Advances in Animal and Veterinary Sciences
Short Communication
Advances in Animal and Veterinary Sciences 2 (2): 78 – 80Simple Purification Method for Beta–Lactoglobulin from Buffalo Milk
Ranjit Aich1, Subhasis Batabyal2, Siddhartha Narayan Joardar2*
- College of Veterinary Science and Animal Husbandry, Mhow–453 446, Madhya Pradesh, India
- West Bengal University of Animal and Fishery Sciences, Belgachia, Kolkata–700 037, West Bengal, India
*Corresponding author: joardar69@gmail.com
ARTICLE CITATION:
Aich R, Batabyal S, Joardar SN (2014). Simple purification method for beta–lactoglobulin from buffalo milk. Adv. Anim. Vet. Sci. 2 (2): 78 – 80.
Received: 2013–12–23, Revised: 2013–12–29, Accepted: 2013–12–30
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.2.78.80
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
It has been observed that 82 percent of milk–allergic patients are sensitive to beta–lactoglobulin (β–lg), a major milk protein that accounts for approximately 10 to 15 percent of total milk proteins. The modification in β–lg is considered a promising venture to mitigate milk allergies. With the aim of standardizing a convenient method for isolation and purification of β–lg from buffalo milk, the present study was designed to keep its antigenicity intact, so that, the purified β–lg can be used to detect buffalo milk protein intolerance. Raw milk was collected from Murrah breed of buffalo from Haringhata farm (West Bengal) and converted to skimmed milk by removing fat globules. Casein protein was removed by acidification to pH 4.6 by adding 3 M HCl. The β–lg was isolated by gel filtration chromatography using Sephacryl S–200 from supernatant whey protein fraction. Further, β–lg was purified by anion–exchange chromatography using DEAE–Sepharose. Molecular weight of the purified buffalo β–lg was 18.05kDa as assessed by the gel documentation system using standard molecular weight marker (range 14.3 to 97.4 kDa) in 15 percent one–dimensional SDS–PAGE. The isolated β–lg was almost in pure form as the molecular weight of purified β–lg monomer is 18kDa. The study revealed a simple and suitable method for isolation of β–lg from whey protein in pure form that might be exploited for any basic and applied studies related to buffalo milk protein intolerance.
INTRODUCTION
The whey proteins in bovine milk consist of β–lactoglobulin (β–lg), α–lactalbumin (α–LA), bovine serum albumin (BSA), immunoglobulins (Ig), and others. Whey is widely used as a food ingredient and as an additive to improve the texture and quality of food. The biological functions of β–lactoglobulin are still not known. However, owing to its three–dimensional structure similarity to that of human retinol–binding protein in serum (Papiz et al., 1986), it could have a role in metabolism of phosphate in the mammary gland and the transport of retinol and fatty acids in the gut (Hill et al., 1997). The complete amino acid sequence of β–lg has been reported and genetic variation in amino acids sequence has been identified (Creamer et al., 1983). Eleven genetic variants which encode different forms of the β–lg protein have been discovered, thus influencing the quality of the milk: A, B, C, D, E, F, G, H, I, J and W. Variant A and B are of the highest interest since they are associated with milk production performance, its quality and processing. The AA genotype of β–lg has a favorable effect on milk and protein production, whereas the BB genotype has a favorable effect on fat content (Ikonen et al., 1999).
β–lactoglobulin seems to be quite resistant to gastric digestion in vivo and apparently remains mostly intact after it passes through the stomach (Yvon et al., 1984). Better understanding of the underlying mechanisms of food hypersensitivity reactions has always necessitated studying antigenic and molecular characteristics of food antigens. To perform such studies, it is imperative to have the antigens in the pure form. Having these antigens, on the other hand, will allow us to determine specific antibodies. The present study was undertaken with the objectives to standardize a suitable and simple procedure of purification of β–lactoglobulin from buffalo milk.
