Dose Dependent Effect of Kisspeptin-10 Administration on Spermatogenesis and Hypothalamic Pituitary Gonadal Axis in Prepubertal Rats
Dose Dependent Effect of Kisspeptin-10 Administration on Spermatogenesis and Hypothalamic Pituitary Gonadal Axis in Prepubertal Rats
Faiqah Ramzan1,2,*, Irfan Zia Qureshi2 and Muhammad Haris Ramzan3
1Gomal Center of Biochemistry and Biotechnology, Gomal University, Dera Ismail Khan 29050, Pakistan
2Animal and Human Physiology Laboratory, Department of Animal Sciences, Faculty of Biological Sciences, Quaid-i-Azam University 45320, Islamabad
3Department of Physiology, Khyber Medical University Institute of Medical Sciences (KMU-IMS), Kohat 26000
ABSTRACT
Discovery of kisspeptin and its receptor has enhanced our understanding of the neurobiological mechanisms that govern the pituitary-gonadal-axis. However, in sexually immature male mammals, the maturation of gonads is not clear yet. The current study tested the hypothesis that continuous kisspeptin administration suppresses gonadotropin and testosterone release and spermatogenesis. Intraperitoneal kisspeptin administration was carried out in 05 weeks old prepubertal male Sprague Dawley rats, twice daily for 12 days, at three different dosage regimens: 10ρg, 1ηg and 1µg compared to control (saline-treated) rats. Effects on pituitary gonadotropins [Follicle Stimulating Hormone (FSH) and Luteinizing Hormone (LH)], secretion of testosterone and spermatogenesis were evaluated on alternate day for one spermatogenic cycle. Major techniques applied were; radioimmunoassay, light microscopy and stereology. Histological study of spermatogenesis was done at stage VII of spermatogenic cycle. After the treatment, in all groups, no alterations were observed in the levels of plasma FSH. The concentrations of testosterone and LH were found to be significantly reduced in kisspeptin treated groups of 1µg dose (p<0.01) and 1ηg dose (p<0.05) whereas the dose of 10ρg had shown no significant change. Kisspeptin doses of 1ηg and 1µg lead to significant reduction in quantity of elongated spermatids and daily sperm production (p<0.05; p<0.001), step 7 spermatids (p<0.05; p<0.001), type A spermatogonia (p<0.05; p<0.01), pachytene spermatocytes (p<0.01; p<0.001) and preleptotene spermatocytes (p<0.05) whereas no significant decrease was observed with a dose of 10ρg. The present findings reveal that during non-pubertal stage; continuous administration of kisspeptin may suppress hypothalamic-pituitary-gonadal axis and spermatogenesis.
Article Information
Received 10 December 2018
Revised 01 March 2019
Accepted 27 March 2019
Available online 28 December 2020
Authors’ Contribution
FR perceived the idea, designed the study, conducted the work and drafted the manuscript. IZQ supervised the study. MHR drafted the manuscript.
Key words
Kisspeptin, Spermatogenesis, Histomorphology, Gonadotropins, Testosterone.
DOI: https://dx.doi.org/10.17582/journal.pjz/20181210171230
* Corresponding author: [email protected]
0030-9923/2021/0001-0323 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
Introduction
Kisspeptins, a family of neuropeptides required for activation and maintenance of the mammalian reproductive axis, are encoded by the Kiss1 gene (Oakley et al., 2009). In humans, the protein Kisspeptin comprises of 145 amino acids and is fragmented into shorter peptides (Kp54, Kp14, Kp13, and Kp10) that share a common RF-amide C-terminal decapeptide sequence. They all are potent stimulators of GnRH release by signaling through the G-protein coupled receptor, GPR54 (also called KISS1R), which is expressed by GnRH neurons. Disruption of kisspeptin signaling causes hypogonadotropic hypogonadism in mice and humans (Lapatto et al., 2007; Seminara et al., 2003; Topaloglu et al., 2012). Mutant mice do not undergo sexual maturation at puberty and have low gonadotropic and sex steroid hormones levels caused by defective GnRH secretion from the hypothalamus. Conversely, activating mutations of GPR54 cause precocious puberty in humans (Teles et al., 2008).
Gonadotropins (FSH and LH) are the primary regulators of spermatogenesis. However initiation and preservation of normal spermatogenesis is governed by indispensable actions of androgens (Singh et al., 2017). A significant increase in the release of gonadotropins (Messager et al., 2005; Navarro et al., 2005a, b; Shahab et al., 2005) and testosterone (Dhillo et al. 2005) was evident by administration of kisspeptin peptide either cereberally or systemically. Sensitivity of LH release to the stimulatory effect of kisspeptin is significantly higher (Gottsch et al., 2004; Navarro et al., 2005b) as compared to sensitivity of FSH release (Navarro et al., 2005b).
Spermatogenesis comprises four elementary processes: 1) the stem cell and mitotic divisions of cells (spermatogonial development); 2) DNA synthesis and yielding of haploid spermatid following two meiotic divisions (meiosis); 3) Development of spermatid leading to differentiation of structures of head and tail (spermiogenesis), and 4) the process of releasing the mature sperms into the lumen of seminiferous tubules (spermiation). All mammals follow the same, above mentioned, patterns of spermatogenesis and are appropriately demonstrated at the morphological level as well (Clermont, 1972; Leblond and Clermont, 1952; de Kretser and Kerr, 1988; Russell et al., 1990). Development of germ cells, in rodents and in some species of primates, happen in such a systemic and distinguishable way, along the seminiferous tubule, that each single stage of spermatogenesis can be easily observed within the cross-section of the tubule (Russell et al., 1990). In humans (Homo sapiens) and a few other primate species (e.g., marmosets) on the other hand; the stages follow the course of complex helical pattern such that six distinct stages may be observed being processed in a cross section of a single seminiferous tubule (Schulze and Rehder, 1984).
