Advances in Animal and Veterinary Sciences
Review Article
Microalgae in Poultry Field: A Comprehensive Perspectives
Wafaa A. Abd El-Ghany*
Poultry Diseases Department, Faculty of Veterinary Medicine, Cairo University, 12211 Giza, Egypt.
Abstract | Many years ago, antibiotics have been added to animals and poultry feed as growth promotor. Hazardous using of antibiotics on long run creates several health problems for animals as well as humans. Therefore, countries searched for natural alternatives that incorporate antimicrobials in livestock and poultry ration without any adverse effect on both productivity and health. One of these important and promising alternatives is microalgae. Microalgae are microscopic, uni- or multi-cellular and photosynthetic algae that grow in marine, fresh and brackish water. They considered as rich sources of proteins, essential fatty acids, carbohydrates, vitamins, minerals, pigments and antioxidants. Several types of microalgae have been used safely in human, animals and poultry. Different species of microalgae as Spirulina, Chlorella and others proved positive influences on poultry nutrition. Therefore, this review article aimed to show the effects of using different types of microalgae on the productive characteristics, immune response, microbial resistance and carcass trait of poultry.
Keywords | Chlorella, Immunity, Performance, Poultry, Spirulina
Received | May 07, 2020; Accepted | July 15, 2020; Published | July 25, 2020
*Correspondence | Wafaa A. Abd El-Ghany, Poultry Diseases Department, Faculty of Veterinary Medicine, Cairo University, 12211 Giza, Egypt; Email: [email protected]
Citation | El-Ghany WAA (2020). Microalgae in poultry field: A comprehensive perspectives. Adv. Anim. Vet. Sci. 8(9): 888-897.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.9.888.897
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 El-Ghany. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Poultry industry is considered as one of the most important source of income all over the world. Due to continuous and extensive increase in human population, the need for safe and protein rich sources of poultry or livestock becomes very necessary. Antibiotics in feed resulted in development of drug-resistant bacteria (Sorum and Sound, 2001), antibiotic residues in products (Burgat, 1999) and decrease the numbers of intestinal microflora (Andremont, 2000). Hazardous using of antimicrobials in poultry field threatens this industry as they affect both birds and humans health. The European Union (European Commission, 2001) banned using of antibiotics growth promoters in poultry nutrition. So, many biologicals alternative strategies have been investigated to incorporate antimicrobials natural compounds without any adverse effect on both productivity and health.
Microalgae are microscopic, uni or multi-cellular and photosynthetic type of algae that present in both salty and fresh aquarium. Microalgae is regarded as a perfect source of valuable and naturally efficient nutrients, it extensively gained particular interest, attention and application among multiple producers worldwide. They have been tested in experimental animals hundreds of years as immunomodulatory, anti-inflammatory, antioxidant, antimicrobial and antiviral and proved their efficacy (Uyisenga et al., 2010; Abdel-Daim et al., 2013; Shokri et al., 2014; Ashgan et al., 2015). Microalgae may also considered as a promising fundamental constituents of animals and poultry ration. Blue-green microalgae; Arthrospira (Spirulina species) as Spirulina platensis (S. platensis) and S. maxima are the most efficient and widely distributed edible feed additive microalgae in humans, animals and poultry (Yoshida and Hoshi, 1980; Vonshak, 2002; Meineri et al., 2009; Kanagaraju and Omprakash, 2016). It has been found that Spirulina contains 50% -70% proteins (Soni et al., 2017) as well as most essential amino acids (Anusuya et al., 1981), so it could be used as alternative to protein sources in poultry ration (Spolaore et al., 2006; Austic et al., 2013). Besides, this type of microalgae showed high contents of fatty acids, carbohydrates, vitamin B complex (thiamine, riboflavin, pyridoxine and vitamin B12), vitamin C, vitamin A, vitamin E, minerals (calcium, iron, zinc, manganese, magnesium, phosphorus, copper, chromium, sodium and potassium), pigments (carotenoids, phycocyanin and xantophylls) and antioxidants (Ross and Dominy, 1990; Khan et al., 2005; Cheong et al., 2010; Holman and Malau-Aduli, 2012; Beheshtipour et al., 2013; Jafari et al., 2014; AbouGabal et al., 2015; Soni et al., 2017; Hynstova et al., 2018).
