Journal of Animal Health and Production
Case Report
Application of Autogenous Vaccine for the Treatment of Bovine Cutaneous Papillomatosis Type 2 in Malaysia
Nurbakyah Khalid, Intan Noor Aina Kamaruzaman, Siti Nor Che Yahya, Ibrahim Abdul Azeez-Okene, Mohd Farhan Hanif Reduan, Nurshahirah Shaharulnizim*
Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia.
Abstract | This is the first case report highlighting the application of an in-house autogenous vaccine for the treatment of bovine cutaneous papillomatosis Type 2 in a Jersey crossbred cow in Malaysia. A sample of papilloma growth was excised, homogenised, treated using 0.5% (v/v) formalin and performed microbiological examinations for sterility check. The solution was then administered subcutaneously to the cattle at seven days interval for four weeks. The papilloma growth on the affected cattle regressed rapidly following treatment, and there were no undesirable side effects and recurrences observed. The use of autogenous vaccine as an alternative treatment for bovine papillomatosis is preferred over surgical excision as it is proven effective, safe, and low chances of recurrence.
Keywords | Autogenous vaccine, Bovine papillomavirus, Papillomatosis, Tumour, Wart
Received | September 07, 2020; Accepted | October 08, 2020; Published | November 27, 2020
*Correspondence | Nurshahirah Shaharulnizim, Faculty of Veterinary Medicine, Universiti Malaysia Kelantan, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia; Email: [email protected]
Citation | Khalid N, Kamaruzaman INA, Yahya SNC, Azeez-Okene IA, Reduan MFH, Shaharulnizim N (2021). Application of autogenous vaccine for the treatment of bovine cutaneous papillomatosis type 2 in malaysia. J. Anim. Health Prod. 9(1): 1-4.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.1.1.4
ISSN | 2308-2801
Copyright © 2021 Shaharulnizim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bovine papillomatosis is a common viral disease of cattle caused by any member of the bovine papillomaviruses (BPV). The disease generally affects the integument system of cattle and indirectly causes economic losses in terms of productivity and depreciation of animals’ value (Freitas et al., 2011). The disease is prevalent in cattle globally and most commonly observed in dairy cattle (Hauck, 2008). Bovine papillomatosis is highly infectious, manifest as neoplastic-like growth on the integument system. The growth on the skin can be characterised as multiple benign wart-like projections (papilloma) or can advance to a malignant form affecting multiple organs of the animal and is potentially lethal (Borzacchiello and Roperto, 2008).
Several risk factors are thought to be associated with BPV such as inheritance, nutritional and hormonal imbalance, exposure to sunlight, and poor host immunity (Ranjan et al., 2013). Although the source of the virus is not well understood, it has been suggested that the virus is present in
the environment and can be transmitted direct (via bodily discharges) or indirect (via fomites or vectors) (Freitas et al., 2011). Female and young cattle (<1 year old) are predisposed to the disease compared to male or adult cattle (Salib and Farghali, 2011). The diagnosis of the disease is made based on cutaneous wart lesions on multiple skin sites such as head, eyes, ears, neck and udder. Additionally, progressive papilloma growth can reach to the adjacent organs such as the alimentary tract, genital mucosa, and mammary gland causing mucosal tumours of which the animals may show signs such as difficulty in breathing, weight loss and reduced milk yield (Campo, 2012; Munday, 2014). Several treatment options for bovine papillomatosis are available such as surgical excision and the use of chemotherapy to remove and regress the tumour growth in infected cattle (Hauck, 2008). This case report highlights a case of bovine cutaneous papillomatosis in cattle, which was successfully treated using a formalin-inactivated BPV autogenous vaccine.
Case Details
An 8-month-old Jersey Crossbred cow was presented to the Veterinary Clinic of University Malaysia Kelantan, with a complaint of multiple wart-like growths around the left eye. The owner claimed that the growth progressively increased in size for several weeks and the animal exhibited vision problems. General physical examination on the cattle exhibited normal vital parameters, except the left eye. A close examination on the left eye revealed multiple periorbital nodular, pedunculated brownish keratinised wart-like growth of sizes ranging from 0.5-3.0 cm (Figure 2A). The left eye vision was normal. The differential diagnosis of this case included bovine cutaneous papillomatosis and squamous cell carcinoma based on the lesion observed, and a biopsy sample from the wart was taken, fixed in 10% (v/v) formalin, and sent for histopathology and molecular detection by polymerase chain reaction (PCR) using specific papillomavirus primers [FAP 59 (Forward: 5’-TAACWGTIGGICAYCCWTATT-3’) and FAP 64 (Reverse: 5’-TACGTGGGAAGCGCCTCGCT-3’)], targeting papillomavirus viral protein L1 (Strugaru et al., 2012). A phylogenetic tree was then constructed to determine the relationship of the PCR positive papillomavirus and related bovine papillomaviruses using Molecular Evolutionary Genetics Analysis version 10.0 (MEGA X) (PSU, USA).
