Journal of Animal Health and Production
Research Article
Differential Expression of Factors Associated with Embryo Quality in Endometrial Epithelial Cells of Superovulated Korean Native Cattle
Enkhbolor Barsuren1, Sang-Hwan Kim2, Jong-Taek Yoon2,3*
1School of School of Animal science and biotechnology, Mongolian University of Life Sciences, 17024 Zaisan, Khan-uul District, Ulaanbaatar, Mongolia; 2Institute of Genetic Engineering, Hankyong National University, 327, Jungang-ro, Ansung, Gyeonggi-do, 17579, Korea; 3Department of Animal Life and Environment Science, Hankyong National University, 327, Jungang-ro, Ansung, Gyeonggi-do, 17579, Korea.
Enkhbolor Barsuren and Sang-Hwan Kim contributed equally to this work.
Abstract | Embryo transfer (ET) has become the most powerful tool for animal breeders and animal scientists to improve the genetic makeup of their herds. In this study, embryos were analyzed and divided into three groups based on their prevalence in the endometrial tissue, and the genes affecting embryo quality were investigated. The aim of this study was to analyze embryo quality-related gene expression in the endometrial epithelial cells (EECs), embryos, uterine milk, and serum. Our results suggested that FSH and LH positively affected embryo quality; however, the specific regulatory mechanisms involved need further investigation. We found a significantly higher expression of MMP-2 in the low-quality group compared to the good-quality group, and MMP-9-dependent regulation of embryo quality in the good- and fair- quality groups. Higher expression of apoptotic genes, Casp-3 and 20α-HSD, promoted apoptosis in the low-quality group, compared with the good-quality group. Thus, in this study, 20α-HSD and Casp-3 and apoptosis-inducing genes were seen to be highly expressed in the low-quality group, suggesting that MMP-2 is involved in ECM degradation. These results indicate that the expression of MMPs and apoptotic genes in the EECs can be used as a genetic marker to select good-quality embryos. Based on the expression of MMPs and apoptotic genes, we proposed that MMPs play a role in determining the quality of the embryo, by regulating the remodeling system in EECs.
Keywords | Endometrial epithelial cell, Pre-implantation, Classification, Embryo quality, Bovine
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | December 26, 2019; Accepted | March 31, 2020; Published | May 04, 2020
*Correspondence | Jong-Taek Yoon, Institute of Genetic Engineering, Hankyong National University, 327, Jungang-ro, Ansung, Gyeonggi-do, 17579, Korea; Email: [email protected], [email protected]
Citation | Barsuren E., Kim S-H, Yoon J-T (2020). Differential expression of factors associated with embryo quality in endometrial epithelial cells of superovulated Korean native cattle. J. Anim. Health Prod. 8(2): 59-70.
DOI | http://dx.doi.org/10.14737/journal.jahp/2020/8.2.59.70
ISSN (Online) | 2308-2801
Copyright © 2020 Barsuren et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Embryo transfer (ET) is a powerful tool to disseminate good genetic traits to improve herd performance (Hasler, 2014). However, embryonic survival rates after ET vary widely, from 40% to 70%. Moreover, ET is an expensive and inefficient technology. The success of ET is determined by many factors, including quality of embryo, season, breed and age of cattle, nutritional status, stress management, and treatment protocols. Although the effects of intrinsic and extrinsic factors on pregnancy rates of ET have been elucidated (Nishigai et al., 2002; Betteridge, 2006; Kawate et al., 2007; Wu et al., 2012), conception rates, following ET, have changed very little over the years (Hasler, 2003). In mammals, the uterus carries the developing fetus (Arai et al., 2013). In most mammals, conceptus implantation to the uterine endometrium consists of blastocyst hatching, migration, apposition/attachment, invasion, and placenta formation. It is known that close to 50% of fertilized pre-implantation embryos in mammals, including humans, fail to implant (Wilcox et al., 1988). Implantation takes place in the endometrium and this layer experiences morphological and functional changes that are closely associated with the cyclic release of hormones (Christine et al., 1999). During estrus, the bovine endometrium undergoes regeneration, preparing for blastocyst implantation (Arai et al., 2013). If implantation does not occur, the endometrium sloughs during diestrus and regenerates from the next estrous cycle (Arai et al., 2013). The mechanism underlying bovine endometrial growth during estrous cycles remains unclear (Zhang et al., 2004, 2018). Degradation and regeneration of the endometrial extra-cellular matrix (ECM) is a vital process that involves matrix metalloproteinases (MMPs). MMPs, including MMP-2 and MMP-9, play an important role in tissue remodeling in various pathological and physiological processes, such as implantation, uterine and ovarian functions during peri-partum, cancer development, and wound healing (Nagase et al., 1999; Takagi et al., 2007). MMP-2 is expressed in stromal cells in cats and humans during the gestation and uterine cycle, respectively (Freitas et al., 1999; Rodgers et al., 1994; Walter et al., 2006), and may also play a role in stimulated uterine recrudescence. MMPs are proteolytic enzymes that depend on zinc and calcium ions to degrade the ECM in various tissues (Nagase et al., 1999). MMP-9 plays an important role in tissue remodeling in various physiological processes, including implantation and ovarian and uterine functions, during estrus and pregnancy (Smith, 1999).