The stem cells for the spermatogenic process are termed spermatogonial stem cells, and these undergo infrequent mitotic divisions. They can undergo several rounds of mitosis without completing cytokinesis, beginning the pathway by which groups of spermatogonia then proceed to enter meiosis and are termed first as primary spermatocytes and then secondary spermatocytes. The secondary spermatocytes then divide to form spermatids, the haploid cells that are transformed during spermatogenesis into spermatozoa (Kerr et al., 2006).
Effect of acute and chronic kisspeptin administrations on testosterone and histology of the testicular tissue has been carried out previously in prepubertal (Ramzan and Qureshi, 2011) and pubertal rats (Thompson et al., 2009). It was established that continuous kisspeptin administration adversely affects spermatogenesis in both prepubertal and adult rats. However, effects of kisspeptin treatment on germ cell number have not reported till date. This study, therefore, is intended to probe the effect of a range of intraperitoneal kisspeptin doses, administered twice daily to prepubertal male rats, on testosterone, plasma gonadotropins and stereology of testicular tissue, at alternate day for one spermatogenic cycle. A spermatogenic cycle includes all of the transformations from spermatogonium to mature spermatozoan and requires about 12-13 days in rat (Clermont, 1972; Hilscher et al., 1969).
Materials and methods
Animals and maintenance
To investigate the role of puberty regulating peptide “kisspeptin” in prepubertal male rats, one hundred and ninety six prepubertal male Sprague Dawley rats, five weeks old (postnatal day, PND 35), having an average weight of 100±10g were purchased from National Institute of Health (NIH), Islamabad, Pakistan and retained in the animal house facility at Quaid-i-Azam University, Islamabad, Pakistan during the course of experimental period. To reduce crowding stress, five rats were housed in one cage (steel mesh cage, 15″ × 11″ × 9″), under standard conditions of 12L: 12D h photoperiod, 25±2ºC temperature controlled with automatic timers and adjustable controls for heating and cooling. Standard rat diet and water were provided ad libitum.
Dosage and treatment
Kisspeptin doses were administered twice daily for continuous 12 days at 07:00 am and 19:00 pm. Kisspeptin (metastin 45-54 or kisspeptin-10; 1mg lyophilized powder) was purchased from Calbiochem (EMD Biosciences, Inc. La Jolla, CA). The stock solution for experimental purpose was made by dissolving 1mg Kisspeptin in 1 ml dimethylesulphoxide (DMSO) (1mg/ml) and was diluted further by the addition of distilled water (dH2O). The solution was later administered intraperitoneally (i.p.).
All rats were randomly organized in twenty eight groups, each comprising of seven rats (n=7). Saline solution, 0.9% weight/volume (w/v), was administered to the control group (saline solution had the same concentration of DMSO as that of kisspeptin stock solution, and was diluted further to the concentration used in experimental doses), while the experimental groups received three different doses of kisspeptin-10: 10ρg, 1ηg and 1µg doses. Both solutions, saline and kisspeptin, were administered i.p. as described in Ramzan and Qureshi (2011). Seven rats each from control and treated groups were sacrificed 3 h after the last dose of kisspeptin after 0 (baseline), 2, 4, 6, 8, 10 and 12 days of kisspeptin treatment. Experimental days on which sampling was done correspond to age of rats as 35 (baseline), 37, 39, 41, 43, 45, 47 days (postnatal), respectively.
This continuous treatment of 12 days was designed because the germ cells advance within the seminiferous epithelium, in a specific 12 to 13-day cycle that begins with mitotic division of spermatogonia and proceeds through meiosis and finally ends with the release of sperms (Leblond and Clermont, 1952).
Collection of blood and tissue samples
Anesthesia (sodium pentobarbital: i.p. 60-80 mg kg-1b.w.) was given to animals prior to taking blood; blood was withdrawn from the left ventricle of the heart of each rat, directly in to the EDTA vaccutainers. Blood was allowed to stand for 1 h and plasma was extracted by a 10 min centrifugation at 1258×g at 4°C (Eppendorff centrifuge 5810 R, Germany). Plasma samples of control and treated rats were aliquoted and stored at –20°C until assayed for hormone concentrations.
For light microscopy, testicular tissues from control and kisspeptin treated rats were excised, weighed, rinsed in phosphate buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA) solution prepared in PBS.
For the determination of daily sperm production (DSP) and elongated spermatid head count, the testes of control and kisspeptin treated rats were snap frozen in liquid nitrogen and stored at -70°C. They were later homogenized and processed.
Testes were weighed and gonadosomatic index (GSI %) was measured with the help of following formula:

Digital vernier caliper was used to find length and width of testis whereas its volume was determined using the ellipsoid equation according to Pochron and Wright (Pochron and Wright, 2002):
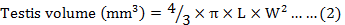
Where, L is length and W is width of the testis. Volume of left and right testis was determined and then average volume of both testes was determined as the representative measurement.
Light microscopy
Tissue samples were fixed overnight at 4°C, in freshly prepared 4% PFA (pH=7.2). Ascending grades of ethanol were used to dehydrate the tissues, followed by cleaning with xylene, and fixation in paraffin wax. A microtome (Shandon finesse 325, Italy) was used to cut 5μm thick sections of the tissue. Staining of the sections was done with Harris’s Hematoxylin and Periodic acid Schiff. A research microscope Nikon Optiphot BH 2 (Japan), was used to observe the sections.