The histopathology findings revealed finger-like projection papillae extending from the epidermis layer to the dermis layer (Figure 1A). On a high magnification view (40X), hyperkeratosis was observed on the epithelial layer and extension of the stratum spinosum with elongated rete pegs (Figure 1B), in addition to koilocytosis formation, which is characterised by giant clumped pleomorphic keratohyalin granules and ballooning degeneration of the keratinocyte, were present on the elongated rete peg in addition to epithelial proliferation (Figure 1C and 1D). These lesions are indicative of papillomavirus (Taylor and Haldorson, 2013; Russo et al., 2020). Moreover, the PCR result of the tissue sample was positive for bovine papillomavirus and further confirmed as BPV Type 2 by sequencing and phylogenetic analysis (Figure 3).
The cow was treated using an autogenous bovine papillomatosis vaccine prepared in the laboratory, as described previously with a slight modification (Mayilkumar et al., 2014). Briefly, ~20g of papilloma tissue was excised aseptically and suspended in 500 ml of 0.9% (w/v) normal saline. The tissue was then homogenised, filtrated, and treated using 0.5% (v/v) formalin (HmBG Chemicals, Germany). The sterility of the suspension was checked by inoculating some solution in a blood agar (Oxoid, UK), McConkey agar and nutrient agar (HiMedia, India) (for bacterial growth) and Sabaroud Dextrose Agar (SDA) (HiMedia, India) (for fungal growth) respectively. Lastly, the sterile suspension was stored at 4°C overnight before use. A 5.0 mL of vaccine solution was administered to the cow subcutaneously at intervals of seven days for four consecutive weeks. The cow responded well to the treatment, resulting in nodule regression. Continuous observation on the lesion was made four more weeks after the last treatment regime, and the cow showed almost complete regression (Figure 2D). There were no side effects and nodule recurrences on the left eye and elsewhere on the body. The owner was advised to keep monitoring for the recurrence and keep the cow in a clean state to avoid flies.
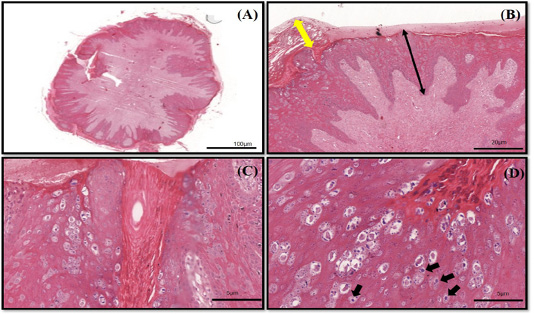
Figure 1 (A-D): The histopathology findings of bovine papilloma tissues in this case. (A) Low magnification showing a well-developed finger-like projecting papillae rising from the surface of the epidermis to the subcutaneous layer. (B) Hyperkeratosis of the epithelial layer (double yellow arrow) with acanthosis formation (double black arrow). (C) Epidermal proliferation with elongated rete pegs and neoplastic fibroblast. (D) Multiple koilocytosis formation on the dermis layer (black arrows). H&E staining 10X and 40X.
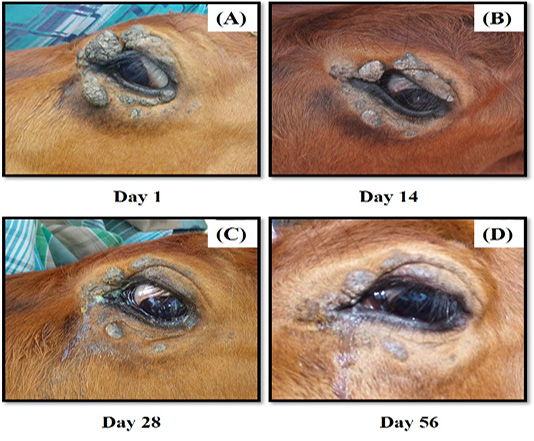
Figure 2 (A-D): The progression of treatment after administration of autogenous BPV vaccine. There was marked regression of the tumour growth from (A) Day 1, (B) Day 14, (C) Day 28 and (D) Day 56.