Many factors, including proteases, growth factors, cytokines, and steroid hormones, play important roles in tissue remodeling and coordination of endometrial function, resulting in a suitable environment for embryo attachment during early pregnancy (Fortier et al., 1988; Stewart et al., 1992; Cross et al., 1994; Lim et al., 1997; Sharkey et al., 1998; Xiao et al., 1999). In days 8 and 17 of cow pregnancy, during which maternal recognition of pregnancy must occur, attenuation of oxytocin-induced PGF2α production is important in determining the success or failure of pregnancy (Bazer et al., 1991; Spencer et al., 1999). Oxytocin receptors are required for the synthesis and secretion of luteolytic PGF2α (Hansen et al., 2017). Previous studies have indicated that an increase in BAX/BCL2 ratio occurs around 4 h after PGF treatment, and precedes the increase in Caspases 8, 9, and 3 activities, which induce apoptosis (Yadav et al., 2005). The selective COX-2 inhibitor, NS-398, induced regression of endometriotic implant through caspase-3-dependent apoptosis in a hamster model (Laschke et al., 2007). Caspase-3, a key downstream effector of apoptosis, belongs to the cysteine protease family, and is known for its function in the mitochondrial apoptotic pathway and death receptor pathway (Zhivotovsky et al., 1997). Growth factors including CTGF, FGF-2, IL-8, TGF-β1, MMP-2, and VEGFA as well as proliferation of epithelial and fibroblast cells are the foundation for endometrial remodeling (Caron et al., 2009; Kizaki et al., 2008; Maybin et al., 2011; Welter et al., 2004). The latter are mainly synthesized and secreted by the ovary following the follicle-stimulating hormone (FSH)-mediated activation of an aromatase that converts androgens to estrogen (Whitlock et al., 1986, 1998). Since endometriotic lesions contain estrogen receptors (Lessey et al., 1989; Bergqvist et al., 1993; Fujishita et al., 1997) and aromatase (Noble et al., 1996; Kitawaki et al., 1997; Ferrero et al., 2014) it has been suggested that local estrogen production may stimulate the growth of lesions. We have established an in vitro co-culture system with bovine trophoblast CT-1 cells and primary uterine EECs that mimic the in vivo attachment process (Bai et al., 2012). However, primary EECs are not ideal for long-term studies, because these cells undergo some de-differentiation in culture, for example, loss of growth factor/cytokine and hormone responsiveness, and have a short life span before senescence. The bovine EEC is a good in vitro model to investigate the mechanism associated with the inhibition of oxytocin-induced PGF2α production by conceptus interferon-tau. The ECM is composed of various molecules, including collagens, heparan sulfate proteoglycans, and laminins, and provides a suitable structural microenvironment for the embryo. It is necessary for the ECM to undergo proteolytic degradation during early pregnancy, both in primates with invasive placentation and ungulates with non-invasive placentation (Ding et al., 2002; Yamada et al., 2002). MMPs contribute to the proteolytic degradation of the ECM in physiological and pathological conditions. A role for MMPs in remodeling uterine tissues during the estrous cycle and pregnancy has been described in several species, including humans (Behrendtsen et al., 1992; Fowlkes et al., 1994; Shimonovitz et al., 1994; Huang et al., 1998; Salamonsen et al., 1999). Insulin-like growth factor-II (IGF-II) is one of the best characterized pro-mitogenic and anti-apoptotic molecule, which in the cow is secreted by the embryo and reproductive tract tissues (Lonergan et al., 2003; Yaseen et al., 2001; Armstrong et al., 2003; Robinson et al., 2000). Using radio-immunoassays, established on human derived antigens, the immunoreactivity of pregnancy-associated plasma protein A (PAPP-A), protein phosphatase 5 (PP5), and placental protein 14 (PP14) was detected in placental extracts and blood of pregnant baboons (Sinosich et al., 1990). The addition of vascular endothelial growth factor (VEGF) and cysteamine in two sequential steps to the maturation medium resulted in an improvement of cytoplasmic maturation, with a positive impact on oocyte development capacity by increasing the efficiency of in vitro blastocyst production (Anchordoquy et al., 2015). 20-Hydroxysteroid dehydrogenase (20α-HSD) plays an important role in controlling the cellular concentration of progesterone by catalyzing its NADPH-dependent reduction into 20α-hydroxyprogesterone. The HSDs, which are members of the aldo-keto reductase (AKR) family, play pivotal roles in the modulation and regulation of steroid hormones, such as androgens, estrogens, and progestins, and are thus considered important targets for drug design (Penning et al., 2004).