Stereology and morphometry
Quantitative study of spermatogenesis was carried out, at stage VII of the seminiferous cycle, by calculating the aggregate numbers of different germ cells. The reason behind selecting stage VII was, the highest expression rate of androgen receptor protein at this stage in rats, backing the contention that mid-spermatogenic stage responds positively to androgen. Besides, immune expression and androgen receptor mRNA are at peak levels in the middle of spermatogenic stages VII–VIII, with a seeming downregulation at stage VIII (Donnel et al., 2006; Lue et al., 2000). A previous study documented by O’Donnell et al. (1996) also suggested that testosterone is essential for spermatogenesis at stage VII-VIII. Suppression of testosterone at these stages can inhibit the development of round spermatids.
A method developed by Leblond and Clermont (1952) was used to count pachytene spermatocytes (P), preleptotene spermatocytes (Pl), type A spermatogonia (SG) and step 7 spermatids (7Sd).
In each treatment group, various germ cells nuclei were calculated in 100 round seminiferous tubules. From every animal, a total of seven slides were selected; from each slide, two sections were chosen for counting of germ cells. From each transverse section, two readings were acquired and average of both values was considered as representative measurement. Entire counts (raw counts) of germ cells were corrected for thickness of sections and variations in the nucleolar or nuclear diameters, complying to the method of Abercrombie (1946) with the help of the below mentioned formula:
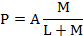
Where, P is the mean count of nuclear points per section, A is the raw count of nuclei in the section, M is the thickness (μm) of the section, and L is the mean length (μm) of the nuclei. The products are indicated as number of cells per cross section of seminiferous tubule.
Cells identification and grouping
Ramzan and Qureshi (2011) was used for grouping Sertoli and germ cells.
Testicular spermatid head count
Spermatid heads were counted on an improved Neubauer chamber as described in Ramzan and Qureshi (2011).
Hormone analyses
Standard solid phase radioimmunoassay (RIA) was used to measure concentrations of plasma luteinizing hormone (LH), follicle stimulating hormone (FSH) and testosterone as documented by Ramzan and Qureshi (2011).
Statistical analysis
Results were presented as mean ± standard error of mean (SEM). The obtained results were analyzed and compared using two-way ANOVA followed by post hoc Tukey’s adjustment with the help of Statistical Package for Social Sciences (SPSS, version 21, Inc., Chicago, Illinois, USA). A p-value less than 0.05 (p< 0.05) was considered to have statistical significance.
Results
Body weight and testicular parameters
No changes occurred in body and testicular weights, testes volume and gonadosomatic index (%) at any of the kisspeptin doses (Figs. 1, 2).
Regression analysis showed a linear increase in body weight (g), testes weight (g), testes volume (ml) and gonadosomatic index (%) with increasing age for control and kisspeptin treated rats over the time course of treatment (Table I).
Table I.- Regression analysis indicating increase in mean body weight and testicular parameters against variable doses of kisspeptin (KP).
|
Parameter |
Treatment |
R2 |
|
Body weight |
Control |
0.97 |
|
KP 10 ρg |
0.97 |
|
|
KP 1 ηg |
0.99 |
|
|
KP 1 µg |
0.93 |
|
|
Testes weight |
Control |
0.93 |
|
KP 10 ρg |
0.93 |
|
|
KP 1 ηg |
0.93 |
|
|
KP 1 µg |
0.85 |
|
|
Testes volume |
Control |
0.90 |
|
KP 10 ρg |
0.94 |
|
|
KP 1 ηg |
0.88 |
|
|
KP 1 µg |
0.73 |
|
|
Gonadosomatic index |
Control |
0.78 |
|
KP 10 ρg |
0.19 |
|
|
KP 1 ηg |
0.06 |
|
|
KP 1 µg |
0.004 |
Seminiferous tubular diameter and epithelium height
No effect was observed on seminiferous tubular diameter at any of the treatment doses. Regression analysis showed a linear increase in seminiferous tubular diameter (µm) (Table II).
Height of seminiferous tubular epithelium (height of the Sertoli cells) was found to have decreased after 12 days of treatment with 1µg doses and 1ηg (p< 0.001; p< 0.01), respectively as compared to controls, whereas effects remained unchanged with 10ρg dose (Fig. 3).
Plasma gonadotropins and testosterone
No effect was seen on the levels of plasma FSH throughout the experiment (Fig. 4).
Significant increase in concentration of plasma LH were seen with 1µg and 1ηg doses (p<0.01) after 4 days of treatment; but thereafter, decreased gradually approaching significant lower level after 12 days of treatment at 1µg (p<0.01) and 1ηg doses (p<0.05) as compared to the control, while remaining non-significantly different from control at 6th, 8th and 10th treatment days. No significant difference was observed at 10ρg dose during the experimental period (Fig. 5).
Table II.- Regression analysis indicating decrease in seminiferous tubular diameter and epithelium height against variable doses of kisspeptin (KP).
|
Parameter |
Treatment |
R2 |
|
Seminiferous tubular diameter |
Control |
0.97 |
|
KP 10 ρg |
0.98 |
|
|
KP 1 ηg |
0.97 |
|
|
KP 1 µg |
0.95 |
|
|
Seminiferous tubular epithelium height |
Control |
0.96 |
|
KP 10 ρg |
0.92 |
|
|
KP 1 ηg |
0.95 |
|
|
KP 1 µg |
0.53 |
Testosterone levels remained unaltered at 10ρg dose. At other doses, no change was observed in plasma testosterone concentration after 2 and 4 days of treatment, but a non-significant decrease (p<0.074) was noticeable after 6 days. The decrease continued and approached significant levels after 8th, 10th and 12th days of treatment with 1µg dose (p<0.01) and 1ηg dose (p<0.05) (Fig. 6).
Quantitative data on spermatogenic cells
Decrease in Type A spermatogonia observed only after 12 days of treatment at both 1µg and 1ηg kisspeptin doses (p<0.01; p<0.05), respectively, but remained unaltered at 2, 4, 6, 8, 10 days of treatment. No significant effect was found at 10ρg dose (Fig. 7).