Fresh raw milk, after collection, was filtrated through multilayer gauze in order to remove impurities. Then the milk was centrifuged at 3000g for 30 min at 4ºC and using a spatula the top fat layer was removed. The skimmed milk was acidified to pH 4.6 by adding 3M HCl (Hahn et al., 1998; Vyas et al., 2002). Furthermore, the solution was incubated for 30 min at 40ºC and caseins were removed by centrifugation at 8000g for 15 min at 4ºC and the supernatant was poured over glass wool. The pH of the acidic whey fraction was raised to pH 7.2 with 1N NaOH, and the material was subsequently centrifuged for 15 min at 10ºC at 8000g. The supernatant was filtered and this material is referred to as whey protein fraction (WPF). The total concentration of buffalo whey protein was determined by Lowry’s method (Lowry et al., 1951) and kept in aliquots at –20ºC until used.
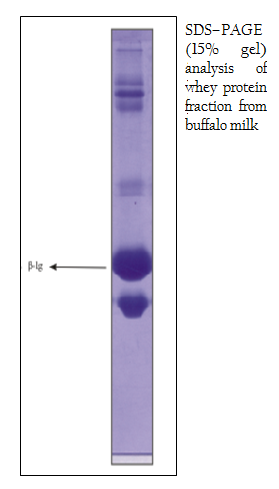
Sephacryl S–200 (Sigma–Aldrich, USA.) was degassed and packed into the column (43.0 cm X 2.0 cm) to isolate β–lactoglobulin from buffalo whey protein fraction (Yoshida, 1990). The matrix was equilibrated with 0.02M phosphate buffer, pH 6.8 (Kinekawa and Kitabatake, 1996) and the sample containing 14.0 mg of protein was loaded. The elution was carried out with equilibrating buffer by using 3ml of fraction at a flow rate of 0.20 ml/min with 0.02M phosphate buffer, pH 6.8. The purity of the fractions was monitored by taking the absorbance at 280 nm in a UV/VIS spectrophotometer (Tech Comp, Korea). The absorbance values were plotted against fraction number. Protein fractions of peak A (test tube no. 27, 28, 29, 30, 31) and peak B (test tube no. 36, 37, 38, 39) were pooled and concentrated. The protein concentration of the pooled sample was determined (Lowry et al., 1951). The concentrated pooled sample was then preserved at –200C in aliquots for further use. Anion–exchange chromatography was performed on DEAE– Sepharose gel packed in a column (13 cm X 1.5 cm). The loading buffer, equilibrating buffer and elution buffer were as follows: 0.02 M phosphate buffer, pH 6.8; 0.02M phosphate buffer, pH 6.8; 0.02M phosphate buffer containing 0– 0.5 M NaCl, pH 6.8, respectively. The matrix was equilibrated with equilibrating buffer, and the sample collected after gel filtration of buffalo whey protein fraction was loaded containing 6.80 mg of protein, pH 6.8. The bound proteins were eluted at a linear gradient by using elution buffer with flow–rate and fraction volume being 0.30 ml/min and 3 ml, respectively. Protein fractions of peak A at 0.2 M NaCl (test tube no. 10, 11) and peak B at 0.3 M NaCl (test tube no. 23, 24) were pooled and concentrated. Finally, the concentration of the target protein of the eluted sample was determined (Lowry et al., 1951) and preserved at –200C in aliquots for further use. Purity of protein preparation was checked by one–dimensional 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) in vertical slab gel electrophoresis chamber (AE–6200) along with power supply (ATTO Corporation, Japan) according to the method described by (Laemmli et al. 1970), with some modification under denaturing and reducing condition. Molecular weights of purified samples were analyzed by the Gel Documentation System (Bio–Rad) using medium range protein markers (PMW–M, GENEI) and staining with Coomassie brilliant blue R250 (Sigma, USA). The total concentration of buffalo whey proteins, determined by Lowry’s method (Lowry et al., 1951), was about 7.0 mg/ml. The SDS–PAGE analysis of whey protein fraction (Figure 1) isolated from buffalo milk showed similar pattern of migration with 6 major bands after staining with Coomassie brilliant blue R250. The β–lg from buffalo whey protein fraction was isolated by gel filtration chromatography using Sephacryl S–200 and the protein concentration was 3.40 mg/ml. The results demonstrated that buffalo β–lg could be separated well from whey proteins (Figure 2). Several methods for isolation of β–lg from whey have been reported, but most of them are expensive and are able give high yields. Among the methods frequently used to separate it from whey are precipitation at low pH (Konrad et al., 2000), peptic hydrolysis followed by selective membrane filtration (Konrad et al., 2000), chromatographic methods (Schlatterer et al., 2004; Ye et al., 2000), and separation by ion–exchange chromatography (Skudder, 1985; Kristiansen et al., 1998).