EECs undergo profound changes in structure and function in preparation for blastocyst implantation. Functional changes occurring during the bovine estrous cycle are of specific importance for the preparation of the reproductive tract, which hosts final maturation of the gamete, fertilization, and embryonic development. Embryonic losses occur predominantly during the preimplantation period (Thatcher et al., 2006). A well-synchronized maternal environment must therefore be present to allow normal development. MMPs and tissue metallopeptidase inhibitors (TIMPs) are most important mediators in the process of ECM remodeling (Visse et al., 2003). MMPs and TIMPs are involved in the regulation of key steps in reproduction, such as ovulation and luteolysis, endometrium function, growth and development of fetal membranes, cervix dilation during parturition, and postpartum regression of the uterus. Uterine receptivity requires timely and spatially controlled changes of the ECM, in which MMPs are involved. The transcription of MMPs may be stimulated through cytokines, growth factors, hormones, and cell-cell or cell-matrix interactions (Nagase et al., 2006; Nagase et al., 1999). FSH, a member of the glycoprotein hormone family, plays a key role in the regulation of reproductive function and ultimately the production of gametes and fertility (Hermann and Heckert, 2007; Minj et al., 2008). Estradiol, progesterone, FSH, and LH, used together, could influence oocyte quality (Zacarias et al., 2018). If the immortalized EECs, in which characteristics of primary EECs could be maintained for longer periods, were established, the in vitro co-culture system (Sakurai et al., 2012) would become a more valuable tool to study interaction and events associated with conceptus attachment, with limited invasion of the maternal endometrium. The biochemical properties of genes associated with embryo quality have not yet been characterized. These events are often overlooked or cannot be studied, even though early embryonic losses occur during this period. We investigated the relationship between the action and expression of the factors involved in embryo quality in three different groups of EECs. Thus, this study analyzed embryo quality related genes expression in the EECs, uterine milk, and serum. Additionally, we investigated the effect of FSH, LH, and FSH+LH on EECs and early developing embryos.
Materials and Methods
Ethical approval of study
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Hankyong National University (Permit Number: 2018-2).
Animals
This study was done with data collected from the embryo transfer program (2017) of a commercial farm rearing Korean native cattle. Females with a normal body condition score (BCS), adequate postpartum period, normal estrous cycle, normal uterus, living under normal conditions with adequate nutrition, and normal health, were used in this study.
Synchronization of donors and recipients
The donors were administered 30 AU of FSH (Antorin, Kawasaki, Japan) once every 12 h for 4 d to 5 d, after heat. Four days after the insertion of the progesterone releasing intravaginal device (CIDR-PLUS, Bioniche Animal Health, Australia) into the vagina of the cows, using a CIDR injector, estrus cycle was induced by the administration of FSH for 4 d, with an interval of 12 h between each FSH injection. PGF2α was administered 2 d after FSH injection. Four days later, CIDR-PLUS was removed. Artificial insemination was carried out using frozen semen, thrice with a 12 h interval between the procedures, after the injection of GnRH (Table 1).
Collection of in vivo-produced embryos
Embryos were collected 7 d after inseminations by transcervical uterine flushing using Dulbecco’s phosphate-buffered saline (D-PBS) media. Local anesthesia was induced by administering 2% lidocaine (Jeil Pharm, Korea) between the first and second lumbar vertebrae and embryos were flushed using a Foley catheter. The recovered embryos were evaluated according to the IETS classification and EECs were collected from Korean native cattle (n = 10).
Uterine flushing, for recovering embryos and EECs, was performed at day 7 post-ovulation, using nonsurgical uterine lavage. Embryos (grade 1, 2) were transferred using a non-surgical technique.
Approximately 20 mL of the solution that remained with the EECs in the collection cup was placed in a petri dish and analyzed under a stereomicroscope at 10X magnification. The EECs were classified into two grades of good and low-quality groups, according to their quality. Treatment of red blood cells with EECs was performed for a duration of 10 min. The retrieved EECs were washed twice in the medium containing EECs, to remove cellular debris.
Table 1: Embryo transfer program for donors and recipients.
| Treatment day | Donor | Recipient | ||
| AM | PM | AM | PM | |
| 0 | P4 device insertion + 50 mg P4 + 1 mg E2 | P4 device insertion + 50 mg P4 + 1 mg E2 | ||
| 4 | 6 mg FSH | 6 mg FSH | - | - |
| 5 | 5 mg FSH | 5 mg FSH | - | - |
| 6 |
4 mg FSH 30 mg PGF2α (dinoprost) |
4 mg FSH 30 mg PGF2α (dinoprost) |
0.625 mg PGF2α (cloprostenol) |
- |
| 7 | P4 device removal 3 mg FSH | 3 mg FSH | P4 device removal | - |
| 8 | Estrus | AI 1 mg GnRH | Estrus | 250 μg GnRH |
| 9 | AI | AI | - | - |
| 15 | Flushing | Embryo transfer | ||
Table 2: Primers for Real-Time PCR analysis of genes associated with embryo quality.
