Journal of Animal Health and Production
Short Communication
Journal of Animal Health and Production. 1 (3): 32 – 35Detection of Bovine Leptospirosis Using Different Conventional Laboratory Tests in Nineveh Province, Iraq
Eva. Aisser Ajaj, Maab Ibrahim AL– Farwachi*
*Corresponding author:[email protected]
ARTICLE CITATION:
Ajaj EA, and AL– Farwachi MI (2013). Detection of bovine leptospirosis using different conventional laboratory tests in Nineveh province, Iraq. J Anim Health Prod. 1 (3): 32 – 35.
Received: 2013–06–01, Revised: 2013–09–23, Accepted: 2013–06–26
The electronic version of this article is the complete one and can be found online at
(http://nexusacademicpublishers.com/table_contents_detail/11/104/html)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
The present study involves evaluation of various techniques such as direct dark field microscopy, direct microscopic examination of urine sediments stained with fountana stain , Congo red and acridine orange stains as well as microbiological isolation for diagnosis of leptospirosis in clinically suspected cases. A total 127 samples comprised of 92 sera and 35 urine samples were collected from adult local breed cattle aged 2 – 7 years old during 7 months period of September 2012 to March 2013. The diagnosis of the disease was performed by direct dark field microscopic examination, direct microscopic examination of urine sediments stained with fountana stain, Congo red and acridine orange stains and microbiological isolation. Serodiagnosis of Leptospira interrogans serovars Hardjo and Pomona was carried out using a commercial indirect ELISA (BOVICHEK® LEPTO kit , Biovet , Canada ). Thirty–three ( 94.3 % ) of 35 urine samples were found positive by each of the direct dark field microscopy, and direct microscopic examination of urine sediments stained with fountana stain , Congo red and acridine orange stains and microbiological isolation. From a total of 92 sera, 6 (6.5 %) were positive for Leptospira hardjo and only one animal (1 %) was seropositive to L. pomona . The present results suggested that direct dark field microscopy, and direct microscopic examination of urine sediments stained with fountana stain, Congo red and acridine orange stains and indirect ELISA form the basis of diagnosis of leptospirosis in clinically suspected cases. L. interrogans serovar hardjo has the highest prevalence in the region under study.
Leptospirosis is a major problem worldwide, particularly in the tropics (World Health Organization 2003; Adler , 2010) Leptospiral serovares that affect bovines more frequently are hardjo, pomona, canicola and icterohaemorrhagiae. Nowadays, serovar hardjo is considered the most frequent and important serovar for bovines (Radostits et al. , 2007). The clinical presentation of leptospirosis in cattle is variable . Bovine leptospirosis causes abortion, stillbirth or weak calves, infertility and a decrease in milk production (Faine et al. 2000; Victoriano et al. 2009).
Diagnosis of leptospiral infection in cattle is difficult because of low specificity, low sensitivity and vague interpretation of various diagnostic tests, plus the frequent absence of specific clinical signs, particularly in non–pregnant and non–lactating cows (Radostits et al. , 2007). Laboratory routine diagnosis of bovine leptospirosis is performed using serological methods and leptospires detection in urine and organs (Wagenaar et al. , 2000; Morgan et al. , 2007). The microscopic agglutination test (MAT) is considered the standard serologic test that is specific and provides useful epidemiologic data in the form of presumptive serogroups (Cole et al., 1973). However, this assay is not suitable for routine laboratories since it is technically demanding, costly, and requires the maintenance of live, hazardous stock serovar cultures and also requires analyses of paired sera to verify the seroconversion which delays the diagnosis (Thiermann , 1984; Dassanayake et al. 2009). Enzyme–linked immunosorbent assay (ELISA) have been developed (Surujballi and Mallory 2004; Mariyar 2006) and several commercial test kits are available mostly using reactive Leptospira antigen obtained from pathogenic L. hardjo (Kavanagh et al. , 2002 ; Leonard et al. , 2004 ; Ryan , 2012). In the present study, researchers aimed to evaluation of different conventional laboratory tests for diagnosis of bovine leptospirosis.
