND |
10(25%) |
0.1-1.34 |
0.788±0.14688 |
10 (25%) |
0.0004-0.0009 |
0.0006±0.00006 |
|
Dimethomorph |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
*n= number of samples; **SE=Standard Error; ***ND =Not Detected
Table 2: Comparison of Acceptable Daily Intake (ADI*) values of detected pesticides with the calculated daily intake from edible eggs
|
Pesticide |
ADI mg/ kg person (ppm) |
ADI/70 kg b wt |
Mean pesticides in total home produced egg samples (ppm) |
Calculated average daily intake of pesticides from consuming 100g/egg/day ( j) |
Mean pesticides in total farm produced egg samples (ppm) |
Calculated average daily intake of pesticides from consuming 100g/egg/day( j) |
||
|
mg/d/person |
% |
mg/d/person |
% |
|||||
|
Diazinon (a) |
0.002 |
0.14 |
0.224 |
0.0224 |
16% |
0.220 |
0.0220 |
15.71% |
|
Dimethioate (b) |
0.002 |
0.14 |
0.542 |
0.0542 |
38.7% |
0.395 |
0.0395 |
28.21% |
|
Heptachlore (c) |
0.0001 |
0.007 |
ND** |
ND |
ND |
ND |
ND |
ND |
|
Heptachlore epoxide (c) |
0.0001 |
0.007 |
0.0022 |
0.00022 |
3.1% |
0.0008 |
0.00008 |
1.14% |
|
Aldrin (c) |
0.0001 |
0.007 |
0.0003 |
0.00003 |
0.43% |
0.0003 |
0.00003 |
0.43% |
|
γ-chlordane(c) |
0.0005 |
0.035 |
ND |
ND |
ND |
ND |
ND |
ND |
|
ṕṕ-DDD (d) |
0.01 |
0.7 |
0.0318 |
0.00318 |
4.5% |
0.0366 |
0.00366 |
5.23% |
|
ṕṕ-DDT (d) |
0.01 |
0.7 |
0.0008 |
0.00008 |
0.11% |
0.0011 |
0.00011 |
0.18% |
|
Deltamethrin(e) |
0.05 |
3.5 |
ND |
ND |
ND |
0.788 |
0.0788 |
2.25% |
|
Permethrin (f) |
0.05 |
3.5 |
ND |
ND |
ND |
ND |
ND |
ND |
|
HCB (g) |
0.00006 |
0.0042 |
ND |
ND |
ND |
ND |
ND |
ND |
|
Methoxychlor(h) |
0.1 |
7 |
ND |
ND |
ND |
0.0021 |
0.00021 |
0.003% |
|
Metrobuzin (i) |
0.013 |
0.91 |
ND |
ND |
ND |
0.0117 |
0.00117 |
0.13 |
a= JMPR(2001); b=JMPR(2003); c=JMPR(1995); d=JMPR(2001); e=JMPR(2002); f=JMPR(1999); g=JMPR(1973); h= JMPR(1977); i=EFSA(2006); j=Daily consumption for adult person according to Nutritional Institute, Egypt (1996 and 2006); *ADI: Accepted Daily Intake; **ND: Not Detected
produced from hens that have outdoor access (free-range, free-run, home-produced, and organic) usually exhibit higher levels of OCPs than those produced from caged hens. On the other hand Rawn et al. (2012) and Luzardo et al. (2013) have found similar results in the concentration and frequency of detected OCPs, with only small differences among examined groups. These higher concentrations of OCPs in farm produced eggs may be due to eating soil and soil’s creatures (worms and other insects) in farms that do not use barn floor, as they are more exposed to environmental pollutants than those are caged or in outdoor home produced with barn floor, where the possibility of eating soil does not exist (Van Overmeire et al., 2006; Windal et al., 2009).
Table 2 showed that the egg-related calculated average daily intake of pesticides for people living in Sharkia Province is extremely lower than the ADI established by the WHO for these contaminants (JMPR) and the level of exposure of this population through this food is lower than estimates recently published for other populations (Darnerud et al., 2006; Van Overmeire et al., 2006; Polder et al., 2010; Luzardo et al., 2013). Unlike other studies we have not found higher levels of exposure for most pesticides in those consumers that choose home produced eggs, and even farm produced eggs showed a tendency of lower intake levels. Dimethioate was found as the higher calculated average daily intake % of pesticides in both home produced and farm produced eggs as it recorded 38.7% in home produced eggs, while it was only 28.21% in farm produced one. But it is still lower than the ADI established by the WHO for these contaminants (JMPR, 2003). On the other hand ṕṕ-DDT was established as the lowest calculated average daily intake % of pesticides in home produced eggs as it was 0.11%, while methoxychlore was the lowest in farm produced one as it recorded only 0.003%.