| No. | Primer name |
Sequence (5ʹ→3ʹ) |
(°C) | |
| 1 | 18Sr | Forward | TCGCGGAAGGATTTAAAGTG | 60 |
| Reverse | AAACGGCTACCACATCCAAG | |||
| 2 | PAPP-A | Forward | ACGACACATGTGGCTTCA | 53 |
| Reverse | CATTGACAGCTCCACTAG | |||
| 3 | VEGF | Forward | TGTAATGACGAAAGTCTGCAG | 55 |
| Reverse | TCACCGCCTCGGCTTGTCACA | |||
| 4 |
20α-HSD |
Forward | GGAAAGCGGATAGTCAGGGTGATC | 59 |
| Reverse | GCCATTGCCAAAAAGCACAAG | |||
| 5 | OT | Forward | ACTCCACCATCAAACCAAGC | 51 |
| Reverse | ACCCCAAGCTCAGACAGCTA | |||
| 6 | MMP-2 | Forward | ATGACCGAGGCGCGAGTGTC | 65 |
| Reverse | TCAGCAGCCCAGCCAATCGGA | |||
| 7 | MMP-9 | Forward | CTTGCCTTCTCATGCTGGGACT | 62 |
| Reverse | GTGAGGATAGCACTTGGTCTGGCT | |||
| 8 | TIMP-2 | Forward | GGGTCTCGCTGGACATTG | 57 |
| Reverse | TTGATGTTCTTCTCCGTGACC | |||
| 9 | TIMP-3 | Forward | CCCTTCCCACTGAGCTTCCCTT | 64 |
| Reverse | CTATCTGCTGGCTGCCCTTGAC | |||
| 10 | Estradiol | Forward | ACAAGCGCCAGAGAGATGAT | 51 |
| Reverse | AGGATCTCTCTAGCCAGGCACA | |||
Embryo transfer and pregnancy diagnosis
The recipients were observed daily for signs of spontaneous estrus, and ET was performed on day 7 after estrus was detected. All recipients had their ovaries palpation per rectum on the day of ET to verify the presence of a CL. All ET were performed non-surgically into the lumen of the cranial portion of the uterine horn, ipsilateral to the CL. Before ET, epidural anesthesia was administered using 4 mL of 2% lidocaine solution. Pregnancy was confirmed by palpation of the uterine tract per rectum at 60 d after ET. The methods described in this study include different steps of in vitro production of bovine embryos up to the 8-cell stage in semi-defined conditions: (1) oocyte maturation, (2) in vitro fertilization, and (3) in vitro development. The first section explains procedures of ovary collection and oocyte aspiration and selection for in vitro maturation. The second section involves methods for the preparation of semen and oocytes for fertilization. The last section explains the best conditions to obtain 8-cell embryos after 3 d of in vitro culture.
Isolation of RNA and cDNA synthesis from EECs
Total RNA was extracted from EECs using the TRIZOL reagent (Invitrogen, USA), according to manufacturer’s recommendations. The concentration of total RNA was determined by absorbance at 260/280 nm, using a Beckman DU 600 spectrophotometer (Beckman Instruments, San Ramon, CA). First-strand cDNA synthesis reaction was catalyzed by SuperScript II Reverse Transcriptase (RT). The RNA/primer mixture was prepared in sterile PCR tubes as follows: 8.5 ug RNA, 10 mM dNTP mix, and oligo primer(dT); the samples were incubated at 65°C for 5 min and placed on ice for at least 1 min. Thereafter, the RT reaction mixture, including 10X RT buffer, 5 mM MgCl2 (4L), 0.1 M DTT, RNaseOut recombinant RNase inhibitor, was added to first standard RNA mixture and mix gently. After incubating at 42°C for 2 min, 0.5ul of SuperScript II RT (50 units) was added to each tube and the tubes were incubated at 42°C for 50 min. Subsequently, the reactions were carried out at 70°C for 15 min on ice, and 1ul of RNase H was added to each tube, which were incubated for 20 min at 37°C.
Expression of embryo quality genes
Total RNA was extracted from EECs using the TRIzol reagent (Invitrogen), treated with DNAse (Ambion, Austin, TX), as per the manufacturer’s instructions, and quantified by UV spectrophotometry. Thereafter, cDNA synthesis reaction mix was added to the SYBR Green (TOYOBO, Jap.) master mix, and PCR amplification was performed with target gene primers (Table 2) and 18Sr (housekeeping gene) primer with an annealing temperature of 51–65°C and 30 cycles. The Rotor-Gene Real-Time Software 6.0 was used to analyses the results, and the cycle threshold (Ct) values plotted in logarithmic scale were used to compare RNA expression. Gene expression levels relative to 18Sr were calculated using the 2-ΔΔCt method.