Significant decrease (p<0.05) in Preleptotene spermatocytes was shown at both 1ηg and 1µg doses of kisspeptin but only after 12 days of treatment. The dose of 10ρg remained ineffective (Fig. 8).
Pachytene spermatocytes decreased significantly at 1ηg and 1µg doses of kisspeptin after treatment day 4 (p<0.01), day 6 (p<0.001; p<0.01, respectively), day 8 (p<0.01; p<0.001, respectively), day 10 (p<0.05; p<0.001, respectively), and day 12 (p<0.01; p<0.001, respectively). No effect was observed with 10ρg dose (Fig. 9).
Significant decrease was observed in step 7 spermatids at both 1ηg and 1µg doses after day 4 (p<0.05; p<0.01, respectively), day 6 (p<0.05; p<0.01, respectively), day 8 (p<0.01; p< 0.001, respectively), day 10 (p<0.05; p<0.001, respectively), and day 12 (p<0.001) of kisspeptin treatment. The decrease, with dose10ρg was detectable only after 12 days of treatment (p<0.05) (Fig. 10).
Elongated spermatid heads and daily sperm production
Elongated spermatid heads (g-1 testis wt.) showed significant decrease at 1ηg and 1µg doses after 8, 10 and 12 days of treatment (p<0.001). The decrease also occurred at 10ρg dose after 8, 10 and 12 days of treatment (p<0.01; p<0.05; p<0.05, respectively). Only a few spermatids were observed before 41 day of age (Fig. 11).
A significant decrease in daily sperm production (g-1 testis weight day-1) was also revealed with both 1ηg and 1µg doses after 8, 10 and 12 days of kisspeptin treatment (p<0.001). A significant decrease was also noticeable at 10ρg dose after 8, 10 and 12 days (p<0.001; p<0.01; p<0.05, respectively). The decrease was greater at higher kisspeptin doses (1ηg and 1µg) while comparatively less significant at 10ρg dose (Fig. 12).
Correlation analysis
A positive correlation (r = 0.55, p<0.01) existed between LH and testosterone concentrations. In contrast a significant negative (r = -0.317, p<0.01) correlation existed between FSH and LH concentration.
A significant positive correlation existed between type A spermatogonia and preleptotene spermatocytes (r = 0.41, p<0.01), type A spermatogonia and pachytene spermatocytes (r = 0.37, p<0.01), type A spermatogonia and step 7 spermatids (r = 0.35, p<0.01). Number of preleptotene spermatocytes correlated positively with pachytene spermatocytes (r = 0.50, p<0.01) and step 7 spermatids (r = 0.47, p<0.01). Pachytene spermatocytes showed a positive correlation with step 7 spermatids and (r = 0.35, p<0.01). A positive correlation was found between FSH levels and Sertoli cell number (r = 0.10).
Elongated spermatid head count was also found to have a positive correlation with daily sperm production (r = 0.12). Similarly, a strong positive correlation existed between testosterone and elongated spermatid head count (r = 0.57, p<0.01), and also between testosterone and daily sperm production (r = 0.48, p<0.01).
Discussion
The kisspeptin/GPR54 interaction has finally been identified in recent years as key regulator of the HPG axis (Seminara et al., 2003; de Roux et al., 2003). Although the acute effects of central and peripheral administration of kisspeptin have been discovered in rats and mice (Gottsch et al., 2004; Navarro et al., 2004, 2005), effects of long term kisspeptin administration on structure and functional aspects of testicular tissue in general and in particular prepubertal testes remain obscure. The present study intended on to explore the gonadal maturation and reproductive hormones status following kisspeptin challenge in reproductively immature male rats. There was a significant reduction observed in count of pachytene spermatocytes, preleptotene spermatocytes, type A spermatogonia and step 7 spermatids, daily sperm production (DSP) and height of seminiferous tubular epithelium after i.p. administration of 1µg kisspeptin dose, continuously for 12 days. No effects were seen on weights of body and testis, volume of testis, GSI %, and diameter of seminiferous tubules. At 1ηg dose, germ cell number, DSP and epithelial height decreased while no significant effect was found on body weight, reproductive organ weights, GSI % and seminiferous tubular diameter. At further low dose of 10ρg decrease in the above parameters did not reach significant levels. A resultant decrease in concentrations of plasma LH and testosterone were the major outcomes.
One of the salient findings of present study is that, despite a significant decrease in the concentrations of LH and testosterone with 1ηg and 1µg doses, continuous administration of kisspeptin-10 did not affect FSH levels, which instead remained comparable to saline treated controls, at the end of the 12 day period. As, FSH helps maintain the spermatogenic epithelium by stimulating Sertoli cells in the male and LH is tropic to the Leydig cells (Smith and Walker, 2014), it appears that significant decrease in the testosterone concentration following the kisspeptin treatment was quite possibly due to significantly lowered LH concentration because secretion of testosterone is under the control of LH. This indicates that active suppression of gonadal testosterone secretion was likely the cause of sharp decline in testosterone concentration. Testosterone release into the bloodstream acts to regulate LH secretion by the pituitary through a negative feedback loop. Elevated circulating testosterone concentrations feedback at the level of the hypothalamus and pituitary to suppress LH secretion by the pituitary, which subsequently reduces luteinizing hormone–chorionic gonadotropin receptor (LHCGR) - mediated stimulation of Leydig cells and thereby reduces testosterone production. Conversely, a fall in circulating testosterone concentrations induces LH secretion by the pituitary and a concomitant increase in testosterone production by the Leydig cells (Smith and Walker, 2014).