In respect to pesticide residues in unhatched eggs, Table 1 cleared that heptachlor was detected only in the non-hatched eggs as it failed to be detected in both kinds of edible eggs in 25% only of examined samples. On the other hand, heptachlor epoxide was found at higher level compared to heptachlor as it detected in 75% of examined non-hatched eggs. None of the detected results exceeded the MRLs of 0.05 mg /kg for heptachlor (sum of heptachlor and heptachlor epoxide) (FAO/WHO, 2006).
Aldrin was detected in 10 (25%) of the analyzed unhatched eggs and its MRL of 0.2 mg /kg (FAO/WHO, 2006) was not exceeded in these samples. For γ- chlordane, it was recorded in 25% of examined unhatched egg samples and none of the contaminated eggs exceeded MRL of 0.02 mg/kg for chlordane (sum of cis- and trans- isomers and of oxychlordane, expressed as chlordane) (2002/32/EC). Little data are available on effect of aldrin on hatchability as chlordane have documented
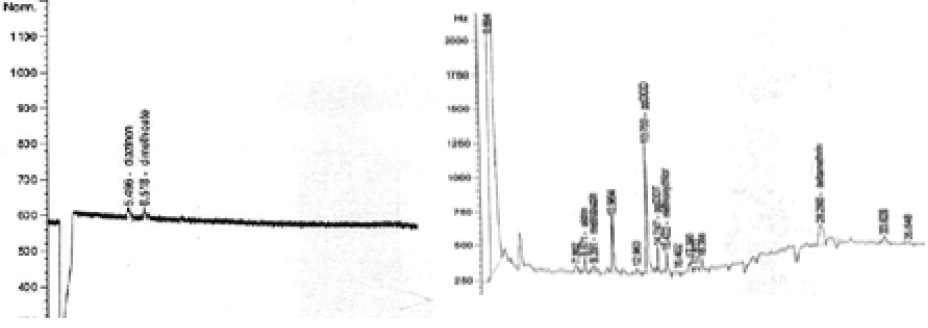
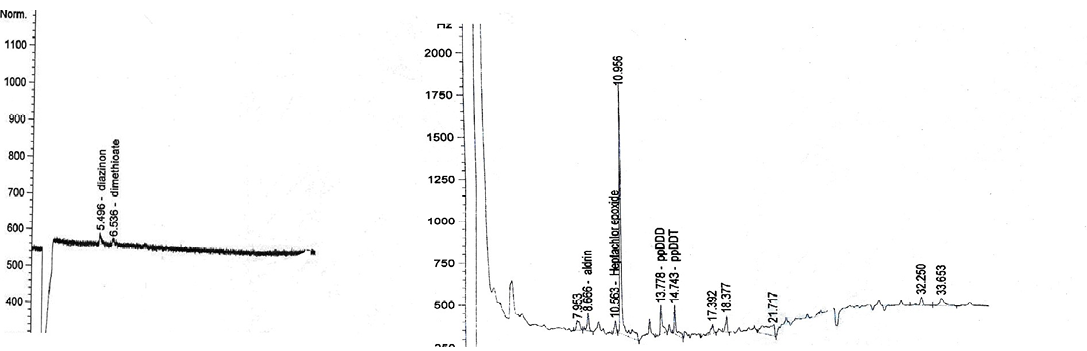
diazinon (5.496min); dimethioate (6.518min); aldrin (8.671min); metrobuzin (9.391min); ppDDD (13.769min); ppDDT (14.747min); methoxychlor (15.422min); deltamethrin (28.290min)
diazinon (5.496min); dimethioate (6.536min); aldrin (8.666min); heptachlor epoxide (10.563min); ppDDD (13.778min); ppDDT (14.743min)
effects on reproduction in birds (Nisbet, 1975).
Regarding to fungicide hexachorobenzene (HCB) has been detected in only unhatched eggs in ten samples of the examined unhatched eggs. None of the contaminated eggs exceeded MRL of 0.01 mg/kg for HCB (2002/32/EC). Avrahami and Steele, (1972) mentioned that feeding of up to 100 p.p.m. It is worth mentioning that HCB in the diet had no detrimental effects on egg fertility or hatchability. Therefore, no negative effect of HCB on hatchability of eggs has been previously reported (Wiemeyer, 1996).
Permethrin results cleared that none of the contaminated eggs exceeded MRL of 0.05 mg/kg for permethrin (EEC. No. 2377/90) as it was detected in 25% of the examined non-hatched eggs. Anwar et al. (2004) reported that chick embryo treated with various concentrations (25, 50, 100 and 200 ppm) of permethrin insecticide resulted in altered biochemical components and liver enzymes level.