Extraction of total protein from EECs
For ELISA, western blot, and gelatin zymography, total protein was extracted from the EECs, serum, and uterine milk using the Pro-prep solution (Intron, Seoul, Korea), according to the manufacturer’s instruction. Total protein was quantified using the Bradford protein assay (Bio-Rad, CA, USA), and protein samples were stored at −80°C.
Western blot analysis
Each sample, containing 30 µg protein, was separated on a 13% gel using SDS-PAGE, in duplicate, and transferred to an Immun-blot PVDF membrane (Bio-Rad, CA, USA). The membrane was blocked using 5% non-fat dry milk as blocking buffer over-night at 4°C. The membrane was washed once for 10 min with washing buffer (0.1% Tween-20, 50 mM Tris-HCl (pH 7.6), and 200 mM NaCl), and incubated for 2 h with TIMP-3 polyclonal antibody (diluted 1:1000; Cat No. sc-6836 (C-20), Santa Cruz, CA, USA), TIMP-2 polyclonal antibody (diluted 1:1000; Cat No. sc-9905 (L-17), Santa Cruz, CA, USA), and rabbit anti-Caspase-3 polyclonal antibody (diluted 1:1000; Cat No. ab4051, Abcam, MA, USA). Thereafter, the membrane was washed thrice for 15 min, with 1X TBS-T buffer, and incubated for 2 h with HRP-conjugated secondary anti-rabbit (sc-2054), anti-mouse (sc-2054 and sc-2031, all from Santa Cruz Biotechnology). Detection was carried out, using the ECL detection kit, after 5 min incubation in dark. Subsequently, the reagent was removed and the membrane was exposed to a sheet of diagnostic film in a film cassette, for 1–30 min; protein expression was normalized to that of β-actin, which served as an internal control, using the software Alpha Innotech ver. 4.0.0 (San Leandro, CA, USA).
Gelatinase zymography
The enzymatic activity of MMP-2 (gelatinase A) and MMP-9 (gelatinase B) was detected by gelatinase zymography, as described previously (Sato et al., 1999; Imai et al., 2003). Briefly, 10 µg protein, in 10µl Tris-buffer, was loaded onto gelatin containing lanes for SDS PAGE (12.5% SDS gel containing 1 mg/mL gelatin A or B) and separated using a mini-gel apparatus (Bio-Rad, NY, USA), under non-reducing conditions. Following electrophoresis, the gels were incubated in 2.5% Triton X-100 for 1 h to remove SDS. The gels were rinsed thrice (20 min per rinse) with water and incubated for 15–18 h at 37–38°C in the incubation buffer (50 mM Tris–HCl (pH 7.5), 10 mM CaCl2, and 5 mM ZnCl2) with or without 12 mM 1,10-phenanthroline (an inhibitor of MMP activity). Thereafter, the gels were stained with 0.5% (w/v) Coomassie Brilliant Blue R250, and proteolytic activity was observed as clear bands on a blue background. The relative molecular mass (Mr) of gelatinolytic MMP was determined by comparison with molecular weight markers (Bio-Rad) in the adjacent lane. Band intensity, representing MMP activity, was quantified by densitometry, using a GS 700 Imaging Densitometer (Bio-Rad).
Enzyme-linked immunosorbent assay (ELISA)
For ELISA, protein samples were diluted in 50% assay buffer. Hormone (FSH, LH, PAPP-A, and IGF) levels were measured using quantitative sandwich ELISA (R and D Systems Europe, Abingdon, UK), according to the manufacturer’s guidelines. All samples were measured in duplicates and the mean was calculated. Hormone (FSH, LH, PAPP-A, and IGF) levels were determined according to a standard curve, which takes into account three parameters based on the following equation: y = (A - D)/(1 + (x/C) ^ B) + D). The standard curve was calculated from seven known values. All values were reported in ng/mL.
Immunofluorescence (IF)
EECs and embryos were fixed in 4% paraformaldehyde overnight at 4°C, washed for 30 min in PBS, and permeabilized with 0.2% Triton X-100 for 30 min at room temperature (RT). The samples were blocked at RT for 3 h in PBS, containing 10% normal horse serum (NGS) and 0.01% Triton X-100, and detected using primary antibodies (diluted 1:100 in blocking buffer) overnight at 4°C. After washing for 30 min with PBS, the samples were incubated with secondary antibodies (diluted 1:500 in blocking buffer) for 2 h at RT and then washed with PBS for 30 min. The samples were mounted on slides with DAPI and observed under a fluorescence microscope (Nikon Corp., Tokyo, Japan) at X200 and X400 magnification. The relative fluorescence intensity was analyzed with the ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
All results were analyzed t-test and general linear model (GLM) using the Statistical Analysis System software (SAS Institute, version 9.4, Cary, NC, USA). The all data are shown as mean ± SD, and the significant difference between groups was determined at the p < 0.05 level.