The current study elaborated that continuous administration of kisspeptin, in prepubertal male rat, may lead to obvious dissociation of gonadotropin levels, with a particular decrease only in the concentration of LH. Loss of LH stimulation after continuous kisspeptin administration accompanied by persistent FSH levels casts doubts on a simple desensitization of the HPG axis as a contributing factor for the phenomenon. It appears that chronic kisspeptin input may have resulted in altered pattern of pulsatile GnRH release in terms of frequency and pulse amplitude, which might preferentially drive FSH secretion (Dalkin et al., 1989). FSH and LH, the pituitary gonadotropin hormones, are essential for fertility. They contain an identical α-subunit (CGA) and a unique β-subunits, FSHβ and LHβ, respectively. These two hormones are regulated by the hypothalamic decapeptide, GnRH, which is released in a pulsatile manner from GnRH neurons located in the hypothalamus. Variable frequencies of pulsatile GnRH stimulate discrete signaling pathways and transcriptional machinery after binding to the receptor, GnRHR, on the cell surface of anterior pituitary gonadotropes (Thompson and Kaiser, 2014). Like FSH and LH secretion, the transcription of the gonadotropin subunits is also dependent on GnRH pulse frequency (Dalkin et al., 1989; Haisenleder et al., 1991; Jakubowiak et al., 1989; Kaiser et al., 1997). A decreased frequency of pulsatile GnRH favors Fshb transcription, whilst an increased frequency favors Lhb transcription. Although levels of Cga mRNA do respond to pulsatile GnRH, the regulation in response to varying frequencies of pulsatile GnRH is less important for overall FSH and LH production, since Cga is produced in excess over Lhb and Fshb at both fast and slow GnRH pulse frequencies (Landy et al., 1991; Weiss et al., 1990). Continuous exposure to GnRH down regulates both mRNA levels and secretion of gonadotropins, compared to pulsatile GnRH; therefore, biosynthesis of both FSH and LH is critically dependent on the pulsatile nature of the GnRH signal (Belchetz et al., 1978; Burger et al., 2004; Ferris and Shupnik, 2006; Gharib et al., 1990; Haisenleder et al., 1991). This might be the reason for significantly decreased LH and testosterone in our case.
The gonadotropin-releasing hormone (GnRH) neuronal network generates pulse and surge modes of gonadotropin secretion critical for puberty and fertility. Kisspeptin neurons in the arcuate nucleus innervate the projections of GnRH neurons in and around their neurosecretory zone are key components of the pulse generator in nearly all mammals while kisspeptin neurons located in the preoptic area project to GnRH neuronal cell bodies and proximal dendrites are involved in surge generation in rodents (Herbison, 2016).
The importance of the differential control of FSH and LH secretion is emphasized by disorders associated with dysregulation of their release from the pituitary. Patients with low levels of GnRH, FSH and LH, for example in association with idiopathic hypogonadotropic hypogonadism or Kallmann syndrome, are infertile (Seminara et al., 1998). Conversely, accelerated GnRH pulse frequency, associated with increased levels of LH over FSH, is associated with polycystic ovarian syndrome (PCOS) (Blank et al., 2007).
In the present study measurement of the hormone levels at alternate days was conducted. FSH levels increased progressively from postnatal day 35 to 47 but remained similar to saline treated controls throughout the study period. In kisspeptin treated rats LH levels peaked after 4 days of treatment, and declined thereafter to become significantly lower than the controls after 12 days of treatment. To follow the general growth related pattern in both the control and treated rats, plasma testosterone levels increased with increasing age of rats, however kisspeptin treatment lowered testosterone levels compared to control animals after 6 days of treatment that remained lower thereafter.
Presently, it seems logical to presume that kisspeptin perhaps acted to suppress an early maturation of gonads as the male gonads have to develop fully at a later stage and sperms are first produced in the testes around day 45 with optimal production occurring at 75 days (Russell, 1992). However, testicular degeneration observed by Thompson et al. (2006) does not conform to this notion since they collected data from adult male rats. Thus it is suggested that suppressed levels of LH and testosterone could simply be the pharmacological effect of kisspeptin treatment.
Significant decreases in germ cells, daily sperm production, total support capacity of Sertoli cells, Sertoli efficiency and meiotic index and an increase in coefficient of mitosis indicated germ cell loss, which may have occurred through apoptotic mechanisms.
At the moment, data at the cellular level completely supports suppression of testosterone and LH concentration, mediated by kisspeptin at stage VII of the spermatogenic cycle, which results in exceeded deterioration of the seminiferous epithelium. Currently, a small amount of elongated and round spermatids were persistently seen, pointing towards stoppage of germ cell maturation. A previous report by Kerr (1995) showed that in the rat testis, testosterone and FSH apparently act on the seminiferous epithelium at various stages of the spermatogenic cycle, FSH possibly on stages I-VI and the testosterone at later stages i.e. stage VII-VIII. If previous stages are maintained properly then stages IX-XIV show normal germ cell development. McLachlan (McLachlan et al., 1994) also revealed that LH withdrawal leads to suppression of spermatogenesis and results in significant reductions in the numbers of pachytene spermatocytes, preleptotene spermatocytes, spermatogonia and round spermatids whereas elongated spermatids remained unnoticeable. In a very similar way a distinct degeneration pattern of spermatogenic cell is also seen in acute removal of the testosterone. A significant decrease was observed in elongated spermatid and spermatid heads at day 8 and afterwards as animals used were prepubertal, they did not have these cells before that age.
Both GPR54 and KiSS-1 are reported to be expressed in both ovaries and testes of rodents (Kotani et al., 2001; Ohtaki et al., 2001; Terao et al., 2004; Castellano et al., 2006), a direct testicular effect may be speculated to provide a functional kisspeptin-GPR54 system for local regulation of reproductive status at the level of gonads. An extra-pituitary direct inhibitory action on the gonads from the effects of GnRH agonists is also reported in some studies (Hsueh and Schaeffer, 1985; van Kroonenburgh et al., 1986). Thompson et al. (2009) observed testicular degeneration after a single intracerebroventricular (i.c.v.) injection of kisspeptin-54, and further suggested that the action was regulated by GnRH. Kisspeptin may act directly on the testes, indirectly through the central nervous system (CNS), or by a combination of the two (Pinto et al., 2012). In the present study route of kisspeptin mediated degeneration on testicular tissue is yet to be determined.