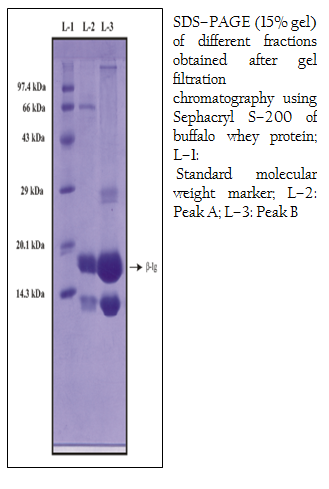
Figure 2: SDS–PAGE (15% gel) of different fractions obtained after gel filtration chromatography using Sephacryl S–200 of buffalo whey protein; L–1: Standard molecular weight marker; L–2: Peak A; L–3: Peak B
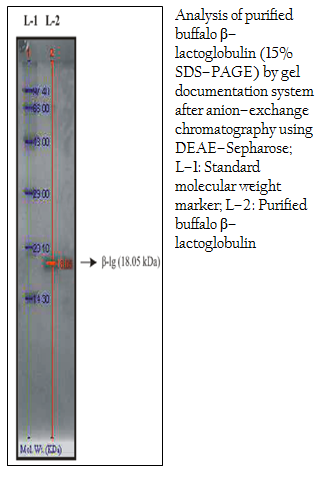
Figure 3: Analysis of purified buffalo β–lactoglobulin (15% SDS–PAGE) by gel documentation system after anion–exchange chromatography using DEAE–Sepharose; L–1: Standard molecular weight marker; L–2: Purified buffalo β–lactoglobulin
Purification of β–lg from gel filtrated samples of buffalo whey protein was done by anion–exchange chromatography on DEAE–Sepharose. The concentration of the target protein of the eluted sample was 3.50 mg/ml. Purity of protein preparation was checked by 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and a single band of purified buffalo β–lg was found (Figure 3). The purified β–lg samples from buffalo origin were analyzed by the Gel Documentation System (Bio–Rad) using standard molecular weight marker (ranging from 14.3 to 97.4 kDa) in 15% one–dimensional SDS–PAGE. It was observed that the molecular weight of buffalo β–lg was 18.05 kDa (Figure 3). The present finding corroborates earlier report of de Jongh and others where MALDI–TOF spectrum of the purified bovine β–lg clearly identified A and B types with molecular masses of 18.371 kDa and 18.284 kDa, respectively (de Jongh et al., 2001). Earlier, electrophoretic analysis of purified cattle β–lg was performed in SDS–PAGE; the molecular weights were estimated at 34,000 Da for β–lg dimmer and 16,000 Da for β–lg monomer (Yoshida, 1990). In contrast, the molecular weight of β–lg isolated by saturated ammonium sulfate precipitation and purified by preparative scale gel filtration was found to be 18,400 Da by SDS–PAGE analysis and 36,800 Da by fplc–GF analysis (Apenten et al., 2002). Taken together, β–lg could be isolated in almost pure form (seen as a single band in gel). The finding is interesting, because for any basic or applied study pertaining to such bio–molecule warrants purity of the starting material. As such these simple steps encompassing column chromatographic techniques might be a useful means in getting lactoglobulin molecule in pure form that might be modulated in the next step to mitigate buffalo milk protein intolerance.
In short, the study revealed a simple and suitable method for isolation of β–lg from whey protein in almost pure–form that might be exploited for any basic and applied study related to buffalo milk protein intolerance and this method could possibly be modified for other proteins.
ACKNOWLEDGEMENT
The authors wish to thank the Vice Chancellor, West Bengal University of Animal and Fishery Sciences, Kolkata for providing necessary research facilities.
REFERENCES
Apenten R K O, Khokhar S, Galani D (2002). Stability parameters for β–lactoglobulin thermal dissociation and unfolding in phosphate buffer at pH 7.0. Food Hydrocol. 16: 95 – 103.
http://dx.doi.org/10.1016/S0268-005X(01)00067-4
Creamer L, Parry D, Malcolm G (1983). Secondary structure of β–lactoglobulin B. Arch. Biochem. Biophys. 227: 98 – 105.
http://dx.doi.org/10.1016/0003-9861(83)90351-X
De Jongh HHJ, Groneveld T, de Groot J (2001). Mild isolation procedure discloses new protein structural properties of β–lactoglobulin. J. Dairy Sci. 84: 562 – 571.
http://dx.doi.org/10.3168/jds.S0022-0302(01)74508-0
Hahn R, Schulz PM, Schaupp C, Jungbauer A (1998). Bovine whey fractionation based on cation–exchange chromatography. J. Chromat. 795(2): 277 –287.
http://dx.doi.org/10.1016/S0021-9673(97)01030-3
Hill JP, Thresher WC, Boland MJ, Creamer LK, Anema SG, Manderson G, Otter DE, Paterson GR, Howe R, Burr RG, Motion RL, Windelman A, Wickham B (1997). The polymorphism of the milk protein β–lactoglobulin. In: Milk composition, production and biotechnology, Eds. Welch Ras and others, CAB International, Wallingford, UK. pp 173 –213.
Ikonen T, Ojala M, Ruottinen O (1999). Associations between milk protein polymorphism and first lactation milk production traits in Finnish Ayrshire cows. J. Dairy Sci. 82: 1026 – 1033.
http://dx.doi.org/10.3168/jds.S0022-0302(99)75323-3
Kinekawa Y, Kitabatake N (1996). Purification of beta–lactoglobulin from whey protein concentrate by pepsin treatment. J. Dairy Sci. 79(3): 350 – 356.
http://dx.doi.org/10.3168/jds.S0022-0302(96)76371-3
Konrad G, Lieske B, Faber W (2000). A large–scale isolation of native β–lactoglobulin: Characterization of physicochemical properties and comparison with other methods. Intl. Dairy J. 10: 713 –721.
http://dx.doi.org/10.1016/S0958-6946(00)00099-6
Kristiansen KR, Otte J, Ipsen R, Quist KB (1998). Large–scale preparation of β–lactoglobulin A and B by ultrafiltration and ion–exchange chromatography. Intl. Dairy J. 7: 805 – 812.
Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680 – 685.
http://dx.doi.org/10.1038/227680a0
PMid:5432063
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951). Protein Measurement with the Folin phenol reagent. J. Biol. Chem. 193(1): 265 –275.
PMid:14907713
Papiz MZ, Sawyer L, Eliopoulos EE, North ACT, Fondlay JBC, Sivaprasadarno R, Jones TA, Newcomer ME, Kraulis PJ (1986). The structure of β–lactoglobulin and its similarity to plasma retinol–binding protein. Nature 367: 383 – 385.
http://dx.doi.org/10.1038/324383a0
PMid:3785406
Schlatterer B, Baeker R, Schlatterer K (2004). Improved purification of β–lactoglobulin from acid whey by means of ceramic hydroxiapatite chromatography with sodium fluoride as a displacer. J. Chromat. 807: 223 – 228.
Skudder PJ (1985). Evaluation of a porous silica–based ion–exchange medium for the production of protein fractions from rennet– and acid whey. J. Dairy Res. 52: 167 – 181.
http://dx.doi.org/10.1017/S0022029900023992
Vyas HK, Izco JM, Jimenez–Flores R (2002). Scale–up of native betalactoglobulin affinity separation process. J. Dairy Sci. 85(1): 1639 –1645.
http://dx.doi.org/10.3168/jds.S0022-0302(02)74236-7
Ye X, Yoshida S, Ng TB (2000). Isolation of lactoperoxidase, lactoferrin, α–lactalbumin, β–lactoglobulin B and β–lactoglobulin A from bovine rennet whey using ion exchange chromatography. Int. J. Biochem. Cell Biol. 32: 1143 – 1150.
http://dx.doi.org/10.1016/S1357-2725(00)00063-7
Yoshida S (1990). Isolation of β–lactoglobulin and α–lactalbumin by gel filtration using Sephacryl S–200 and purification by Diethylaminoethyl ion–exchange chromatography. J. Dairy Sci. 73: 2292 – 2298.
http://dx.doi.org/10.3168/jds.S0022-0302(90)78910-2
Yvon M, Van Hille I, Pellisier JP (1984). In vivo milk digestion in the calf abomasum. II. Milk and whey proteolysis. Rep. Nut. Dev. 24: 835 – 839.
http://dx.doi.org/10.1051/rnd:19840702