Results
Effects of in vivo embryo production
The number of embryos produced in vivo, during April-June 2017, are shown in Table 3. Bovine embryos, EECs, and uterine milk were recovered 7 d after the first insemination by flushing the uterus with D-PBS and collection of blood from the cattle.
Table 3: Number and quality of embryos recovered non-surgically from donor cattle on day seven.
| No. | Donor |
No. of embryos |
|||
| Total | Transferable | Degenerated | Un-fertilized | ||
| 1 | 002 0488 6915 2 | 20 | 16 | 2 | 2 |
| 2 | 002 0455 5739 1 | 35 | 20 | 7 | 8 |
| 3 | 002 3061 3726 4 | 27 | 26 | - | 1 |
| 4 | 002 3082 9997 3 | 28 | 26 | - | 2 |
| 5 | 002 0430 5351 7 | 23 | 1 | - | 22 |
| 6 | 000 1787 5387 6 | 15 | - | 2 | 13 |
| 7 | 000 1867 1876 8 | 13 | 4 | 9 | - |
| 8 | 002 0430 5351 7 | 11 | 3 | - | 8 |
| 9 | 000 2016 5679 2 | 2 | 2 | - | - |
| 10 | 002 3134 5818 0 | 4 | - | 3 | 1 |
The relationship between embryo quality and pregnancy rate, after transfer
The quality of embryo influences pregnancy rate, after transfer. Therefore, the relationship between embryo quality and pregnancy rate, after ET, was investigated. As shown in Table 4, the pregnancy rate increased with good-quality embryos, and was very low when fair- and low-quality embryos were used.
Table 4: Effects of groups (good, fair, and low) on pregnancy rates in cows.
| Groups | No. of cows with ET | No. of cows pregnant | Conception rate (%) |
| Good | 55 | 35 | 63.6 |
| Fair | 3 | 1 | 33.3 |
| Low | 2 | - | 0 |
Change in mRNA expression level of factors associated with embryo quality in the EECs of good, fair, and low-quality groups
The mRNA expression levels of factors involved in embryo quality were detected in the good, fair, and low-quality groups (Figure 1). The mRNA expression level for PAPP-A and VEGF was statistically higher in the good-quality group compared to the low- and fair-quality groups. High expression levels of mRNA were also detected for 20α-HSD and OT, in the low-quality group compared to the good- and fair-quality groups. A significantly higher level of E2 mRNA was found in the fair-quality group compared to the EECs classified as good- and low-quality. The mRNA expression of MMP-2, TIMP-2, MMP-9, and TIMP-3 in the EECs of good-, fair-, and low-quality groups is shown in Figure 1. MMP-9 expression in the EECs remained stable throughout the estrous cycle and gestation but was higher in the good- and fair-quality groups in comparison to the low-quality group. In contrast, the level of TIMP-3 (MMP-9 inhibiting factor) mRNA in the EECs was higher for the low-quality group than for the good-quality group. Moreover, the expression of MMP-2 mRNA was more in the EECs of the low-quality group and that of TIMP-2 (MMP-2 inhibitor factor) mRNA was higher in good- and fair-quality groups.
Protein expression patterns in EECs
MMP-2, MMP-9, TIMP-2, TIMP-3, and Caspase-3 protein expression was evaluated in the EECs of good-, fair- and low-quality groups (Figure 2). Protein expression pattern was similar to mRNA expression pattern. The expression of active MMP-9 was lower in the low-quality group than in the good- and fair-quality groups (Figure 2A). For the low-quality group, the expression of TIMP-3 was lower and that of TIMP-2 and Casp-3 was higher than that in the other groups (Figure 2B). Thus, apoptotic proteins were highly expressed in the low-quality group. FSH and PAPP-A protein expression was low in the uterine milk of the low-quality group. FSH, LH, and IGF proteins were detected in the serum of good-, fair- and low-quality groups (Figure 2C). Further, high FSH and LH serum protein levels were observed in the good- and fair-quality groups compared with the low-quality group.