Testicular degeneration was highlighted by Ramzan and Qureshi (2011) previously, after administration of kisspeptin, intraperitoneally for 12 days, in prepubertal rats. This study is, therefore; first of its kind that present quantitative data on the effect of kisspeptin on spermatogenesis in prepubertal male rats. Appropriate comparison of the present data cannot therefore be made from the existing studies as this is the first study of kisspeptin effects on the spermatogenic cell population. It has been concluded from present results that kisspeptin might cause dose dependent degeneration of testicular tissue when administered continuously.
Acknowledgments
The first author received scholarship grant for this study from the Higher Education Commission, H-9, Islamabad, Pakistan.
Statement of conflict of interests
The authors hereby declare no conflict of interest with any institute or individual.
References
Abercrombie, M., 1946. Estimation of nuclear population from microtome sections. Anat. Rec., 94: 239-247. https://doi.org/10.1002/ar.1090940210
Belchetz, P.E., Plant, T.M., Nakai, Y., Keogh, E.J. and Knobil, E., 1978. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science, 202: 631-633. https://doi.org/10.1126/science.100883
Blank, S.K., McCartney, C.R., Helm, K.D. and Marshall, J.C., 2007. Neuroendocrine effects of androgens in adult polycystic ovary syndrome and female puberty. Semin. Reprod. Med., 25: 352-359. https://doi.org/10.1055/s-2007-984741
Burger, L.L., Haisenleder, D.J., Dalkin, A.C. and Marshall, J.C., 2004. Regulation of gonadotropin subunit gene transcription. J. mol. Endocrinol., 33: 559-584. https://doi.org/10.1677/jme.1.01600
Castellano, J.M., Gaytan, M., Roa, J., Vigo, E., Navarro, V.M., Bellido, C., Dieguez, C., Aguilar, E., Sanchez-Criado, J.E., Pellicer, A., Pinilla, L., Gaytan, F. and Tena-Sempere, M., 2006. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology, 147: 4852-4862. https://doi.org/10.1210/en.2006-0117
Clermont, Y., 1972. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev., 52: 198-236. https://doi.org/10.1152/physrev.1972.52.1.198
Dalkin, A.C., Haisenleder, D.J., Ortolano, G.A., Ellis, T.R. and Marshall, J.C., 1989. The frequency of gonadotropin-releasing-hormone stimulation differentially regulates gonadotropin subunit messenger ribonucleic acid expression. Endocrinology, 125: 917-924. https://doi.org/10.1210/endo-125-2-917
de Kretser, D.M. and Kerr, J.B., 1988. The cytology of the testis. In: The physiology of reproduction (eds. E. Knobil and J. Neill). Raven Press Ltd., New York, pp. 837-932.
de Roux, N., Genin, E., Carel, J.C., Matsuda, F., Chaussain, J.L. and Milgrom, E., 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. natl. Acad. Sci. U.S.A., 100: 10972-10976. https://doi.org/10.1073/pnas.1834399100
Dhillo, W.S., Chaudhri, O.B., Patterson, M., Thompson, E.L., Murphy, K.G., Badman, M.K., McGowan, B,M., Amber, V., Patel, S., Ghatei, M.A. and Bloom, S.R., 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J. clin. Endocrinol. Metab., 90: 6609-6615. https://doi.org/10.1210/jc.2005-1468
Donnel, L.O., Meachem, S.J., Stanton, P.G. and Machlachlan, R.I., 2006. Endocrine regulation of spermatogenesis. In: The physiology of reproduction (eds. E. Knobil and J.D. Neill). Elsevier Academic Press, New York, pp. 1017-1148. https://doi.org/10.1016/B978-012515400-0/50026-9
Ferris, H.A. and Shupnik, M.A., 2006. Mechanisms for pulsatile regulation of the gonadotropin subunit genes by GNRH1. Biol. Reprod., 74: 993-998. https://doi.org/10.1095/biolreprod.105.049049
Gharib, S.D., Wierman, M.E., Shupnik, M.A. and Chin, W.W., 1990. Molecular biology of the pituitary gonadotropins. Endocrionol. Rev., 11: 177-199. https://doi.org/10.1210/edrv-11-1-177
Gottsch, M.L., Cunningham, M.J., Smith, J.T., Popa, S.M., Acohido, B.V., Crowley, W.F., Seminara, S., Clifton, D.K. and Steiner, R.A., 2004. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology, 145: 4073-4077. https://doi.org/10.1210/en.2004-0431
Haisenleder, D.J., Dalkin, A.C., Ortolano, G.A., Marshall, J.C. and Shupnik, M.A., 1991. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: Evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology, 128: 509-517. https://doi.org/10.1210/endo-128-1-509
Herbison, A.E., 2016. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol., 12: 452-466. https://doi.org/10.1038/nrendo.2016.70
Hilscher, B., Hilscher, W. and Maurer, W., 1969. Autoradiographic studies on the modus of proliferation and regeneration of the seminiferous epithelium of Wistar rats. Z. Zellforsch. Mikrosk. Anat., 94: 593-604. https://doi.org/10.1007/BF00936064
Hsueh, A.J. and Schaeffer, J.M., 1985. Gonadotropin-releasing hormone as a paracrine hormone and neurotransmitter in extra-pituitary sites. J. Steroid Biochem., 23: 757-764. https://doi.org/10.1016/S0022-4731(85)80011-X