In Nineveh province, a total of 127 samples comprised of 92 sera and 35 urine samples were collected from adult local breed cattle aged 2 – 7 years old during 7 months period of September 2012 to March 2013. Cows which have shown at least one of the clinical signs suggestive for leptospirosis, such as jaundice, haemoglubinuria, abortion or mastitis, during sample collection or some months before were included in this study Nineveh province is located in the north of Iraq. The climate of the Nineveh province is hot, dry in summer and cool rainy at winter. Blood samples were taken aseptically using sterile 10 ml anticoagulant free vacutainers from the jugular vein. Serum was separated by centrifugation of blood at 3000 x g for 10 min at room temperature.The sera were kept on – 200 C until used. Midstream urine samples were collected from 35 clinically suspected cases in sterile bottles after cleaning of the vulva and perineal region and encouraging the animal to urinate by massage method. All urine samples were placed in the ice container in the dark and transported to the laboratory as soon as possible.
This test was carried out as described by Abdollahpour ( 1995 ) as follows : one ml of urine samples was centrifuged at 12000 X g for 20 min. at 40 C. Supernatant was removed and the pellets were resuspended in 100 micrometer of remaining urine. One drop of urine per animal was placed on a glass slide and covered with a coverslip. The material was examined by optical microscope (Olympus® – Model Bx40) at 200x magnification. A positive result was made by visualization of cells presenting morphology and motility compatible with leptospires ( Fain 1982 ).
Direct microscopic examination of urine sediments stained with fountain stain and Congo red stain (Collee et al. 1996) and acridine orange stain (Clark 1973) were also used. The slides stained by fountana and Congo red were examined under x100 oil immersion of light microscope , while The slides stained with acridine orange were examined under a fluorescence microscope (Olympus BX 50 ) at a magnification of x200. Culture and isolation: The Ellinghausen, McCullough, Johnson and Harris – EMJH (Difco®–USA) with the addition of 5–fluoruracil (300 mg/L) and rifampicin (20 mg/L), and incubated at 30ºC for 3– 6 weeks. Cultures were examined weekly under dark field microscopy and tubes showing contaminants were discarded. Microorganisms that were isolated have been also identified by biochemical characteristics ( catlase and peroxidase tests) (Benson 2002).
A commercial indirect ELISA kit for detection of antibodies against L. Leptospira interrogans serovar hardjo and L. interrogans serovar pomona in serum was used, the kit has been supplied from BOVICHEK® LEPTO kit, Biovet, Canada. All sera were tested according to the manufacturer's instructions, then read the optical densities in the microwells using a micro plate reader at a wavelength of 450 nm. ELISA optical density (O D) reading were transformed to serum / positive percentage (S / P) according to a specific equation cited by the manufacturer.
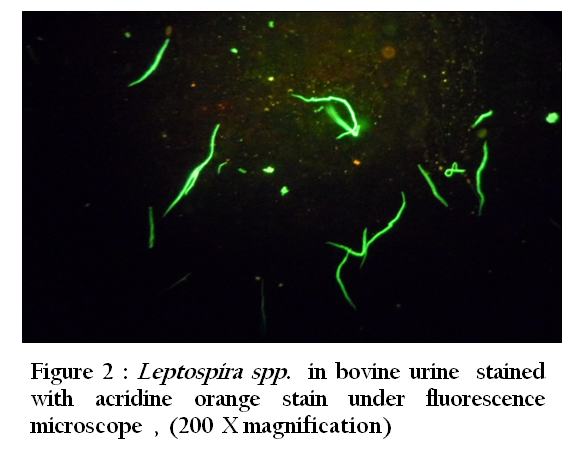
Figure 2: Leptospira spp. in bovine urine stained with acridine orange stain under fluorescence microscope , (200 X magnification)
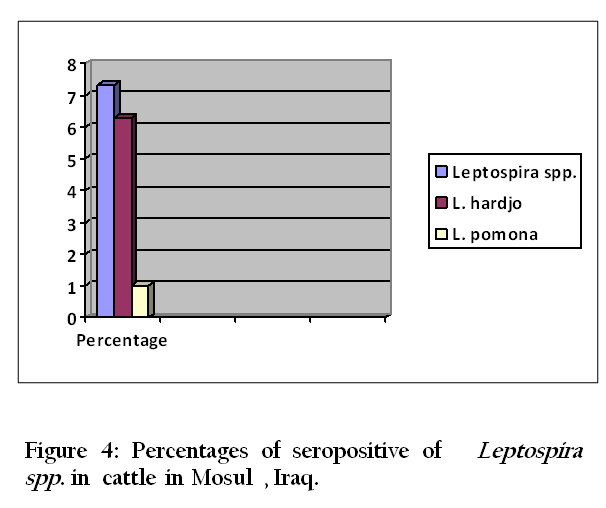
Thirty–three (94.3 %) of 35 urine samples were positive by each of the direct dark field microscopy ( Figure 1) ,direct microscopic examination of urine sediments stained with fountana stain , acridine orange ( Figure 2) and Congo red stains ( Figure 3) and microbiological isolation. The results of study showed that the total percentage of seropositive of Leptospira spp. antibodies was 7.3 (mean 7 seropositive out of 96 sera). Six (6.3%) of 96 sera were found positive for Leptospira interrogans serovar hardjo and only one (1 %) of 96 sera were seropositive to L. interrogans serovar pomona (Figure 4).
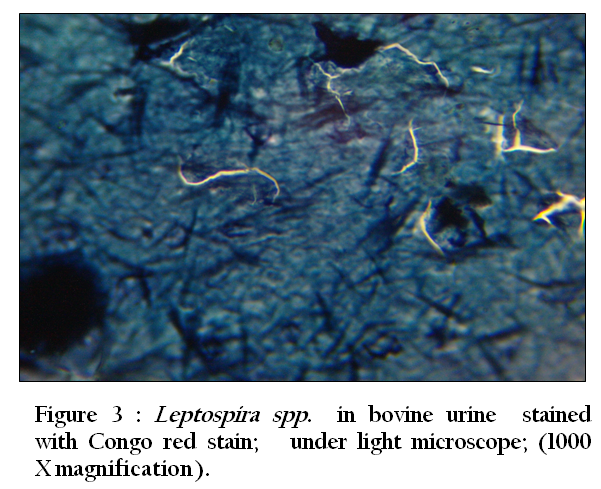
Figure 3: Leptospira spp. in bovine urine stained with Congo red stain; under light microscope; (1000 X magnification)
This is the first diagnostic study of leptospirosis in cattle in the Nineveh province , Iraq. Thirty–three ( 94.3 % ) of 35 urine samples were positive by each of the direct dark field microscopy, direct microscopic examination of urine sediments stained with fountana stain , acridine orange and Congo red stains and microbiological isolation. Leptospirosis is one of the most important zoonotic disease spreading throughout the world with numerous reservoir hosts (World Health Organization 2003; Adler , 2010). However, despite the fact that Nineveh province is one of major livestock husbandry centers in Iraq, there is no published data on the epidemiology of bovine leptospirosis in this province.
There are only one report in cattle in Baghdad. In this study the seroprevalence to one or more serovars of L.interrogans was 57.3 % in cattle. MAT was the only test that had been used for serological survey of leptospiral infection and in that study the highest prevalence was for serovar hardjo (Al–Badrawi et al. , 2010).
In our study, an antibodies against L. interrogans serovar hardjo and L. interrogans serovar pomona were detected in the 6 of 96 sera and 1 of 96 sera respectively. Hardjo is a host–adapted serovar for cattle, which can become chronic carriers of hardjo and serve as reservoirs for infection of other cattle and humans (Bolin, 2003). L. interrogans serovars pomona, as accidental hosts, causing acute disease and abortion (Miller et al.,1991; Peregrine et al. , 2006). Serological surveys indicate wide spread exposure to L.serovar hardjo in cattle as in Ireland (Ryan , 2012), United Kingdom (Pritchard ,1986), Portugal (Rochat , 1998), Nigeria (Ezeh , 1989) , Tanzania (Machang’U , 1997) ,West Malaysia (Bahaman , 1987) , Turkey (Kocabiyik and Cetin, 2004), and United States (Larson et al. , 2007).
Leptospiral antibodies appear within a few days of onset of illness and persist for weeks or months and, in some cases, years. Unfortunately, antibody titers may fall to undetectable levels while animals remain chronically infected. To overcome this problem, sensitive methods are needed to detect the organism in urine or the genital tract of chronic carriers (Radostits et al . 2007). The variability in the prevalence between reports could be attributed to environmental differences between geographical areas and topographical reasons. Herd management may affect the overall seroprevalence of the disease and the distribution of serovars, and the prevalence is generally higher in dairy than beef cattle (Faine et al. 2000), and the higher prevalence of hardjo found in our study could be explained by the fact that the cattle had close contact with the reservoirs of this serovar (Radostits et al . 2007) .In our study serodiagnosis of bovine leptospirosis based on the results of the indirect ELISA test. This diagnosis of leptospirosis in the live animal can be a achieved by detection of antibodies using MAT.
ELISA was sensitive and could detect antibodies to multiple pathogenic Leptospira serovars. It is a good assay for bovine leptospirosis screening (El Jalii , 2008) The ELISA has some advantages over the MAT. It is relatively sensitive and specific and semiautomated, it uses killed antigens, and the results can be read objectively (Surujballi and Mallory 2004). In conclusion, direct dark field microscopy ,and direct microscopic examination of urine sediments stained with fountana stain , Congo red and acridine orange stains and indirect ELISA form the basis of diagnosis of leptospirosis in clinically suspected cases and serovar hardjo infections were determined to be more common leptospiral infections in cattle in Nineveh provinces , Iraq.
ACKNOWLEDGEMENT
This study was supported by the College of veterinary medicine, Mosul University, Mosul, Iraq.
REFERENCES
Al–Badrawi T Y G, Habasha F G and Sultan S H (2010). Sociological study of Leptospirosis in cattle, sheep and goats in Baghdad Province . Al– Anbar J. Vet. Sci., 3(1):78–82.
Adler B, Moctezuma A (2010). Leptospira and leptospirosis. Vet. Microbiol. 140(3): 287–296.
http://dx.doi.org/10.1016/j.vetmic.2009.03.012
PMid:19345023
Bahaman AR, Ibrahim AL and Adam H (1987) Serological prevalence of leptospiral infection in domestic animals in West Malaysia. Epid. Infect. 99(1): 379–392.
http://dx.doi.org/10.1017/S0950268800067868
Benson HJ (2002). Microbiological applications, The laboratory manual in general microbiology. 8th. Ed., McGraw–Hill Companies, Inc., New York: 186.
Bolin CA (2003). Leptospirosis. In: Fowler, M.E.& Miller, R.E., Zoo and wild animal medicine. St. Louis, Missouri: WB Saunders: 699–702.
Clark G (1973). Staining procedures. 3rd. Ed., Williams and Wilking Company, Baltimore, USA. 183.
Cole JR, Sulzer CR and Pursell AR (1973). Improved microtechnique for the leptospiral microscopic agglutination test. Applied Microbiol. 25(4): 976–980.
PMid:4736794 PMCid:PMC380950
Collee JG, Fraser AG, Marmino BP and Simmons A (1996). Mackie and McCarteny practical medical microbiology. 14th . Ed., Churchill Livingstone Inc., New York: 3 – 926.
Ezeh AO, Addo PB, Adesiyun AA, Bello CSS and Makinde A (1989) Serological prevalence of bovine leptospirosis in Plateau State, Nigeria., Rev. Elev. Med. Vet. Pays Trop. 42(4):505–508.
Dassanayake DLB, Wimalaratna H, Agampodi SB, Liyanapathrirana VC, Piyarathna TACL and Goonapienuwala BL (2009). Evaluation of surveillance case definition in the diagnosis of leptospirosis, using the Microscopic Agglutination Test: a validation study. BMC Infectious Diseases 9:48.
http://dx.doi.org/10.1186/1471-2334-9-48
PMid:19386085 PMCid:PMC2679753
El Jalii IM (2008). Comparison between ELISA and the Microscopic Agglutination a test for the Diagnosis of Bovine Leptospirosis. Revue Élev. Méd. vét. Pays trop. 61 (2): 73–75.
Faine S (1982).Guidelines for the control of leptospirosis. Geneva: World Health Organization. 171. (WHO Offset Publication 67).
Faine S, Adler B, Bolin C and Perolat P (2000). Leptospira and Leptospirosis, 2nd Ed., Med. Sci., Melbourne, Australia:112-114
Kavanagh OV, Skibinska A, Mackie DP, Montgomery JM, Logan EF, Ellis WA (2002).In Bovine leptospirosis: Validation of an ELISA to detect antibodies to Leptospira borgpetersenii serovar hardjo. Res. Vet. Sci. 72:26–26
http://dx.doi.org/10.1016/S0034-5288(02)90075-5
Kocabiyik AL and Cetin C(2004) . Bovine leptospirosis in south Marmara region of Turkey : A serological survey .Revue Méd. Vét. 155)12: (606–608
Larson RL, Maas J, Richey E and Bolin C (2007). Herd Prevalence and Risk Factors of Leptospira Infection in Beef Cow/calf Operations in the United States: Leptospira borgpetwerseni Serovar Hardjo. Bovine Practitioner. 41 (1) :15–23.
Leonard N, Mee JF, Snijders S and Mackie D (2004). Prevalence of antibodies to Leptospira interrogans serovar hardjo in bulk tank milk from unvaccinated Irish dairy herds. Ir. Vet. J. 57(3):226–231.
http://dx.doi.org/10.1186/2046-0481-57-4-226
PMid:21851657 PMCid:PMC3113821
Miller DA, Wilson MA and Beran GW(1991). Survey to estimate prevalence of Leptospira interrogans infection in mature cattle in the United States. Am. J. Vet. Res. 52(11):1761–1765. PMid:1785719
Machang U RS, Mgdde G and Mpanduji D (1997). Leptospirosis in animals and humans in selected areas of Tanzania. Belg. J. Zool. 127: 97–104.
Mariyar C P, Kumar A A, Thangapandian E, Amuthar R and Srivastavava SK (2006). Evaluation of a recombinant LipL41 antigen of Leptospira interrogans serovar canicola in ELISA for serodiagnosis of bovine leptospirosis. Comp. Immunol. Microbiol. Infect Dis. 29(5):269–277.
http://dx.doi.org/10.1016/j.cimid.2006.06.007
PMid:16979238
Morgan L H, Mark A W, Tammy M A, Corrie L T and David A M (2007). Comparative serological study of Leptospira serovar hardjo genotypes for use in the microscopic agglutination test J. Vet. Diagn. Invest. 19(1):84–87 .
http://dx.doi.org/10.1177/104063870701900113
Peregrine AS, Martin SW and Hopwood DA (2006). Neospora caninum and Leptospira serovar serostatus in dairy cattle in Ontario. Can. Vet. J .47(3):467–470.
PMid:16734373 PMCid:PMC1444906
Pritchard DG (1986) National situation of leptospirosis in the United Kingdom. In: W.A. Ellis and T.W.A. Little (ed.) : Present state of leptospirosis diagnosis and control. Martinus Nijhoff, Dordrecht, The Netherlands : 221–233
Radostits OM, Gay CC, Hinchcliff KW and Constable PD (2007). In: Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses,sheep, Pigs and Goats. Saunders (Elsevier), Edinburgh : 1094–1110.
Rochat T (1998). A review of leptospirosis in farm animals in Portugal.Rev. Sci. Tech. 17: 699–712.
Ryan E G, Leonard N, Luke O'G, Michael L D and Simon J M (2012). Herd–level risk factors associated with Leptospira Hardjo seroprevalence in Beef/Suckler herds in the Republic of Ireland. Ir. Vet. J. 65(1):6.
http://dx.doi.org/10.1186/2046-0481-65-6
PMid:22449264 PMCid:PMC3342215
Surujballi OM, Mallory M (2004) . An indirect enzyme linked immunosorbent assay for the detection of bovine antibodies to multiple Leptospira serovars. The Can. J. of Vet. Res. 68 (1):1–6.
PMid:14979428
Thiermann A B (1984). Leptospirosis: current developments and trends. J. Am. Vet. Med. Assoc. 184(6):722–725.
PMid:6725107
Victoriano A F, Smythe L D, Gloriani–Barzaga N, Cavinta L L, Kasai T, Limpakarnjanarat K, Ong B L, Gongal G, Hall J, Coulombe C A, Yanagihara Y, Yoshida S and Adler B (2009). Leptospirosis in the Asia Pacific region. BMC Infectious Diseases . 9:147.
http://dx.doi.org/10.1186/1471-2334-9-147
PMid:19732423 PMCid:PMC2749047
Wagenaar J, Zuerner R L, Alt D, Bolin CA (2000).Comparison of polymerase chain reaction assays with bacteriologic culture, immunofluorescence, and nucleic acid hybridization for detection of Leptospira borgpetersenii serovar hardjo in urine of cattle. Am. J. Vet. Res. 61(3): 316–320.
http://dx.doi.org/10.2460/ajvr.2000.61.316
PMid:10714525
World Health Organization. (2003). Human Leptospirosis: Guidance for Diagnosis Surveillance and Control. Geneva: World Health Organization :109, ISBN 92–4–154589–5.