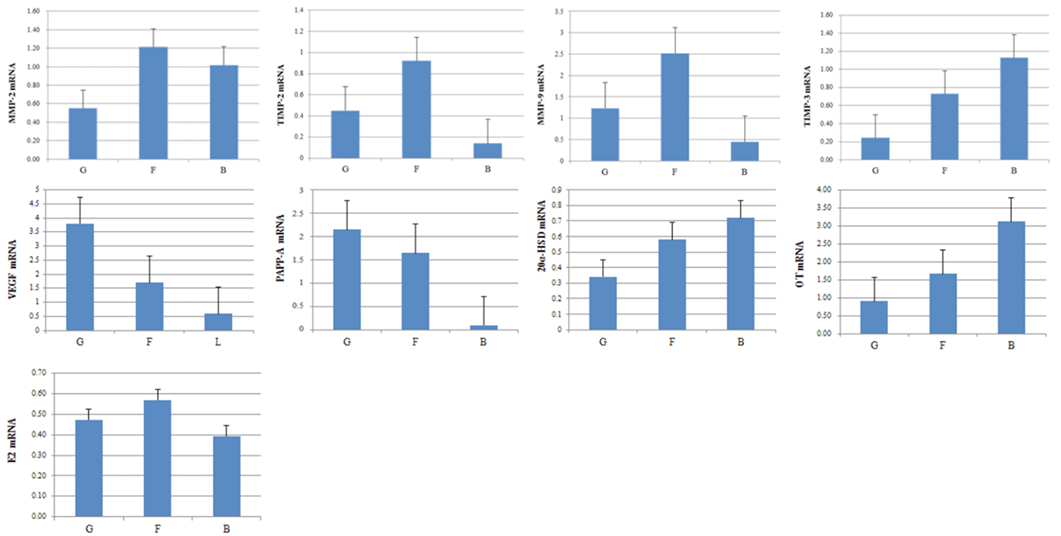
Figure 1: Expression of pregnancy-associated plasma protein A, VEGF, 20-Hydroxysteroid dehydrogenase, OT, E2, MMP-2, MMP-9, TIMP-2 and TIMP-3 mRNA in EECs. Expression of embryo quality-associated mRNAs in the EECs. Samples from good- (G), fair- (F), and low-quality group (B).

Figure 2: Protein expression analysis of MMP-2, MMP-9, TIMP-2, TIMP-3 and Casp-3. Gelatin zymography (A) of embryo quality in uterine milk. Western blot (B) of embryo quality in EECs. ELISA-based analysis of FSH, LH, and IGF concentration in the serum of good-, fair- and low-quality groups (C). Samples of good- (G); fair- (F) and low-quality group (B).
Effect of FSH, LH, and FSH+LH treatment on the expression of embryo quality-related factors in bovine embryos and EECs
Bovine embryos and EECs were cultured to confluence in Dulbecco’s modified Eagle’s medium (DMEM, Gibco), supplemented with 10% bovine calf serum (BCS, Gibco) and 3% antibiotic, and treated with FSH, LH, and FSH+LH in a humidified atmosphere with 5% CO2 at 38°C. For the treated embryos and EECs, viability was expressed as the percentage of control group. The relative levels of PCNA and PAPP-A in embryos and EECs was measured by immunofluorescence (Figure 3).
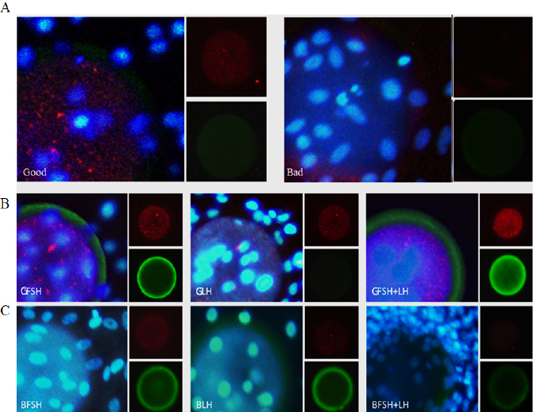
Figure 3: Immunofluorescence analysis of embryo quality-related proteins in embryos and EECs incubated for 24 h. Expression of PCNA and pregnancy-associated plasma protein A (PAPP-A) in the embryo and EECs of the good (Good) and low-quality (Bad) groups (A). The large panel shows the merged picture. Grading of EECs: Good- (B) and low-quality (C) groups. GFSH: FSH-treated embryos and EECs; GLH: LH-treated embryos and EECs; GFSH+LH: FSH + LH-treated embryos and EECs. Embryos were developed in the absence or presence of hormones. The large panel shows the merged picture. The small panel shows the protein detected (red: PCNA), (green: PAPP-A).
Effect of FSH, LH, and FSH+LH treatment on the secretion of FSH, LH, and PAPP-A by embryos and EECs into the culture medium
We examined the effect of drugs (FSH, LH, and FSH+LH) on the levels of secreted FSH, LH, and PAPP-A secreted by the embryos and EECs into the culture medium by ELISA (Figure 4). There was a significant difference in the level of FSH and LH hormone after culturing for 24 h among the four groups. The level of FSH and LH significantly increased in the FSH and FSH+LH-treated good-quality group in comparison with the low-quality group.
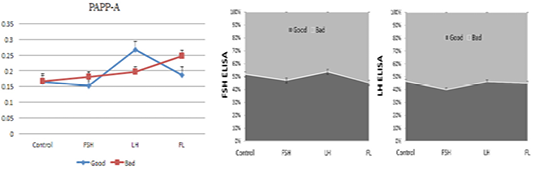
Figure 4: ELISA-based analysis of pregnancy-associated plasma protein A, FSH and LH in the embryo and EEC culture conditioned media. Samples of good- (blue) and low-quality groups (red). (con: control group; FSH: follicle stimulating hormone; LH: luteinizing hormone; FL: follicle stimulating hormone + luteinizing hormone).