Deltamethrin was detected in 25% of examined unhatched eggs. The results recorded didnot exceed MRLs of 0.02 mg/kg for deltamethrin (FAO/WHO, 2004).
Concerning DDT and its metabolites in unhatched eggs, the ṕṕ-DDT was detected in 10 (25%) of non hatched egg, while ṕṕ-DDD was detected in 20 (50%) which recorded higher concentration than ṕṕ-DDT. None of examined eggs samples, contaminated with either ṕṕ-DDT or ṕṕ -DDD was higher in concentration than MRL for DDT (sum of ṕṕ-DDT, oṕ -DDT, ṕṕ-DDE and ṕṕ-DDD) of 0.1 mg /kg (FAO/WHO, 2006). DDT and its derivatives are most frequently cited as a cause of reproductive failures in avian species. Rubin et al. (1947) observed that DDT impaired hatchability of chicken eggs when its concentration in the diet reaches (620 ppm). Smith et al. (1969) “found 400 ppm ṕṕ-DDT in feed significantly reduced fertility and hatchability of egg from Japanese quail (Coturnix coturnix japonica)”. Some studies reported
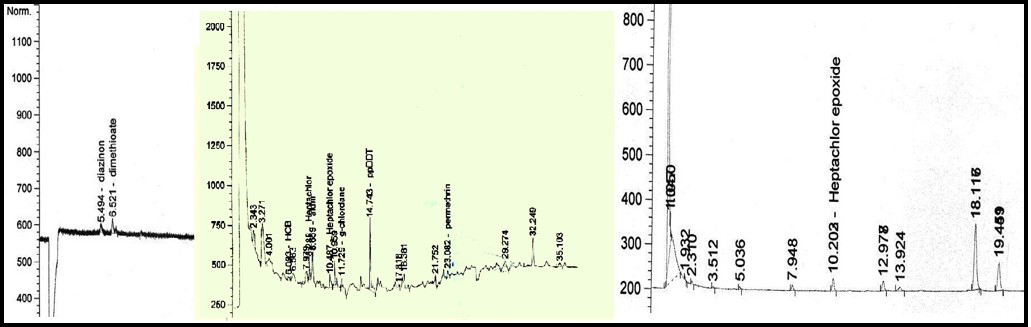
diazinon (5.494min; dimethioate (6.521min); HCB (6.093min); heptachlor (8.288min); aldrin (8.659min); heptachlor epoxide (10.487min); g-chlordane (11.729min); ppDDT (14.743min), permethrin (23.082min); heptachlor epoxide (10.203min); deltamethrin (28.551min)
significantly decreased fertility and hatchability (Wilson et al., 1973; Vangilder and Peterle 1980), while others do not document significant effects (Scott et al., 1975; Shellenberger, 1978). Exposure to DDT/DDD/DDE is clearly linked with the decreased embryonic survival rate (Keith and Mitchell, 1993).
With regard to diazinon, it was detected in 20 (50%) of examined unhatched eggs, but all the recorded results were less than MRLs of 0.2 mg /kg for diazinon (FAO/WHO, 1999), on the subject of the effect of diazinon on chicken egg hatchability, it is observed that feeding of diazinon containing rations for a period of 10 weeks to White Leghorn pullets resulted in a significant decrease of hatchability (Sauter and Steele, 1972).
Dimethioate residues were detected in 75% of the examined unhatched eggs. Thus dimethioate exceeded MRLs of 0.05 mg/kg (FAO/WHO, 2003). Dimethoate detected in the non-hatched eggs may result in embryo mortality as reported by (Budai et al., 2003) who reported an increase in rate of emberyonic mortality in incubated chicken embryos exposed to 0.1% of (38%) dimethoate containing insecticide formulations.
In conclusion, the level of contamination of edible eggs by the recorded pesticides in this study in all subjects, were extremely low, unlike the observations in other studies and none of the samples exceeded ADI established by the WHO for these contaminants (JMPR) and we could consider that the contribution of eggs to the total daily intake for inhabitants of the Sharkia province is negligible. Furtherrmore, for those of unhatched eggs, pesticide residues detected in our area does not exceed MRL for the recorded pesticides except for dimetheoate which exceed MRL in most of examined eggs, thus further experimental toxicological studies is required to determine dimethoate supposed toxic mechanism by which it affect fertile eggs and developing chick embryos.
AUTHOR’S CONTRIBUTION
Ehsan Hashim Abu-Zeid carried out the experimental trial, performed the statistics. Eman Nabil Abdelfatah drafted the manuscript. Both authors revised the manuscript and participated in its design, coordination, proof reading and approved the final manuscript.
ACKNOWLEDGEMENT
All the authors of the manuscript, thank and acknowledge their respective Universities and Institutes.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
REFRENCES