Journal of Animal Health and Production
Research Article
Pathogenesis of Different Genotypes of Egyptian Virulent Newcastle Disease Virus (NDV) Previously Isolated from Chickens in Japanese Quails (Coturnix coturnix japonica)
Ahmed A. H. Ali1, Fatma Abdallah1*, Ahmed Abdelaziz2, Yahia Madbouly3, Gamilat Kotb1
1Department of Virology, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Egypt; 2Free Veterinarian graduated from Mansoura University; 3Poultry Viral Vaccines Research Department, Veterinary Serum and Vaccine Research Institute (VSVRI), Al Abbassia, Cairo, P.O 11381, Box 131, Egypt.
Abstract | Newcastle disease (ND) is a highly infectious transboundary avian notifiable disease caused by Newcastle disease virus (NDV). This study was aimed to assess the pathogenesis of two Egyptian NDV strains of different genotypes in quails based on clinical signs, gross pathology, histopathology, virus nucleic acid detection in different organs using reverse transcriptase polymerase chain reaction (RT-PCR) and virus re-isolation as well as measurement of the antibody titer in serum samples. Two week-old Japanese quails (n=90) were equally allocated into three groups, the quails in the groups A and B were experimentally inoculated via oculonasal route with 0.2 mL of 107 EID50 (embryo infectious dose 50) units of NDV VIb (EG/SR/76/CH/1967) and NDV VIId (NDV/Chicken /Egypt/1/2015) strains, respectively. Group C was kept as a control group, which received phosphate-buffered saline (PBS). Our results denoted that NDV VIId was shown to cause only very mild clinical signs with no microscopic lesions detected in different organs in contrast to the NDV VIb. Additionally, NDV VIId nucleic acid was detected only in two tissue samples (spleen and brain) of two different quails at 7 days post infection (dpi) using RT-PCR without any success in isolating the virus from these tissue samples. From our results, we suggested that the decreased pathogenicity caused by NDV VIId was correlated with limited virus replication in different organs of infected quails, although it contained multiple basic amino acids at fusion protein cleavage site.
Keywords | Newcastle disease virus, Quails, Pathogenesis, Oculonasal route, Virulence, Genotype, Egypt.
Received | November 17, 2020; Accepted | December 01, 2020; Published | December 27, 2020
*Correspondence | Fatma Abdallah, Department of Virology, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Egypt; Email: [email protected]
Citation | Ali AAH, Abdallah F, Abdelaziz A, Madbouly Y, Kotb G (2020). Pathogenesis of different genotypes of egyptian virulent newcastle disease virus (NDV) previously isolated from chickens in japanese quails (coturnix coturnix japonica), Egypt. J. Anim. Health Prod. 9(s1): 90-96.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.90.96
ISSN | 2308-2801
Copyright © 2020 Abdallah et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Newcastle disease (ND) is one of the most dangerous and destructive viral diseases in the poultry worldwide (Miller and Koch, 2013). It has plagued the poultry industry causing huge economic losses since 1926 (Alexander, 1997). Newcastle disease virus (NDV) belongs to the genus Orthoavulavirus within the subfamily Avulavirinae, family Paramyxoviridae and order Mononegavirales (Amarasinghe et al., 2019). All NDV isolates belong to a single serotype (Aldous et al., 2003). Phylogenetically, NDV strains are categorized into 2 distinct classes, I and II (Diel et al., 2012). Class I viruses have one genotype that typically involves non-virulent strains that isolated from aquatic birds and live-bird markets (Diel et al., 2012). Class II viruses are subdivided into 18 genotypes and multiple subgenotypes that are responsible for destructive diseases in poultry (Snoeck et al., 2013). NDV strains are categorized into three main pathotypes: velogenic, mesogenic and lentogenic (Alexander, 2000). In spite of the firm vaccination policy for prevention and control of ND, it is considered an endemic disease in various countries of the world causing a continuous hazard to the poultry industry. ND has severe impacts on domestic and wild avian species and it continues to reemerge all over the world (Momayez et al., 2007). In view of the increasing interest in quail farming by numerous farmers in Egypt, significant information about the susceptibility of the Japanese quails to ND infection and immunization approaches for prevention and control of NDV infections should be kept in mind. In addition, other studies proved that quails had the susceptibility to natural infection with velogenic NDV strain (Czirják et al., 2007). So that this study aimed to estimate, the pathogenesis and ability of two velogenic Egyptian NDV strains of distinctive genotypes previously isolated from chickens to induce the disease in 2-week-old Japanese quails.
MATERIALS AND METHODS
NDVs propagation and titration
Two NDVs were utilized in the present study. The first, velogenic NDV class II, genotype VIId (NDV/chicken/Egypt/1/2015) under an accession number of KX231852 was friendly provided by Dr. Ola Hassanin, Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Meanwhile, the second, velogenic NDV class II, genotype VIb (EG/SR/76/CH/1967) under an accession number of AY968809 was gently provided by Dr. Yahia Madbouly, Department of Poultry Viral Vaccines Research, Veterinary Serum and Vaccine Research Institute, Al Abbassia, Cairo, Egypt. Prior to use in experimental infection, virus propagation and titration were carried in embryonated chicken eggs according to (Alexander, 1989), as well as, virus titers were calculated according to (Reed and Muench, 1938).
Birds and handling
Ninety, 11-days old Japanese quails (Coturnix coturnix Japonica), were obtained from a commercial farm in Dakahlia Province. Upon arrival, serum samples from all quails were collected, and tested against LaSota NDV strain to confirm that quails were non-immunologically for NDV infection using hemagglutination inhibition (HI) (Alexander and Senne, 2008). The quails were reared on well ventilated cages in the experimental animal houses at the Faculty of Veterinary Medicine, Zagazig University under standardized environmental conditions. The quails were acclimated three days to adapt to the rooms before infection at 14 days old.
Experimental design
Quails were randomly divided into three equal groups (A, B and C), 30 quails/each. Groups A and B were infected at 14 days old with 200 ul of 107 embryo infective dose 50 (EID50) of EG/SR/76/CH/1967 and NDV/chicken/Egypt/1/2015 per quail, respectively. The 200 ul of the virus inoculum was divided equally between the ocular and nasal routes. Meanwhile, the control group (C) was inoculated similarly with 200 ul of phosphate-buffered saline (PBS). At 2, 4, 6 and 7 days post infection (dpi), 3 quails were euthanized to assess the virus replication in different tissues from each group. The residual quails were checked daily up to 14 days to record the clinical signs and mortalities. Additionally, quails were euthanized at the point at which they showed clinical signs. Moreover, tissues for histopathology including trachea, spleen, proventriculus, and brain were fixed in formalin. For molecular analysis and virus isolation, approximately 30 mg of brain, spleen, trachea and proventriculus were collected into tubes containing PBS and stored at - 80 οC until required. A 2 mL blood was collected from the wing vein of quail prior to infection and at 7 and 14 dpi, as well as, the serum was separated and stored at -20 οC until testing. The experimental design and care of the quails were approved ahead by the Ethics Committee for Animal Studies at the Faculty of Veterinary Medicine, Zagazig University, Egypt.
Histopathology
After tissue fixation in formalin for 24 hours, the fixed tissues were then transferred in 70% ethanol solution before processing by conventional methods as described previously (Kim, 2018). Tissue sections of fixed lesions were stained with hematoxylin and eosin, and then examined microscopically.
Hemagglutination inhibition assay
The antibody titers were measured by HI assay using the conventional microtiter plate method (OIE, 2012). The micro HI test was performed using 8 hemagglutinating units (HAUs) of inactivated homologous antigen and 0.5% chicken red blood cells.
Molecular detection by reverse transcriptase-polymerase chain reaction (RT-PCR) assay
The extraction of total RNAs were carried from approximately 30 mg of tissues (brain, spleen, trachea, and proventriculus) as well as EG/SR/76/CH/1967 and NDV/chicken/Egypt/1/2015 virus suspensions as positive controls using Gene JETTM viral RNA Purification Kit (Thermo Fisher Scientific Inc., USA). RNA from each sample was reverse-transcribed to produce cDNA using a Quantitect® Reverse Transcription kit (Qiagen, Germany). Afterwards, PCR for amplification of 900 bp from fusion (F) gene was performed using designed primers with the following sequences: forward: 5’-ATG CTC ATC ACT CGG ATT ATG C-3’ and reverse: 5’-GAC TAA TGC TGA GGC ATA TCC T-3’. Amplification was done using Dream TaqTM Green PCR Master Mix (2X) (Fermentas, Glen Burnie, MD). The amplified fragments were separated on agarose gels (1%) and 1-kbp DNA marker (Fermentas, Glen Burnie, MD) was used as standard and the amplified products were visualized using ultraviolet light transilluminator (Spectroline). Tissues of the negative control group were included for detection of any contamination.
Virus Isolation
It was performed on PCR-positive tissue samples only. Briefly, 0.2 mL of the supernatant of the tissue homogenate was inoculated onto the allantoic sac of 11-day-old embryonated chicken eggs from commercial non-vaccinated flocks in three replicates. Allantoic fluid was then harvested and tested for hemagglutination (HA) activity (Alexander, 1989).
Statistical analysis
The geometric mean titers of NDV HI were expressed as a mean ± standard deviation of Log2 HI titer and were compared between groups via One-way ANOVA.
RESULTS
Clinical signs
All quails in group A that infected with NDV VIb strain (EG/SR/76/CH/1967) and group B that infected with NDV VIId strain (NDV/chicken/Egypt/1/2015) survived until the end of the experiment. For the group A, five quails had general inactivity in the morning along with decreasing in feed intake at 2 dpi. Three quails from this group along with other three quails from the control group were then euthanized at this time point. Eight quails showed clinical signs at 4 dpi including dullness, abnormal movement as backward movement and then they were euthanized at that time. At 6 dpi, seven quails displayed torticollis, ataxia and paralysis (Fig.1a, b). These quails were then euthanized at this time point. Additionally, five quails showed moderate depression, dullness and ruffling feather at 6 dpi (Fig.1c). Moreover, moderate clinical signs were observed at 7, 8 and 9 dpi, but with increasing in the rest of the infected quails. At 10 dpi, no clinical abnormalities were detected in the remainder quails. On the other hand, no clinical abnormalities were observed in any of the quails in group B at 2 and 4 dpi. Three quails at 2 and 4 dpi were then euthanized. At 6 dpi, five quails showed mild clinical signs including dullness, moderate depression. These quails were then euthanized at this time point. Meanwhile at 7 dpi, thirteen quails displayed dullness, ruffling feather and abnormal movement. These quails were then euthanized at this time point. There is a gradual decrease in the appearance of clinical signs and their severity at 8, 9 and 10 dpi. Generally, either no abnormalities were noticed clinically or no other deaths were recorded in the control quails throughout the experiment.

Figure 1: Clinical signs of infected quails with velogenic class II genotype VIb NDV strain (EG/SR/76/CH/1967) at 6 dpi. The infected quails showed torticollis (a), paralysis of the legs (b) and ruffling feather (c).
Gross pathology
The most reliable gross pathological findings were hemorrhagic cecal tonsils with pale mottling and hemorrhage at the proventriculus and hemorrhagic enteritis at 2, 4, 6 and 7 dpi in the group infected with NDV VIb strain (EG/SR/76/CH/1967). Additionally, congestion of thigh and breast muscle, mild splenic and kidney enlargement were also observed only at 7 dpi (data not shown). There were no gross pathological lesions upon necropsy found in quails inoculated with the NDV VIId strain (NDV/chicken/Egypt/1/2015) or quails in the control group.
Histopathology
All quails infected with the NDV VIId strain (NDV/chicken/Egypt/1/2015) showed the minimal histological lesions starting from 7 dpi. In quails infected with the NDV VIb strain (EG/SR/76/CH/1967), histological lesions were mainly seen in spleen and proventriculus. In spleen, there was depleted white pulp with pericytoma accompanied with hypertrophic epithelium lining the central arteries of the white pulp (Fig.2a). However, proventriculus showed mild congestion of submucosal blood vessels and necrotic epithelial lining replaced by chronic inflammatory cells (Fig.2b). Moreover, the examined sections from trachea showed necrotic respiratory epithelium and subepithelial and submucosal round cell infiltration (Fig.2c). The other significant lesions seen in the brain as were degenerated neuron, satellitosis, engulfment of degenerated neuron by glia cell (neuronophagia) and replacement of degenerated neurons by glia cells (gliosis) (Fig.2d).
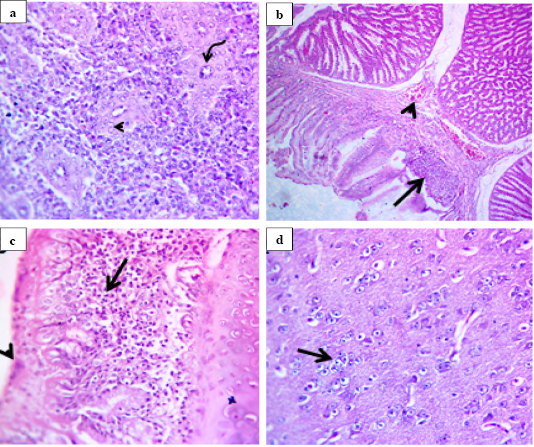
Figure 2: Histological lesions of spleen, proventriculus, trachea and brain of infected quails with velogenic class II genotype VIb NDV strain (EG/SR/76/CH/1967) at 7 dpi (H and E, 400X). Spleen showed pericytoma (arrow head) with hypertrophic epithelium lining the central arteries of white pulp (curved arrow) (a). Proventriculus showed mild congestion of submucosal blood vessels (arrow head) and replacement of necrotic epithelial lining by inflammatory cells (arrow) (b). Trachea showed necrotic respiratory epithelium (arrow head) with subepithelial and submucosal round cell infiltration (arrow) (c). Brain showed replacement of degenerated neuron by glia cells (arrow) (d).
Serology
All quails were serologically negative for NDV in serum antibodies by HI test prior to infection with either NDV VIb or VIId genotypes. Seroconversions were noticed in quails at 7 dpi and continued in increasing till the end of experiment at 14 dpi in both infected groups. There were no statistical differences between groups A and B at 7 and 14 dpi (Table 1).
Table 1: Hemagglutination inhibition antibody response of quails before and after infection with different genotypes of velogenic NDV strain
|
Group |
Days post infection (dpi) | ||
| 0 | 7 | 14 | |
| A |
0±000c |
2.7±25a |
4.5±31b |
| B |
0±000c |
2.4±43a |
4.1±27b |
| C |
0±000c |
0±000 c |
0±000 c |
Values represent means of antibody titers ± standard deviation of Log2 HI titer. Means denoted by different superscript letters (a,b,c) indicate significant differences.
Molecular detection by RT-PCR
Four tissue samples (brain, spleen, proventriculus, and trachea) from clinically infected quails were examined for detection of NDV using RT-PCR (Figure 3) at 2, 4, 6 and 7 dpi. Most positive tissue samples for NDV RNA (39/48 samples) that were collected from quails infected with the NDV VIb strain (EG/SR/76/CH/1967) as shown in Table 2. Briefly, at 2 dpi, the spleen samples from the three euthanized quails were positive, but all tissues from the remaining quails were positive at 4, 6 and 7 dpi. Only 2 tissues were NDV positive from the quails infected with the NDV VIId strain (NDV/chicken/Egypt/1/2015) consisting of 1 spleen and 1 brain samples at 7 dpi.
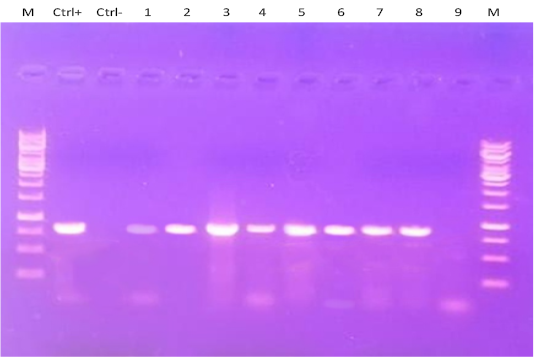
Figure 3: RT-PCR amplification of F gene from clinically infected quails with two NDV strains in the present experiment. Lane M: 1-kbp DNA marker; Ctrl+: control positive; Ctrl: control negative; Lanes 1-6: Amplified products of NDV detected in different tissues of group A that infected with NDV strain (EG/SR/76/CH/1967). Lanes 7-9: Amplified products of NDV detected only in one spleen (lane 7) and one brain (lane 8) tissue samples of group B that infected with NDV strain (NDV/chicken/Egypt/1/2015) at 7 dpi, while lane 9 represented negative NDV infected tissue sample.
Virus re-solation
Virus re-isolation was conducted for different tissues (brain, spleen, proventriculus, and trachea) that were subjected to RT-PCR as shown in Table 2. NDV was re-isolated from all positive RT-PCR, but it was not re-isolated from the two RT-PCR-positive tissues (spleen and brain) of 2 different infected quails in the group B at 7 dpi.