Chlorella species as Chlorella vulgaris is another type of natural green microalga that showed growing importance owing to beneficial nutritional and functional characteristics (Grau and Klein, 1957; Pulz and Gross, 2004; Sugiharto and Lauridsen, 2016). Chlorella is rich in proteins (48%), omega 3 and 9 fatty acids and phosphorus (Tokusoglu et al., 2003; Yaakob et al., 2014; EL-Mohsnawy et al., 2020). Chlorella has been sold as healthy food, cosmetics, antioxidants and as animal feed (Becker, 2007; Lee et al., 2010). In addition, other types of microalgae were defined and used for nutrition of broilers and layers without adverse effect on production or health.
Therefore, this review article was planned to deeply investigate the effects of using different types of microalgae on the productive characteristics, immune response, microbial resistance and carcass trait of poultry.
Influence of using microalgae in poultry field
Productive characteristics
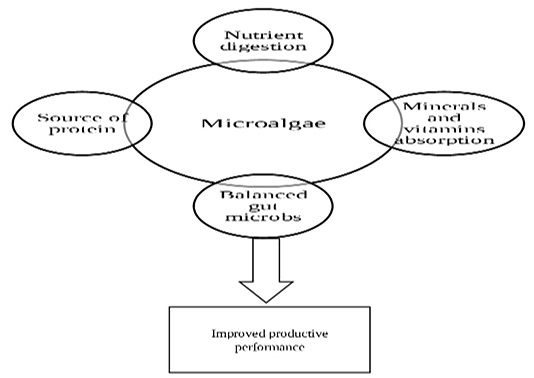
The mode of action of microalgae for improving the performance parameters is illustrated in Figure 1. Dietary incorporation of S. platensis for broilers were associated with improvement in live body weight, body weight gain and the feed conversion rate of broilers (Kharde et al., 2012; Shanmugapriya and Babu, 2014; Shanmugapriya et al., 2015; Park et al., 2018). The early study of Saxena et al. (1983) demonstrated that Spirulina at levels of 111 g/kg and 166 g/kg diet of White Leghorn chicks could replace groundnut cake with improving the weight gain of the birds. Similarly, Venkataraman et al. (1994) detected that addition of Spirulina (140 and 170 g/kg diet), without additional vitamins/minerals, could substitute groundnut cake and fishmeal and had no adverse effects on chicken’s performance. Addition of Spirulina at a level of 12% to broiler ration could replace the source of protein in it as well as enhance the growing and feed conversion rate (Ross and Dominy, 1990). Spirulina showed an improvement of nutrient digestion, minerals absorption and protection from diarrhea (Gružauskas et al., 2004). According to Nikodémusz et al. (2010), feeding of pheasants on ration containing 0.3 g Spirulina/ kg diet induced superior productive performance when compared with non-fed control birds. The same results were obtained by Mariey et al. (2014) who found that supplementation of broilers with very low level of Spirulina (0.02 or 0.03%) improved the performance. Kaoud (2015) recorded similar improvement in performance parameters induced by dietary inoculation of S. platensis when compared with prebiotic treated controls. It was demonstrated that incorporation of Spirulina algae up to 16% in broiler starter ration induced no negative effects on the chick’s performance (Evans et al., 2015). The authors determined that Spirulina contains high levels of both energy and proteins which representing 90% and 76%, consequently. In Japanese quails, addition of Spirulina powder in diet at level of 1% or in drinking water at 0.25% had beneficial significant effects on body weight, weight gain, conversion rate and fertility when compared with control groups (Abouelezz, 2017). Fathi et al. (2018) demonstrated that broilers diet supplemented by 0.7 and 0.9 g/kg of feed S. platensis could improve the growth performance parameters, in addition, S. platensis supplementing level (0.7g/kg) was economically recommended. Moreover, Abd El-Hady and El-Ghalid (2018) recorded that the final body weight, weekly body weight gain and feed conversion rate of broilers fed on 6% S. platensis were significantly higher than birds fed on 3% of this algae at 42 days of age. Treatments of ration with 0.25, 0.5, 0.75, or 1.0% Spirulina enhanced broilers body weight gain, feed conversion, and/or European production efficiency index during 8 to 21, 22 to 35, and overall 1 to 35 days of age (Park et al., 2018).
Regarding the effect of Chlorella on bird’s performance, a very early experiment conducted in chickens using 10% dried Chlorella into feed revealed increased performance in birds (Combs, 1952). The authors referred this result to the presence of pigments (carotene) and vitamins (B2 and B12) in Chlorella if the diet of birds didn’t contain these components. As well, Chlorella at dietary level of 5 or 10% provoked no adverse effects on broilers growth performance as algae considered as an excellent protein source (Lipstein and Hurwitz, 1983). In a subsequent study, Kang et al. (2013) concluded that addition of fresh liquid form of Chlorella at 1% dietary level beneficially affected body weight gain. The positive effect of feed supplementation of ducks with 0.1 or 0.2% Chlorella on body weight gain and feed intake was confirmed by Oh et al. (2015). Broiler chicken watered with culture of Chlorella vulgaris showed less consumption of food and the highest percentage of performance index and relative economic efficiency (Niamat and Ragaa, 2017).
Considering other microalgae, it was observed that addition of 6% of Micractinium spp. to broilers diet also induced positive effect on performance parameters (Lipstein and Hurwitz, 1981).
The improvement in performance characters may be related to the role of Spirulina in inducing balanced gut microbial population that enhances absorption of dietary vitamins and minerals and consequently increases the feed utilization efficiency (Tsuchihashi et al., 1987; Mariey et al., 2012).
The effect of using microalgae to improve the productive performance was contradictive, certain study showed that there was no improvement in the body production of chickens after feeding on ration containing 4 or 8% of Spirulina (Toyomizu et al., 2001). Zahroojian et al. (2013) also found that there was no difference in feed intake of laying hens fed on 1.5, 2 and 2.5% Spirulina and non-supplemented control group. Likewise, the cumulative feed intake in the 1% Spirulina treated birds was lesser than that of the control group (Kanagaraju and Omprakash, 2016). Water treatment of laying hens with Chlorella for 9 weeks induced no significant positive effect on feed intake or conversion ratio (Moradikor and Mohamadi, 2015).
Considering egg production performance parameters, it was noticed that supplementation of laying Japanese quails diet with Spirulina (1.5, 3.0, 6.0, or 12.0%) produced an increase in egg production with good egg quality (Ross and Dominy, 1985, 1990). Spirulina is considered as a very good source of pigments like carotenoids and xanthophylls which are very important of the intensity of egg yolk colour, as feeding of quails on dietary 1% Spirulina induced optimal colour of the egg yolk (Anderson et al., 1991). It was demonstrated that addition of 2.0-2.5% Spirulina to the diet of layers improved the absorption and accumulation of carotenoids and consequently increase the yolk colour intensity (Zahroojian et al., 2011, 2013). Similar experiment was carried out by Sujatha and Narahari (2011) who demonstrated that boiled eggs from Spirulina treated layer chickens exhibited sensorily more acceptable yolk colour than control birds. Increasing in egg performance, egg yolk colour and hatchability have been observed in hens fed on ration with of 0.10.2% Spirulina (Mariey et al., 2012). The effect of microalgae on fatty acids contents of eggs was also studies by Ginzberg et al. (2000) who found that addition of Spirulina valorized egg products by decreasing their contents of cholesterol and saturated fatty acid contents while increasing levels of omega-3 poly unsaturated fatty acids (PUFA). The same authors indicated significant increase in fertility and hatchability percentage of the produced eggs. Very recently, 29 to 40 weeks old layer chicken hens received 3 g S. platensis /kg produced significant higher egg number, egg weight and egg mass than control (Mobarez et al., 2018). Aljumaily and Taha (2019) reported that inoculation of quail’s eggs with Spirulina liquid extracts in the late stages of incubation could enhance the percentages of eggs hatchability, increase the chances of chick’s survival as well as strengthen their immunity and antioxidants status.
Feeding of laying hens with Chlorella has a beneficial influence egg quantity and quality as well as intestinal contents of lactic acid producing bacterial populations (Zheng et al., 2012). It was detected that Chlorella enhanced the production of lutein enriched eggs that inhibit macular destruction in elder human. In this context, supplementation of hens with conservative or Chlorella rich in lutein could effectively increase the egg quantity, lutein content and the yolk colour of eggs (An et al., 2014). Moreover, Chlorella increased lutein and zeaxanthin concentrations as well as improved the external egg quality and the oxidative constancy of fats in yolk of eggs (Englmaierova et al., 2013). Carotenes and xantophylls from the microalgae affect the egg yolk colour (Arakawa et al., 1960). Layer chickens taken ration containing Chlorella vulgaris produced more intensive yellow colour of the egg yolk as it is a rich source of carotenoid content (Lipstein et al., 1980; Batista et al., 2013). Spray dried or bullet milled and spray dried Chlorella vulgaris increased the number of the produced eggs, yolk colour and shell weight as well as hatchability performance (Halle et al., 2009). Janczyk et al. (2009) suggested that Chlorella encouraged increasing the verities of microflora in the intestinal tract which is important for the quality of eggs.