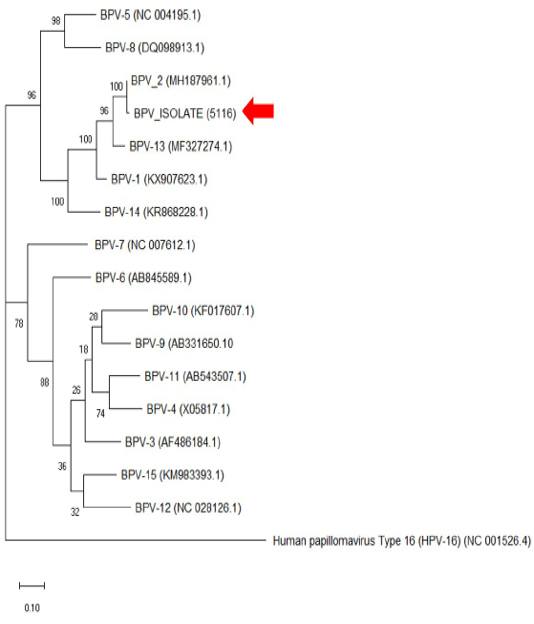
Figure 3: The phylogenetic analysis of the genome sequence of bovine papillomaviruses using the maximum-likelihood method by 1,000 bootstrap replications. A positive sample in this study is indicated by an arrow.
Discussion
Bovine papillomatosis is an economically important disease affecting cattle production worldwide. Although the disease does not pose a zoonotic risk to humans, its malignancy effects reported in animals are equivalent to human papillomavirus (HPV) (Araldi et al., 2017). The papilloma or fibropapilloma growth can occur on the skin and multiple internal organs depending on the diversity of infecting BPV genotypes. The BPV-1 and BPV-2 cause cutaneous fibropapillomas and can also cause cancer of the urinary bladder; BPV (-1,-5,-6,-9 and -10) can cause papillomatosis of the teat and udder, BPV-4 is associated with upper gastrointestinal cancer, BPV-3 and BPV-8 can cause cutaneous papillomatosis; (Borzacchiello and Roperto, 2008; Freitas et al., 2011). From the phylogenetic analysis in Figure 3, it was clear that the cow in this case was infected with BPV-2, which corresponds to the skin lesion shown by the animal. Interestingly, the result is similar to the BPV isolates (Genbank accession no: MH187961.1) in the Amazon region found in a recent study (Daudt et al., 2019).
Typically, the affected animals may recover without treatment. However, some BPV cases can progress to a malignant form, followed by a secondary bacterial infection (Muday, 2014). Besides histopathology, electron microscopy would be an ideal method for the detection of the virus from the skin sample, and molecular methods such as PCR and viral genetic analysis are useful to identify the type of BPV corresponds to the infection (Lange et al., 2009; Yhee et al., 2010; van Dyk et al., 2011).
An autogenous vaccine is considered as an excellent choice of treatment for bovine papillomatosis. It is thought that the vaccine stimulates tumor necrosis factor-α and interleukin 1 (IL-1), which will trigger the production of interleukin-6 (IL-6) and leukocyte as a result of inflammatory responses. Increasing the level of IL-6 leads to the activation of both cellular and humoral immunity causing regression of the papilloma tissues (Aydin et al., 2020). The use of autogenous vaccines has reported a high efficacy rate up to 94% (Lesnik, 1999) and has been successfully used to treat bovine papillomatosis cases over the last two decades (Turk et al., 2005; Ranjan et al., 2013; Mayilkumar et al., 2014). Furthermore, autogenous BPV vaccines are proven safe and can be used as a field vaccine to prevent BPV infection in the cattle herd within a farm. Another treatment option, such as surgical excision of pedunculated warts, is not recommended due to possible recurrence (Shimakura et al., 2018). To our knowledge, this is the first case report describing the use of an in-house autogenous vaccine for the treatment of bovine cutaneous papillomatosis in Malaysia.
Conclusion
Bovine cutaneous papillomatosis is an oncogenic viral disease that can be managed using chemotherapy applications. The autogenous vaccine was proven safe and effective to regress the tumours size and to prevent the growth recurrence, which was successfully attempted in this case.
Acknowledgment
All authors wish to thank the staff of Veterinary Clinic of Universiti Malaysia Kelantan and laboratories for their technical assistance during the handling of this case.
Conflict of interest
All authors declare that there is no conflict of interest.
Authors’ contribution
All authors contributed equally in the preparation of the manuscript.
References