Jakubowiak, A., Janecki, A. and Steinberger, A., 1989. Similar effects of inhibin and cycloheximide on gonadotropin release in superfused pituitary cell cultures. Biol. Reprod., 41: 454-463. https://doi.org/10.1095/biolreprod41.3.454
Kaiser, U.B., Jakubowiak, A., Steinberger, A. and Chin, W.W., 1997. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology, 138: 1224-1231. https://doi.org/10.1210/en.138.3.1224
Kerr, J.B., 1995. Macro, micro, and molecular research on spermatogenesis: The quest to understand its control. Microsc. Res. Tech., 32: 364-384. https://doi.org/10.1002/jemt.1070320503
Kerr, J.B., Loveland, K.L., O’Bryan, M.K. and de Kretser, D.M., 2006. Cytology of the testis and intrinsic control mechanism. In: The physiology of reproduction (eds. E. Knobil and J.D. Neill). Elsevier Academic Press, New York, pp. 1017-1148. https://doi.org/10.1016/B978-012515400-0/50023-3
Kotani, M., Detheux, M., Vandenbogaerde, A., Communi, D., Vanderwinden, J.M., Le Poul, E., Brezillon, S., Tyldesley, R., Suarez-Huerta, N., Vandeput, F., Blanpain, C., Schiffmann, S.N., Vassart, G. and Parmentier, M., 2001. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. biol. Chem., 276: 34631-34636. https://doi.org/10.1074/jbc.M104847200
Landy, H., Boepple, P.A., Mansfield, M.J., Whitcomb, R.W., Schneyer, A.L., Crawford, J.D., Crigler, Jr. J.F. and Crowley, Jr. W.F., 1991. Altered patterns of pituitary secretion and renal excretion of free alpha-subunit during gonadotropin-releasing hormone agonist-induced pituitary desensitization. J. clin. Endocrinol. Metab., 72: 711-717. https://doi.org/10.1210/jcem-72-3-711
Lapatto, R., Pallais, J.C., Zhang, D., Chan, Y.M., Mahan, A., Cerrato, F., Le, W.W., Hoffman, G.E. and Seminara, S.B., 2007. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology, 148: 4927-4936. https://doi.org/10.1210/en.2007-0078
Leblond, C.P. and Clermont, Y., 1952. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Annls. N.Y. Acad. Sci., 55: 548-573. https://doi.org/10.1111/j.1749-6632.1952.tb26576.x
Lue, Y., Hikim, A.P., Wang, C., Im, M., Leung, A. and Swerdloff, R.S., 2000. Testicular heat exposure enhances the suppression of spermatogenesis by testosterone in rats: The “two-hit” approach to male contraceptive development. Endocrinology, 141: 1414-1424. https://doi.org/10.1210/en.141.4.1414
Mclachlan, R.I., Wreford, N.G., Meachem, S.J., de Kretser, D.M. and Robertson, D.M., 1994. Effects of testosterone on spermatogenic cell populations in the adult rat. Biol. Reprod., 51: 945-955. https://doi.org/10.1095/biolreprod51.5.945
Messager, S., Chatzidaki, E.E., Ma, D., Hendrick, A.G., Zahn, D., Dixon, J., Thresher, R.R., Malinge, I., Lomet, D., Carlton, M.B., Colledge, W.H., Caraty, A. and Aparicio, S.A., 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. natl. Acad. Sci. U.S.A., 102: 1761-1766. https://doi.org/10.1073/pnas.0409330102
Navarro, V.M., Castellano, J.M., Fernandez-Fernandez, R., Barreiro, M.L., Roa, J., Sanchez-Criado, J.E., Aguilar, E., Dieguez, C., Pinilla, L. and Tena-Sempere, M., 2004. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology, 145: 4565-4574. https://doi.org/10.1210/en.2004-0413
Navarro, V.M., Castellano, J.M., Fernandez-Fernandez, R., Tovar, S., Roa, J., Mayen, A., Barreiro, M.L., Casanueva, F.F, Aguilar, E., Dieguez, C., Pinilla, L. and Tena-Sempere, M., 2005a. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology, 146: 1689-1697. https://doi.org/10.1210/en.2004-1353
Navarro, V.M., Castellano, J.M., Fernandez-Fernandez, R., Tovar, S., Roa, J., Mayen, A., Nogueiras, R., Vazquez, M.J., Barreiro, M.L., Magni, P., Aguilar, E., Dieguez, C., Pinilla, L. and Tena-Sempere, M., 2005. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology, 146: 156-163. https://doi.org/10.1210/en.2004-0836
O’Donnell, L., McLachlan, R.I., Wreford, N.G., de Kretser, D.M. and Robertson, D.M., 1996. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol. Reprod., 55: 895-901. https://doi.org/10.1095/biolreprod55.4.895
Oakley, A.E., Clifton, D.K. and Steiner, R.A., 2009. Kisspeptin signaling in the brain. Endocrinol. Rev., 30: 713-743. https://doi.org/10.1210/er.2009-0005
Ohtaki, T., Shintani, Y., Honda, S., Matsumoto, H., Hori, A., Kanehashi, K., Terao, Y., Kumano, S., Takatsu, Y., Masuda, Y., Ishibashi, Y., Watanabe, T., Asada, M., Yamada, T., Suenaga, M., Kitada, C., Usuki, S., Kurokawa, T., Onda, H., Nishimura, O. and Fujino, M., 2001. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature, 411: 613-617. https://doi.org/10.1038/35079135
Pinto, F.M., Cejudo-Roman, A., Ravina, C.G., Fernandez-Sanchez, M., Martin-Lozano, D., Illanes, M., Tena-Sempere, M. and Candenas, M.L., 2012. Characterization of the kisspeptin system in human spermatozoa. Int. J. Androl., 35: 63-73. https://doi.org/10.1111/j.1365-2605.2011.01177.x
Pochron, S.T. and Wright, P.C., 2002. Dynamics of testis size compensates for variation in male body size. Evol. Ecol. Res., 4: 577-585.