Discussion
The best indicator of the success of an ET program is the number of live calves born per donor, over a given period, and the number of viable embryos produced per donor is frequently used as an indicator for the success of multiple ovulation and embryo transfer (MOET) (Armstrong, 1993). Embryo viability in the MOET program depends not only on the ovulation rate but also on whether normal fertilization occurs and on the oviductal and uterine milieu in which the embryo develops, prior to embryo recovery (Armstrong, 1993). Thus, to understand the efficiency of blastocyst implantation in the uterine endometrium, one must first understand how intercellular biochemical communication works. The EECs could be a useful experimental model for studying biochemical communication between the embryo and the endometrium and the conditions required for implantation to proceed. This suggests systemic changes that would occur beyond the uterine compartment. According to the basic principles of embryo evaluation (IETS), embryos are classified on the basis of a coding system according to their stage of development (1 to 9) and quality (1 to 4). Based on IETS, embryos (EECs) were divided into three categories: good-, fair- and low-quality. This study demonstrated that the pregnancy rate in cows is influenced by the quality of the embryos. Previous studies have shown that the interaction between the uterine cells with the fertilized egg determines the success of blastocyst development through implantation-related genetic changes, such as induction of MMP expression (Huang et al., 2010). However, a lack of experimental systems has limited the study of the embryo-endometrial relationship. The EECs, could overcome such limitations and are ideal for studying these interactions. We hypothesized that evaluating differential expression of survival signals, such as MMPs, hormones, and apoptotic proteins, during endometrial remodeling may help understand the effects of successful implantation on embryo development. The change in endometrial cells depends on survival signal (MMPs, hormones, and apoptotic proteins) and significantly impacts embryo development. Our results showed that distinct types of MMPs and survival associated genes are differentially expression in the epidermal cells of good- and low-quality groups. In this study, we showed that endometrium quality affected embryo development. Based on this observation, we hypothesized that differences in endometrial gene expression may positively affect embryo quality. We found that high-quality blastocyst formation rate was higher for EECs in the good-quality group than for those of other groups. Moreover, expression of PAPP-A, VEGF, FSH, LH, MMP-9, and TIMP-2 varied among the three groups of EECs, with a higher level of expression in good-quality EECs compared with the fair- and low-quality groups. Levels of VEGF, PAPP-A, and FSH, which are embryo activating factors, began to increase substantially in EECs of the good-quality group. In the present study, the activity of MMP-9 and survival signals, which induce reconstruction of cells, increased in the good-quality group, while the expression levels of MMP-2 and apoptosis associated genes were high for the fair- and low-quality groups. Moreover, a change in the levels of MMPs was observed during the development of the embryo and the metrium cells; similar to results reported by Qin et al. (2003). Thus, detection of MMP-9 in the vaginal mucus is important because endometrial remodeling is essential for successful implantation (Blankenship et al., 1994; Salamonsen, 1999; Bjorn et al., 2000; Qin et al., 2003). In the present study, we detected a higher expression of apoptotic Casp-3, 20α-HSD, and TIMP-3 in the low-quality group in comparison to the good-quality group (Devireddy and Jones, 1999). We found a higher expression of 20α-HSD in the EECs of the low-quality group in comparison to the fair- and good-quality groups. Our results agree with those of Madore et al., 2003, who reported that 20α-HSD is responsible for PGF2α production in cattle. Moreover, we found higher levels of VEGF, PAPP-A, and TIMP-2 in the good-quality group compared to the low-quality group. We suggest the presence of a mechanism controlling the balance between different groups, based on the regulation of FSH and LH gene expression. In our study, we demonstrated a strong positive correlation between the expression of FSH and LH, found in the uterine milk, serum, and conditioned media of the good- and fair-quality groups. This study showed the direct effect of FSH and LH on embryo quality. Previously, it has been shown that EECs cultured in the presence of FSH and LH improved embryo quality. In this study, embryo quality was less affected by E2. Accordingly, bovine IGF1 has been found to be involved in the regulation of LH release (Yamada et al., 2002). These results showed that FSH and LH could determine embryo quality and improve in vivo embryo development and implantation.
Conclusion
In this studies suggest that the FSH/LH hormone-receptors are highly expressed in the EECs of the good-quality group and MMPs and survival signal genes are expressed. In addition, the effect of FSH on embryo quality may be dependent on the species. However, for the EECs of the low-quality group, expression of MMPs and apoptosis associated genes increases due to the rapid influx of LH hormone-receptors. Differential gene expression is important for uterine remodeling, according to functional changes in the EECs. Moreover, the interaction between MMP and hormone-receptor associated genes, involved in endometrium formation during embryo development, needs to be elucidated. Understanding these processes will be useful for successful MOET to create high-quality embryos for animal breeding.
Authors Contribution
All authors contributed equally to the manuscript.
Conflict of interest
There is no conflict of interest.
References