Marine microalgae (Schizochytrium limacinum) powder also exhibited positive effect on laying performance and egg quality as it enhanced docosahexaenoic acid (DHA) yolk concentration without alteration of sensory characteristics of eggs (Parpinello et al., 2006; Rizzi et al., 2009; Park et al., 2015). Addition of red seaweeds C. crispus and Sarcodiotheca gaudichaudii was found to be effectively act like prebiotics to improve chicken gut health, productivity, and egg quality (Kulshreshtha et al., 2014). In addition, Japanese quail layers fed on diet containing 0.5% Schizochytrium spp. microalgae produced eggs with high DHA concentration, low n-6/n-3 PUFA proporation and cholesterol level in the yolk fats (Gladkowski et al., 2014; Trziszka et al., 2014). As a source of n-3 PUFA, a dietary level 2.4% of Isochrysis galbana microalgae was considered as a rich source in egg yolk (Lemahieu et al., 2013, 2014). In the same context, diet containing Nannochloropsis gaditana might be used as an alternative source of omega-3 PUFA to produce DHA enriched eggs (Bruneel et al., 2013). The effect of feeding of layers on different concentrations of defatted Staurosira microalgae (soybean replacer) was tested (Leng et al., 2014) and the authors concluded that a level 7.5% of algae had no adverse effects, however, levels of 15% had bad effects on egg performance, feed intake and feed conversion rate. Two species of microalgal biomass (Desmodesmus and Staurosira) when used in laying hen ration at level up to 25% were considered as rich sources of protein with without bad effects on laying (Ekmay et al., 2015).
However, the effect of micro algae on egg performance parameters were also conflicting. The study of Moradikor and Mohamadi (2015) indicated that inoculation of different levels of Chlorella (0, 100, 200, 300 and 400 ppm) to the drinking water of laying hens for 9 weeks experimental period had no significant impact on egg production and egg mass. Moreover, Spirulina powder at level 1% in the feed and at 0.25% in the drinking water of 14-days-old Japanese quails did not show any significant effect on egg laying rate, egg weight and daily egg mass (Abouelezz, 2017).
The decrease in mortality rate of birds fed on microalgae reflects the improvement of the general health condition of birds. This speculation is supported by Cheong (2014) who noticed that supplementation of quails diet with 2% Spirulina resulting in significant reduction in the mortality rate.
Immune response
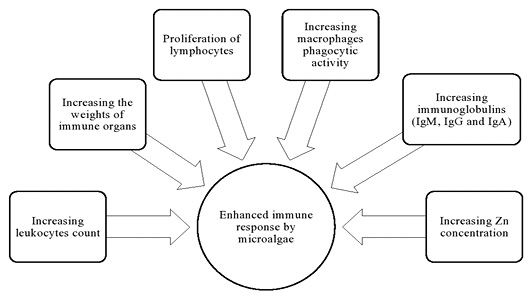
Enhancement of the immune response by microalgae is showed in Figure 2. The effect of supplementation with microalgae in the diet of chickens on the immune response was tested (Qureshi et al., 1994, 1996; Raju et al., 2004). The authors noticed an enhancement of phyto-haemagglutinin-intermediated the propagation of lymphocytes and the phagocytosis of macrophages as well as development of lymphoid organs. Birds received dietary Spirulina showed good health conditions indicating enhancement of immune response against diseases (Baojiang, 1994; Holman and Malau‐Aduli, 2012). Furthermore, addition of Spirulina at level of (0.05%) to broiler ration could partially reduce the adverse properties of mycotoxins on the weights of bursa, thymus and spleen (Raju et al., 2005). This enhancement was expressed by increasing the ability to kill microbes, synthetization of antigens and increasing of T cell activity. Broilers treated with S. platensis algae appeared to have an improved immune system as demonstrated by a significant increase in white blood cell count and enhanced macrophage phagocytic activity (Al-Batshan et al., 2001; Mariey et al., 2014). The health beneficial effect of S. platensis in poultry is related to the antimicrobial, immune-modulatory, anti-inflammatory and antioxidant capacity potentials of algae (Farag et al., 2016). Kaoud (2015) indicated an increase in the relative and absolute thymus and bursa weights of chicken groups fed diet containing Spirulina compared to the control group. In this respect, addition of S. platensis at levels of 0.7 and 0.9 g/kg broiler ration displayed significant increase in bursa, thymus and spleen weights as well as the increasing the level of serum globulin when compared with un-treated control (Fathi et al., 2018). Lokapirnasari et al. (2016) and Widya et al. (2016) revealed that treatment with S. platensis increased the number of leukocytes and it could be recommended as feed additive to increase the immunity of infected chickens against avian influenza (AI) H5N1 virus. Zeweil et al. (2016) detected that using of S. platensis at levels (0.5 and 1 g/kg diet) and vitamin E (75 mg/kg diet) enhanced the total antibody production specific for Newcastle disease virus (NDV) vaccine in heat stressed broiler chicks. Furthermore, Mobarez et al. (2018) indicated that dietary supplementation of layer diet with 3 g S. platensis /kg provoked antibody titers against NDV, AI, sheep red blood cells (SRBCs) and increased interferon proteins concentration compared to the control group. The enhancement of cellular immunity may be correlated to an increase of Zn concentration in Spirulina (Mohamed, 1998; Abdel-Daim et al., 2013; Abou-Gabal et al., 2015).
The effect of different forms of Chlorella algae in improving of immune indices was also showed by Kang et al. (2013). The results revealed that 1% dietary level of fresh liquid form of Chlorella had a positive effect on the immune response of the birds and it was expressed by increasing the count of white cells and lymphocytes as well as the level of immunoglobulins (IgG, IgM and IgA) in the blood. Stimulation of immune response by dietary Chlorella may be due to several mode of actions, the content of fibers and presence of polysaccharide (immurella), polypeptides as well as glycoprotein. In agreement, it has been demonstrated that chickens taken ration containing 0.5% Chlorella showed increasing in leucocytes phagocytic activity and lymphatic tissue development (Kotrbacek et al., 1994). Numerical rise in the reaction to phyto-hemagglutinin had been detected in broilers fed on diet with Chlorella (Rezvani et al., 2012). Moradikor and Mohamadi (2015) demonstrated that addition of 400 ppm of Chlorella to the drinking water of laying hens increase the antibody titers against SRBCs as expressed by increasing the levels of IgM and IgG.
Microbial resistance
Early findings of Tsuchihashi et al. (1987) suggested that feeding on ration containing Spirulina may boost population of Lactobacilli and increase vitamins absorbability. Concomitantly, Kulshreshtha et al. (2008) concluded that Spirulina is useful for the beneficial intestinal flora. It was demonstrated that S. platensis shared probiotics characters being possess a stimulating effect on the growth of Lactobacilli and Streptococcus thermophilus (Bhowmik et al., 2009). Dietary supplementation of broilers with Spirulina induced hypo-cholesterolmic properties due to reducing the synthesis and/or absorption of cholesterol in the intestinal tract as well as increasing the Lactobacillus population (Mariey et al., 2012). Chickens diets containing S. platensis demonstrated an increase in the number of intestinal lactic acid bacteria and a decrease in Escherichia coli (E. coli) (Shanmugapriya et al., 2015). As well, an increase in Lactobacilli count in the gut of Japanese quails after feeding with S. platensis was recorded (Yusuf et al., 2016). Recently, the results of Fathi et al. (2018) indicated significant decrease in the total intestinal bacterial and E. coli counts but significant increase in Lactobacillus count in broilers fed 0.9 and 0.7g S. platensis/kg ration. Kang et al. (2013) concluded that dietary supplementation of 1 % fresh liquid Chlorella improved body weight gain and increased the production of Lactobacillus bacteria in the intestinal microflora of broiler chickens. Inoculation of broiler diet with 1 % Spirulina powder increased the caecal Lactobacillus count (Park et al., 2018).
Feeding Chlorella microalgae resulted in increased Lactobacillus diversity in the crop or cecum or both of laying hens (Janczyk et al., 2009). Correspondingly, ducks fed on diet containing 0.1 or 0.2% Chlorella showed positive influence on the caecal microflora (Oh et al., 2015).
As regards the in-vitro antimicrobial effect of micro-algae, it was documented that S. platensis crude extract inhibited the growth of certain organisms including; Klebsiella pneumoniae, Shigella shigae, Pseudomonas aeruginosa, E. coli, Proteus vulgaris, Salmonella typhi (S. typhi) and Staphylococcus aureus (S. aureus) (Mala et al., 2009). Similar results were obtained by Kaushik and Chauhan (2008) and El-Baz et al. (2013) as they recorded antibacterial activities of S. platensis against S. aureus, E. coli, S. typhi and Enterococcus faecalis. In vivo study of Nuhu (2013) revealed S. platensis bacterial clearance capacity after experimental infection of chicks with E. coli and S. aureus. In Campylobacter jejuni experimentally infected broiler chickens, the efficiency of elevating the ration inoculation dose of Haematococcus pluvalis (astaxanthin source) was evaluated. The results revealed that the tissue concentrations of astaxanthin were increased by increasing the levels of dietary algae, no effect of algal meal on performance and the caecal population of Campylobacter jejuni was also not affected, but a level of dietary 0.18% of algae reduced the count of caecal Clostridium perfringens (Waldenstedt et al., 2003). Lorenz and Cysewski (2000) concluded that incorporation of poultry feed with microalgae Haematococcus spp. as a source of protein could improve their health and productivity. The antimicrobial effect of micro-algae may be related to presence of some acids like γ linolenic acid, active fatty acid, lauric and palmitoleic acid (El-Sheekh et al., 2014) or due to existence of alkaloids and lipopolysaccharides (Rania and Hala, 2008).
Nevertheless, results recorded in the study of Sugiharto et al. (2018) detected that treatment of broilers with S. platensis did not affect the ileal and caecal populations of Lactobacilli.
Carcass trait
It was shown that replacement of groundnut protein or fish meal by up to 170 or 140 g/kg of broiler ration Spirulina induced deeper color pigmentation of skin, breast, and thigh muscles (Venkataraman et al., 1994). Addition of Spirulina in broiler ration influenced the yellow and red colour of meat (Toyomizu et al., 2001). Accumulation of yellow pigmentation in flesh is related to presence of zeaxanthin pigments of Spirulina. Mariey et al. (2014) found an increase in dressing percentage, meat colour score and decreased relative abdominal fat weight after raising of birds on dietary 0.02 or 0.03% Spirulina. Similarly, feeding of ducks on dietary 0.1 or 0.2% Chlorella was able to improve the meat quality (Oh et al., 2015). The improvement of meat quality may be related to antioxidants activity of Spirulina which enhances integrity of muscle fibers and consequently capability of muscles to retain water (Dal Bosco et al., 2014). Bonos et al. (2016) documented that dietary Spirulina (5 g/kg of ration) influenced improving in the meat quality of broiler chicks through increasing the contents of eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in the thigh muscles of birds. Lately, Altmann et al. (2018) concluded that soybean meal in broiler ration could be replaced safely by Spirulina without deteriorating meat quality.
To study the effect of Crypthecodinium cohnii microalgae on the carcass traits of Muscovy ducks, Schiavone et al. (2007) found that addition of 0.5% of this algae induced positive effect on the contents of fats in the breast muscles with no bad effects on production, carcass quality, and biochemical characteristics like pH, colour, oxidative constancy as well as sensory features of the breast muscles. Another study on Schizochytrium microalgae demonstrated that incorporation of this algae in broiler diet at levels 0.1 or 0.2% enriched the contents of fatty acids in the lipid of breast muscles with no adverse effect on the body weight gain (Yan and Kim, 2013). Supplementation of 3% green seaweed Ulva lactucain to broiler chickens increased the yield of breast muscle in comparison with birds fed corn diet only (Abudabos et al., 2013).
Some studies should that addition of microalgae has no influence on the carcass trait. Cheong et al. (2016) found that 15-35 days old Japanese quails supplemented with up to 8% of Spirulina in feed exhibited no significant effects on carcass composition or meat quality by decreasing the drip loss. In the same context, Spirulina in diet or in the drinking water of Japanese quails did not display significant differences considering the dressing percentage, the relative weights of heart, gizzard, liver, abdominal fat, testes, spleen and ovary as well as the length of caeca, small intestine and oviduct (Abouelezz, 2017). There was no significant effect of 1% Spirulina on relative organ weight and breast meat quality of broilers fed with diets (Park et al., 2018).
CONCLUSION
From above mentioned, it could be concluded that different types microalgae could be used as useful and safe alternatives to many constituents of poultry ration like proteins, fatty acids and other essential elements. Microalgae proved great efficacy in improving the productive performance of broilers and layers, enhancement of cellular and humoral immunity, maintaining the beneficial intestinal bacteria and destroy the pathogenic ones as well as improving the carcass traits. So, in the near future, there is a great demand to use microalgae in a large scale of poultry industry.
AUTHOR CONTRIBUTION
Wafaa A. Abd El-Ghany collected the data, wrote and prepared the manuscript.
CONFLICT OF INTEREST
The author have declared no conflict of interest.
REFERENCES