Ramzan, F. and Qureshi, I.Z., 2011. Intraperitoneal kisspeptin-10 administration induces dose-dependent degenerative changes in maturing rat testes. Life Sci., 88: 246-256. https://doi.org/10.1016/j.lfs.2010.11.019
Russell, L.D., 1992. Normal development of the testis. In: Pathobiology in the aging rat (eds. U. Mohr, D.L. Dungworth and C.C. Capen). ILSI Press, Washington DC, pp. 395-405.
Russell, L.D., Ettlin, R.A., Sinha-Hikim, A.P. and Clegg, E.D., 1990. Histological and histopathological evaluation of the testis. Cache River Press, Clearwater, Florida.
Schulze, W. and Rehder, U., 1984. Organization and morphogenesis of the human seminiferous epithelium. Cell Tissue Res., 237: 395-407. https://doi.org/10.1007/BF00228424
Seminara, S.B., Hayes, F.J. and Crowley, Jr. W.F., 1998. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): pathophysiological and genetic considerations. Endocrinol. Rev., 19: 521-539. https://doi.org/10.1210/edrv.19.5.0344
Seminara, S.B., Messager, S., Chatzidaki, E.E., Thresher, R.R., Acierno, Jr. J.S., Shagoury, J.K., Bo-Abbas, Y., Kuohung, W., Schwinof, K.M., Hendrick, A.G., Zahn, D., Dixon, J., Kaiser, U.B., Slaugenhaupt, S.A., Gusella, J.F., O’Rahilly, S., Carlton, M.B., Crowley, Jr. W.F., Aparicio, S.A. and Colledge, W.H., 2003. The GPR54 gene as a regulator of puberty. N. Engl. J. Med., 349: 1614-1627. https://doi.org/10.1056/NEJMoa035322
Shahab, M., Mastronardi, C., Seminara, S.B., Crowley, W.F., Ojeda, S.R. and Plant, T.M., 2005. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc. natl. Acad. Sci. U.S.A., 102: 2129-2134. https://doi.org/10.1073/pnas.0409822102
Singh, V., Agrawal, N.K., Verma, R. And Singh, K., 2017. HPG axis: The central regulator of spermatogenesis and male fertility. In: Male infertility: Understanding, causes and treatment (eds. R. Singh and K. Singh) Springer Singapore, Singapore, pp. 25-36. https://doi.org/10.1007/978-981-10-4017-7_3
Smith, L.B. and Walker, W.H., 2014. Hormone signaling in the testis. In: Knobil and Neill’s physiology of reproduction: Two-Volume Set. Elsevier North-Holland, Inc., pp. 637-690. https://doi.org/10.1016/B978-0-12-397175-3.00016-8
Teles, M.G., Bianco, S.D., Brito, V.N., Trarbach, E.B., Kuohung, W., Xu, S., Seminara, S.B., Mendonca, B.B., Kaiser, U.B. and Latronico, A.C., 2008. A GPR54-activating mutation in a patient with central precocious puberty. N. Engl. J. Med., 358: 709-715. https://doi.org/10.1056/NEJMoa073443
Terao, Y., Kumano, S., Takatsu, Y., Hattori, M., Nishimura, A., Ohtaki, T. and Shintani, Y., 2004. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochim. Biophys. Acta, 1678: 102-110. https://doi.org/10.1016/j.bbaexp.2004.02.005
Thompson, E.L., Amber, V., Stamp, G.W., Patterson, M., Curtis, A.E., Cooke, J.H., Appleby, G.F., Dhillo, W.S., Ghatei, M.A., Bloom, S.R. and Murphy, K.G., 2009. Kisspeptin-54 at high doses acutely induces testicular degeneration in adult male rats via central mechanisms. Br. J. Pharmacol., 156: 609-625. https://doi.org/10.1111/j.1476-5381.2008.00061.x
Thompson, E.L., Murphy, K.G., Patterson, M., Bewick, G.A., Stamp, G.W., Curtis, A.E., Cooke, J.H., Jethwa, P.H., Todd, J.F., Ghatei, M.A. and Bloom, S.R., 2006. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am. J. Physiol. Endocrinol. Metab., 291: E1074-1082. https://doi.org/10.1152/ajpendo.00040.2006
Thompson, I.R. and Kaiser, U.B., 2014. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol. Cell. Endocrinol., 385: 28-35. https://doi.org/10.1016/j.mce.2013.09.012
Topaloglu, A.K., Tello, J.A., Kotan, L.D., Ozbek, M.N., Yilmaz, M.B., Erdogan, S., Gurbuz, F., Temiz, F., Millar, R.P. and Yuksel, B., 2012. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N. Engl. J. Med., 366: 629-635. https://doi.org/10.1056/NEJMoa1111184
Van Kroonenburgh, M.J., Beck, J.L., Vemer, H.M., Rolland, R., Thomas, C.M. and Herman, C.J., 1986. Effects of a single injection of a new depot formulation of an LH-releasing hormone agonist on spermatogenesis in adult rats. J. Endocrinol., 111: 449-454. https://doi.org/10.1677/joe.0.1110449
Weiss, J., Duca, K.A. and Crowley, Jr. W.F., 1990. Gonadotropin-releasing hormone-induced stimulation and desensitization of free alpha-subunit secretion mirrors luteinizing hormone and follicle-stimulating hormone in perifused rat pituitary cells. Endocrinology, 127: 2364-2371. https://doi.org/10.1210/endo-127-5-2364
To share on other social networks, click on any share button. What are these?