DISCUSSION
Newcastle disease (ND) is considered as a significant threat for chickens, however, there are relatively limited information is accessible concerning the pathogenesis of NDV with different genotypes in quails. In the present study, the pathogenesis was assessed on the basis of certain parameters such as clinical signs, gross pathology, histopathology, molecular detection of virus nucleic acid
Table 2: Distribution of NDV in tissues of clinically infected quails based on RT-PCR / virus isolation
| NDV genotype | Quail ID number | Euthanization time (dpi) | Brain | Spleen | Trachea | Proventriculus |
| EG/SR/76/CH/1967 | ||||||
|
|
11 | 2 | -/- | /+ + | -/- | -/- |
| 20 | 2 | -/- | /+ + | -/- | -/- | |
| 15 | 2 | -/- | /+ + | -/- | -/- | |
| 16 | 4 | /+ + | /+ + | /+ + | /+ + | |
| 19 | 4 | /+ + | /+ + | /+ + | /+ + | |
| 3 | 4 | /+ + | /+ + | /+ + | /+ + | |
| 5 | 6 | /+ + | /+ + | /+ + | /+ + | |
| 2 | 6 | /+ + | /+ + | /+ + | /+ + | |
| 23 | 6 | /+ + | /+ + | /+ + | /+ + | |
| 4 | 7 | /+ + | /+ + | /+ + | /+ + | |
| 17 | 7 | /+ + | /+ + | /+ + | /+ + | |
| 9 | 7 | /+ + | /+ + | /+ + | /+ + | |
| NDV/chicken/Egypt/1/2015 | ||||||
| 13 | 2 | -/- | -/- | -/- | -/- | |
| 12 | 2 | -/- | -/- | -/- | -/- | |
| 5 | 2 | -/- | -/- | -/- | -/- | |
| 16 | 4 | -/- | -/- | -/- | -/- | |
| 21 | 4 | -/- | -/- | -/- | -/- | |
| 29 | 4 | -/- | -/- | -/- | -/- | |
| 18 | 6 | -/- | -/- | -/- | -/- | |
| 25 | 6 | -/- | -/- | -/- | -/- | |
| 6 | 6 | -/- | -/- | -/- | -/- | |
| 14 | 7 | -/+ | -/- | -/- | -/- | |
| 26 | 7 | -/- | -/+ | -/- | -/- | |
| 1 | 7 | -/- | -/- | -/- |
-/- |
|
NDV: Newcastle disease virus, dpi: days post infection, /+ +: positive PCR/virus isolation, -/-: negative PCR/virus isolation, -/+: positive PCR/negative virus isolation.
in different organs and virus re-isolation as well as the expression of the antibody titers. We conducted this experiment via oculo-nasal inoculation of Japanese quails with two NDV strains, NDV VIb (EG/SR/76/CH/1967) in group A and NDV VIId (NDV/Chicken /Egypt/1/2015) in group B. Primarily, from our results, we found that no mortality in quails infected via oculo-nasal route by two NDV strains. Similar findings were documented previously (Lima et al., 2004). The NDV VIId strain (NDV/chicken/Egypt/1/2015) caused mild clinical disease including dullness, moderate depression and a reluctance to move away from 6 dpi. Microscopically, the lesions were minimal starting from 7 dpi. They involved mild infiltrates of heterophils in the epithelium and submucosa around sites of inoculation. The sites of replication were restricted, where NDV RNA was detected only in spleen and brain of two different quails at 7 dpi. This minimal pathogenicity could be attributed to the low level of viral replication in quails (Sedeik et al., 2019). However, the clinical signs were initially observed at 6 dpi in the quails inoculated with NDV VIb strain (EG/SR/76/CH/1967) including paralysis, torticollis, ataxia, depression, dullness and ruffling feather. These clinical signs were progressed in intensity at 7 dpi and involved more quails. The neurological clinical signs as a result of tropism of virulent NDV strains to the nervous tissues were also previously reported (Ecco et al., 2011; Susta et al., 2018). Our results showed that the quails inoculated by NDV VIId failed to develop a severe systemic infection in comparison to NDV VIb, although using the same dose (107 EID50 /quail) as well as the same route (oculo-nasal route). Results from this study demonstrated that the decreased pathogenicity was correlated to limited virus replication. The detection of virus nucleic acid in different organs as measured by RT-PCR and virus isolation denoted that the NDV was capable to replicate systemically (Bergfeld et al., 2017). Compared with the virulent NDV VIb strain (EG/ SR/76/CH/1967), the NDV RNA of the NDV VIId strain (NDV/ chicken/Egypt/1/ 2015) was not detected in all tissues except only two tissue samples (spleen and brain) at 7 dpi. This was consistent with no histopathological lesions as well as negligible clinical signs. In general, the increasing of virus replication and abundant inflammatory responses within lymphoid tissues has been accompanying with increased pathogenicity of NDVs (Hu et al., 2015). The low pathogenicity of the NDV VIId strain (NDV/chicken/Egypt/1/2015) in the present study may be assigned to regions of NDV genome other than the fusion protein cleavage site given that the cleavage site contains a virulent motif (Dortmans et al., 2011; Paldurai et al., 2014). