Journal of Animal Health and Production
Research Article
Prevalence of Clostridium perfringens in Retail Meat and Meat Products with Some Decontamination Trials by some Essential Oils
Rasha M. El-Bayomi1*, Yasmen M. El-Mesalamy1, Abdelsalam E. Hafez1, Heba A. Ahmed2
1Food Control Department, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt; 2Zoonoses Department, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt.
Abstract | Meat is a valuable contribution to diets because of its high nutritional values. Clostridium perfringens is a commensal inhabitant of animals and human intestinal tract as well as a common foodborne pathogen associated with food poisoning. The present study was carried out to investigate the prevalence of C. perfringens in meat and its products at Zagazig city, Sharkia Governorate, Egypt. In addition, toxin genes, antimicrobial susceptibility testing and biofilm formation of C. perfringens strains isolated from meat and its products were determined. The achieved results revealed the contamination of meat and meat products by C. perfringens with varying degrees. Alpha toxin gene was found in all isolated strains, while enterotoxin gene not detected. By disc diffusion method, C. perfringens isolates were found resistant to most of the tested antibiotics with high multiple antibiotic resistance (MAR) indices. Most of C. perfringens isolates were able to form biofilms at different temperatures. Finally, marjoram oil 2% was more effective than thyme oil 5% in reduction of C. perfringens count in vitro. The results suggested appropriate food safety practices in meat and meat product production and processing facilities in Zagazig city, Egypt.
Keywords | C. perfringens, Enterotoxin, Antibiotic, Biofilm, Essential oils
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | November 02, 2020; Accepted | November 23, 2020; Published | December 27, 2020
*Correspondence | Rasha M. El Bayomi, Department of Food Control, Faculty of Veterinary Medicine, Zagazig University, El-Zeraah str. 114; 44519-Zagazig, Egypt; Email: [email protected]
Citation | El-Bayomi RM, El-Mesalamy YM, Hafez AE, Ahmed HA (2020). Prevalence of clostridium perfringens in retail meat and meat products with some decontamination trials by some essential oils. J. Anim. Health Prod. 9(s1): 61-68.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.61.68
ISSN | 2308-2801
Copyright © 2020 El-Bayomi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Meat and meat products are valuable sources of various nutrients; mainly protein, fat, B-vitamins, iron, zinc and vitamin A, in addition to the essential amino acids. Meat products are preferred by many peoples because they are easily prepared meals of low price (Shaltout et al., 2016).
Clostridium perfringens is a Gram-positive, non-motile, spore-forming, anaerobic bacillus that commensally inhabits the intestinal tract of animals and humans. It is a cytotoxin producing bacterium with an optimum growth temperature of 35°C–40°C (Dawson et al., 2009). C. perfringens type A strains are implicated in numerous human diseases such as food poisoning and gastrointestinal illness (Fisher et al., 2005).
The extent use of antimicrobial agents for growth promotion and disease control is the main cause of antimicrobial resistance spread particularly in the normal enteric flora, including C. perfringens. Resistance of C. perfringens isolates to various antibiotics has been reported in different countries (Slavic et al., 2011).
Biofilm is structured communities of the bacterial cell enclosed in a self-produced extracellular polysaccharide matrix that provides an increased resistance to environmental stresses. Biofilm formed by C. perfringens protects it from exposure to the atmospheric oxygen and to the high concentrations of antibiotics (Charlebois et al., 2014).
Essential oils (EOs) are aromatic volatile oily liquids obtained from plant materials, particularly. Thyme (Thymus vulgaris L.) is an aromatic plant belongs to family Labiateae, used mainly for several culinary purposes. Marjoram (Origanum majorana L.) is another essential oil belongs to Lamiaceae family and has a broad inhibitory spectrum against wide range of Gram-negative and Gram-positive bacteria (Mohamed and Mansour, 2012).
Thus, the present study was planned for isolation of C. perfringens from meat and meat products, as well as detection of C. perfringens virulence genes, antimicrobial susceptibility test, and investigation of C. perfringens ability to form biofilms at different temperatures. In addition to investigate the antibacterial effect of some essential oils on C. perfringens.
MATERIALS AND METHODS
Collection of samples
A total of 40 fresh meat samples and 160 meat products (minced meat, burger, sausage and luncheon, 40 each) were randomly collected from different outlets at Zagazig city, Sharkia Governorate, Egypt.
Isolation of C. perfringens
Under complete aseptic conditions, 10 g of each sample were aseptically homogenized in a sterile blender containing 90 ml of 0.1 % sterile Buffered Peptone Water (BPW, OXOID, CM9). The enrichment of each sample was performed by transferring 1 ml of each homogenized sample into a tube containing 9 ml of sterile Cooked Meat Broth (CMB; TM MEDIA) (ISO6887, 2003). The tubes were anaerobically incubated at 37ºC for 24 h. For isolation of C. perfringen, a loopful from the enriched cultures were streaked on to the surface of Reinforced Clostridial Agar (RCA; Oxoid, CM0151), and were anaerobically incubated at 37ºC for 24-48 h in anaerobic jar containing gas generating kits (anaeroGen, OXOID ltd, England). The suspected colonies of C. perfringens (shiny, pin headed and translucent) were picked and subjected to Gram staining and biochemical tests (Tizhe et al., 2015).
Molecular characterization of C. perfringens virulence genes
The extraction of the bacterial DNA was carried using QIAamp DNA Mini kit (Qiagen GmbH, Hilden, Germany, Cataloge no. 51304) according to the manufacturer’s instructions. The amplification of C. perfringens plc gene encoding phospholipase C (alpha-toxin) and C. perfringens enterotoxin (cpe) was performed using primers from Midland Certified Reagent Company oilgos (USA). The alpha-toxin primers were sense 5′GTTGATAGCGCAGG ACATGTTAAG′3 and antisense 5′CATGTAGTCATCTGTTCCAGCATC′3 with a product size of 402 bp (Yoo et al., 1997). The primers used for detection of cpe gene were sense 5′ACATCTGCAGATAGCTTAGGAAAT′3 and antisense 5′CCAGTAGCTGTAATTGT TAAGTGT ′3 with a product size of 247 bp. (Kaneko et al., 2011).
Antibiotic susceptibility testing
It was performed by the disc diffusion method according to Bauer et al. (1966). The types of antibiotics and concentrations of each antibiotic disc are listed in a supplementary Supplementary Table 1. The results were used to calculate the Multiple Antibiotic Resistance (MAR) index for the total number of isolates as: MAR index=a/b, where a is the number of antibiotics to which the isolate is resistant, and b is the total number of tested antibiotics.
Detection of biofilm production by microtiter plate assay (MtP)
The biofilm production of C. perfringens isolates at different storage temperatures was determined by MtP assay as previously performed by Kırmusaoğlu (2019).
Trials to improve meat quality and decrease C. perfringens count
Nine Essential oils of watercress (Eruca Sativa), argan (Argania spinosa), marjoram (Origanum majorana), black seed (Nigella sativa), thyme (Thymus vulgaris), cumin (Cuminum cyminum), lemon grass (Cymbopogon citratus), rosemary (Rosmarinus officinalis) and lettuce (Lactuca sativa) were purchased from National Research Center, Dokki, Giza. These were used to determine the most effective oil on C. perfringen, by the disk diffusion method on Brain Heart Infusion agar (Nuno et al., 2016). The best effective oils in disk-diffusion method were thyme and marjoram essential oils, which were selected for further investigation in different concentrations and to calculate their minimum inhibitory concentration (MIC). The following concentrations: 10, 5, 2, 1, 0.5 and 0.25% v/v were prepared from these two oils. The MIC was taken from the lowest dosed disc concentration showing no growth after 24 h.
For the experimental trails was repeated in triplicates, a total of 1200 g of fresh meat was aseptically minced and divided into four groups (100 g, each). One ml of the C. perfringens broth that was adjusted to 0.5 McFarland was inoculated by pipetting over each 100 g of minced meat. The inoculated samples were left for 30 min at room temperature (25oC). Then it was divided in to four groups ;1st group, was inoculated with 1 ml sterile distilled water as a positive control ;2st group, was inoculated with thyme oil 5%; 3ndgroup, was inoculated with marjoram oil 2% and 4rdgroup, minced meat without inoculation of the microorganism as a negative control group. The control as well as the treated groups were examined for determination of the antimicrobial effect of the above-mentioned oils against C. perfringens after treatment for 0.5, 1, 2, 4, 6 and after 24 h.
Statistical analysis
All values of bacteriological analysis are presented as means ± standard error (S.E). Data were analyzed by SPSS and One-Way Analysis of Variance (ANOVA) at 95% level of confidence. Significant differences among the means were determined by DUNCAN test considering p<0.05 as significant.
Results and Discussion
Occurrence of C. perfringens in the examined meat and meat products
The bacteriological analysis of meat and meat product samples showed the presence of C. perfringens in 29 (14.5%) of the examined samples (Table 1). It was found that all examined C. perfringens isolates were positive for alpha toxin, while C. perfringens enterotoxin (cpe), not detected in all examined C. perfringens isolates (Figure 1).
Table 1: Occurrence of C. perfringens in the examined meat and meat product samples (N= 40, each).
| Samples | Positive samples | |
| Number | Percentages | |
| Fresh meat | 6 | 15% |
| Frozen minced meat | 6 | 15% |
| Burger | 8 | 20% |
| Sausage | 4 | 10% |
| Luncheon | 5 | 12.5% |
| Total | 29 | 14.5% |
N: Number of examined samples (40, each).
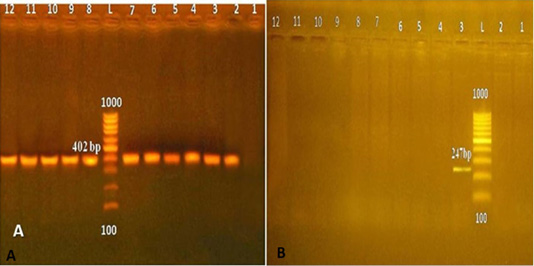
Figure 1: (A) C. perfringens Alpha toxin in 1.5% agarose gel (L: 100 bp ladder; 1: negative control; 2: positive control, from 3 to 12: C. perfringens positive for Alpha toxin). (B) C. perfringens cpe gene (L: 100 bp ladder; 2: negative control; 3: positive control; 4-12: negative C. perfringens for cpe).
Antimicrobial susceptibility of C. perfringens
Data presented in Table 2 shows 100% resistance of the tested C. perfringens isolates to oxytetracyclin and 90% resistance to erythomycin, neomycin and amoxicillin. Resistance profile of multidrug resistant of C. perfringens isolated from meat and meat product samples revealed that the MAR ranged from 0.1 to 1 with an average of 0.68 (Table 2).
Biofilm formation in C. perfringens isolates at 4ºC, 25 ºC and 35 ºC
The results presented in Table 3 show the percentages and degrees of biofilm production by C. perfringens isolates. At the three tested temperatures (4 °C, 25 °C, and 37 °C), 50%, 40% and 100% of the isolates were biofilm producers, respectively. At refrigeration temperature (4 °C) and at room temperature (25°C), isolates were classified as weak and moderate producers, while 50 % and 60% of the isolates were unable to produce biofilm. All C. perfringens isolates were biofilm producer at 37 °C.
Antibacterial effect of some essential oils C. perfringens
The obtained results in Table 4 revealed that from the tested nine oils, marjoram and thyme oils were the highest effective antibacterial oils against C. perfringens.
The data presented in Table 5 revealed the effect of different concentrations of thyme and marjoram essential oils on C. perfringens by the disk diffusion method. The mean inhibition zone diameter from the effect of thyme oil 5% and marjoram oil 2% against C. perfringens was 10±2.7 and 15.33±0.34 mm that were moderately inhibitory (10-20 mm) (Nuno et al., 2016). Thus, marjoram oil 2% and thyme oil 5% were chosen to investigate the chicken meat inoculated with C. perfringens for 0.5, 1, 2, 4, 6 and 24 h at 4 ºC.
After 0.05 h, the mean count of C. perfringens in the control group was 5.59±0.36 log10 CFU/g, while the mean values of treated groups with thyme oil 5% and marjoram oil 2% was decreased to 4.87±0.13 and 4.65±0.09 log10 CFU/g. After treatment for 24 h, the mean C. perfringens count in control group increased to 5.73±0.30 log10 CFU/g, while the mean counts of treated groups with thyme oil 5% was reduced to 4.99±0.17 log10 CFU/g with a reduction percentage of 81.8% and 4.89±0.17 log10 CFU/gin marjoram oil 2%. Statistical analysis by ANOVA revealed significance differences between control and treated samples at p<0.05 (Table 6).
C. perfringens is a normal inhabitant of the animals’ intestinal tract which can contaminate the carcass during unhygienic slaughtering process. The prevalence of C. perfringens in the current study is higher than 11.2% in India (Gurmu et al., 2013) and 5.6% in Turkey (Yibar et al., 2018).
Table 2: Antimicrobial susceptibility and Resistance profile of C. perfringens (N=10).
| Antimicrobial agent | Sensitive | Intermediate | Resistant | |||
| No. | % | No. | % | No. | % | |
| Oxytetracyclin (T) | 0 | 0 | 0 | 0 | 10 | 100% |
| Erythomycin (E) | 1 | 10% | 0 | 0 | 9 | 90% |
| Neomycin (N) | 1 | 10% | 0 | 0 | 9 | 90% |
| Amoxicillin (AX) | 0 | 0 | 1 | 10% | 9 | 90% |
| Ampicillin (AM) | 0 | 0 | 2 | 20% | 8 | 80% |
| Streptomycin (S) | 1 | 10% | 1 | 10% | 8 | 80% |
| Novobiocin (NV) | 1 | 10% | 2 | 20% | 7 | 70% |
| Gentamicin (CN) | 4 | 40% | 0 | 0 | 6 | 60% |
| Norfloxacin (NOR) | 3 | 30% | 2 | 20% | 5 | 50% |
| Enrofloxacin (ENR) | 5 | 50% | 0 | 0 | 5 | 50% |
| Pattern | Resistance profile | Number of isolates (%) | Number of antibiotics | MAR | ||
| I | T, E, N, AX, AM, S, NV, CN, NOR, ENR | 3(30%) | 10 | 1 | ||
| II | T, E, N, AX, AM, S, NV, NOR, ENR | 2(20%) | 9 | 0.9 | ||
| III | T, E, N, AX, AM, S, NV, CN | 2(20%) | 8 | 0.8 | ||
| IV | T, E, N, AX, AM, S, NV | 1(10%) | 7 | 0.7 | ||
| V | T, E, N, AX, AM, S | 1(10%) | 6 | 0.6 | ||
| VI | T | 1(10%) | 1 | 0.1 | ||
| Average | 0.68 | |||||
N: Number of C. perfringens isolates; No.: Number of sensitive, intermediate or resistant C. perfringens isolates
%: Percentage of sensitive, intermediate or resistant C. perfringens isolates; MAR: Multiple Antibiotic Resistance index (a b), where (a) is the number of antibiotics to which the isolates are resistant. (b): is the total number of tested antibiotics (10).
Table 3: Biofilm formation in C. perfringens isolates at 4ºC, 25 ºC and 35 ºC.
| Temperature | Non-producer | Degree of biofilm production (No, %, Average OD±SD) | Overall biofilm producers | ||
| Weak | Moderate | Strong | |||
| 4 ºC |
5(50%) 0.025249±0.04891 |
2(20%) 0.170283±0.06859 |
3(30%) 0.273894±0.0362 |
- | |
| 5(50%) | |||||
| 25ºC |
6(60%) 0.09496±0.069 |
1(10%) 0.26357±0.004 |
3(30%) 0.62969±0.009 |
- | 4(40%) |
| 35 ºC | - |
3(30%) 0.241909±0.05810 |
5(50%) 0.526731±0.173907 |
2(20%) 0.713631±0.03229 |
10(100%) |
OD: Optical Density; SD: Standard Deviation.
Table 4: Antibacterial effect of the used essential oils.
| Pure oil | Mean inhibition zone diameter (mm)±SE |
|
Watercress (Eruca Sativa) |
- |
|
Argan (Argania spinosa) |
- |
|
Thyme (Thymus vulgaris) |
22.67±7.5 |
|
Black seed (Nigella sativa) |
- |
|
Marjoram (Origanum majorana) |
23.33±6.8 |
|
Cumin (Cuminum cyminum) |
16.66 ±7.4 |
|
Lemon grass (Cymbopogon citratus) |
13.33±4.7 |
|
Rosemary (Rosmarinus officinalis) |
5±0.59 |
|
Lettuce (Lactuca sativa) |
- |
Table 5: Antibacterial effect of different concentrations of thyme and marjoram essential oils.
| Concentrations | Mean inhibition zone diameter of thyme(mm) | Mean inhibition zone diameter of marjoram (mm) |
| 10% | 18±0.59 | 16±0.59 |
| 5% | 10±2.7 | 15.67±0.34 |
| 2% | 5±0.58 | 15.33±0.34 |
| 1% | 5±0.58 | 5±0.58 |
| 0.5% | 5±0.58 | 5±0.58 |
| 0.25% | 5±0.58 | 5±0.58 |
Table 6: Effect of thyme oil 5% and marjoram oil 2% on C. perfringens count (log10 CFU/g) after 0.5, 1, 2, 4, 6 and 24h at 4°C.
| Storage hours | Control | Thyme oil 5% | Marjoram oil 2% | ||||
| 0.5 h |
Mean±S.E |
5.59±0.36a |
4.87±0.13b |
4.65±0.09b |
|||
| Reduction count (%) | - | 0.72(80.95%) | 0.94(88.52%) | ||||
| 1 h |
Mean±S.E |
5.54±0.33a |
4.79±0.12ab |
4.48±0.15b |
|||
| Reduction count (%) | - | 0.75(82.22%) | 1.06(91.29%) | ||||
| 2h |
Mean±S.E |
5.66±0.28a |
4.69±0.21b |
4.45±0.17b |
|||
| Reduction count (%) | - | 0.97(89.28%) | 1.21(93.83%) | ||||
| 4 h |
Mean±S.E |
5.67±0.28a |
4.69±0.09b |
4.52±0.18b |
|||
| Reduction count (%) | - | 0.98(89.53%) | 1.15(92.92%) | ||||
| 6 h |
Mean±S.E |
5.68±0.37a |
4.73±0.12b |
4.58±0.16b |
|||
| Reduction count (%) | - | 0.95(88.78%) | 1.1(92.06%) | ||||
| 24 h |
Mean±S.E |
5.73±0.30a |
4.99±0.17ab |
4.89±0.17b |
|||
| Reduction count (%) | - | 0.74(81.8%) | 0.84(85.55%) | ||||
CFU/g: Colony forming unit per gram; S.E: Standard error of mean; Min: Minimum; Max: Maximum. Means within the same row with different superscript letters are significantly different (P< 0.05). Reduction count= log mean of control samples- log mean of treated samples. Reduction%: Mean of control samples- Mean of treated samples Mean of control samples X100.
However, C. perfringens was not detected in a study conducted on sausage in Morocco (Malti and Amarouch, 2008). Higher prevalence of C. perfringens (30%) was reported in western Massachusetts (Lin and Labbe, 2003), while in Kalyobia Governorate, Egypt, Hassanien (2004) reported that C. perfringens was isolated from 40%, 28% and 20% of sausage, beef burger and luncheon samples. In Japan, C. perfringens was isolated from 71% of the examined retail raw meat samples (Miki et al., 2008). Meanwhile, in Menoufiea and Gharbia Governorates, Egypt, Atwa and Abou EI-Roos (2011) reported that C. perfringens was isolated from ready to cook and ready to eat meat products with the percentages of 48.8% and 21.3%, respectively.
The differences in the prevalence of C. perfringens could be attributed to the variation in the unsanitary conditions, poor personal hygiene and cross contamination between raw and cooked meat products (McClane et al., 2006).
Depending on the four major toxins (alpha, beta, epsilon, and iota toxins), C. perfringens is classified into different five genotypes from A to E (Fohler et al., 2016). It was found that all the examined C. perfringens isolates were positive for alpha toxin, while C. Perfringens enterotoxin (cpe), not detected in all isolates. Similar findings were reported by Engstrom et al. (2003) who found that all strains of C. perfringens were non enterotoxin producers. Moreover, Lin and Labbe (2003) reported that all C. perfringens isolates possessed alpha toxin gene, but none of the isolates were identified as carrying the cpe gene. Heikinheimo and Korkeala (2005) who found that C. perfringens strains possessed alpha encoding genes and were negative for enterotoxin encoding genes. In India, 15.15% of C. perfringens isolates were positive for cpe (Gurmu et al., 2013). Meanwhile, Yibar et al. (2018) reported that 27.2% of C. perfringens isolated from raw, ready to cook and ready to eat meat and meat-based products in Turkey carried the cpe gene.
The variations of the results may be attributed to the method of manufacture and contamination level during the processing, packaging and storage (Borch and Arinder, 2002).
The occurrence of multidrug resistant C. perfringens in meat and its products raises the public health concerns. In the current study, the tested C. perfringens revealed high antimicrobial susceptibility.
C. perfringens isolates showed a relatively lower resistance rate (66% and 56.2%) to tetracycline (Tansuphasiri et al., 2005). Shojadoust et al. (2010) reported the resistance of C. perfringens isolates to neomycin (87.5%) and tetracycline (80%). Meanwhile, the percentage of resistance to amoxicillin and ampicillin was less than 7% (Osman and Elhariri, 2013). Silva et al. (2014) reported lower resistance of 22.2% and 27.8% to erythromycin and oxytetracycline respectively. Lower resistance to tetracycline (25.0%) was reported by Chon et al. (2018).
Higher resistance of 100% to gentamycin, erythromycin and streptomycin than the current study as well as 93% to neomycin was reported by Osman and Elhariri (2013). Hamza et al. (2017) reported that C. perfringens isolates from processed meat in Cairo, Egypt showed strong resistance (100%) to streptomycin. Mwangi et al. (2019) found the prevalence of C. perfringens resistance was 98% and 73% to streptomycin and gentamicin.
The MAR index of 0.2 or more indicates contamination from high risk sources, thus, posing risks to human consumers (Tambekar et al., 2006). More than 50% of C. perfringens strains were resistant to more than five antibiotics (Shojadoust et al., 2010). While, in Iran 34.17% of C. perfringens strains showed MDR (Akhi et al., 2015). Mehdi and Wannas (2017) reported that all C. perfringens isolates showed MDR. High resistance rate of C. perfringens may be due to the wide spread of the antimicrobials.
The process of biofilms formation involves different stages including attachment, maturation and dispersion. The obtained results in this study are in accordance with the results obtained by Varga et al. (2008) where C. perfringens strains were shown to form biofilms with optical density values between 0.07 and 0.5. On the other hand, Donelli et al. (2012) were able to obtain a higher biofilm formation for C. perfringens strain with a mean optical density value of 3.2. Most of the C. perfringens were able to form biofilm (230/277) in Canada (Charlebois et al., 2014).
This study showed that the incubation temperature had a significant effect on the ability of C. perfringens to produce biofilm. Incubation at 35°C enhanced biofilm formation significantly more than incubation at 4°C and 25°C. Time-temperature abuse in markets could result in the propagation of the bacteria to dangerous levels (Sudha et al., 2012). Temperature has an adverse effect on biofilm formation by affecting flagellar motility, which enhances the movement of bacteria towards the biofilm surface (Bonsaglia et al., 2014). Thus, C. perfringens can produce biofilm at markets where temperature abuse occurs, resulting in probable risks to consumers.
Various studies have been conducted on the antimicrobial effects of plant essential oils and their constituents against foodborne pathogens. In a study conducted in Brazil, marjoram essential oil exhibited antibacterial activities against C. perfringens (Radaelli et al., 2016). Moreover, Nevas et al. (2004) reported the inhibitory effect of thyme oil against C. perfringens and Silva et al. (2013) used the disc diffusion methods to evaluate the antibacterial activity of thyme oil and reported a high antimicrobial activity (inhibition > 95%) against C. perfringens.
The best effective lowest concentration dosed of thyme oil was 5% and 2% for marjoram. The results obtained in the current study are comparable to Juneja et al. (2006) who reported that adding 0.1 to 2% thyme to the meat inhibited germination and outgrowth of C. perfringens spores at 12 h exponential chill rates. While, Juneja and Friedman (2007) reported that addition of thyme 2% on cooked ground beef and turkey, resulted in 3-5 log CFU/g reduction of spore germination and outgrowth. In addition, Du et al. (2015) reported strong antibacterial effects of thyme oil in vitro against a panel of pathogenic bacteria, including C. perfringens. In Brazil, a study conducted by Radaelli et al. (2016) revealed that marjoram and thyme with MIC of 5 mg/ ml and 1.25 mg/ ml exhibited antimicrobial activities against C. perfringens.
Antimicrobial activities of EOs are related to chemical characteristics such as their hydrophobicity which enables them to interact with the lipids of the bacterial cell membrane thus disturbing bacterial metabolism and cell wall and membrane permeability, leading to extensive leakage of critical molecules and ions from bacterial cells (Diaz Carrasco et al., 2016).
Conclusions and Recommendations
Our study revealed a higher prevalence level of C. perfringens harbored Alpha toxin gene in raw meat and meat products. These results show that there is a need for applying appropriate food safety practices in meat and meat products production and processing facilities. The inhibition of biofilm formation needs for strict precautions to store food under different conditions. Our results suggest that treatment of meat with thyme oil 5% and marjoram oil 2% can greatly reduce C. perfringens, thus enhancing overall food safety. The reduction count was directly proportional.
Author’s Contribution
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Supplementary Table 1: Supplementary: Concentration and diameter of inhibition zone of antibiotics used for sensitivity test (n=10)
| Antibiotic groups | Antimicrobial agent | Conc. (µg) | inhibition zone | ||
| (R) | (I) | (S ) | |||
| Penicillins | Amoxicillin (AX) | 25 | ≤22 | 23-30 | ≥31 |
| Ampicillin (AM) | 10 | ≤22 | 23-30 | ≥31 | |
| Quinolones | Enrofloxacin (ENR) | 10 | ≤ 15 | 16-20 | ≥ 21 |
| Norfloxacin (NOR) | 10 | ≤15 | 16-20 | ≥21 | |
| Aminoglycosides | Gentamicin (CN) | 10 | ≤12 | 13-14 | ≥ 15 |
| Neomycin (N) | 30 | ≤12 | 14-16 | ≥ 17 | |
| Streptomycin (S) | 10 | ≤11 | 12-14 | ≥ 15 | |
| Aminocoumarin | Novobiocin (NV) | 30 | ≤17 | 18-21 | ≥22 |
| Tetracyclines | Oxytetracyclin (T) | 30 | ≤14 | 15-18 | ≥ 19 |
| Macrolides | Erythromycin (E) | 15 | ≤13 | 14-22 | ≥23 |
n: Number of tested antibiotics; Conc.: Concentration; R: Resistant; I: Intermediate; S: Sensitive.






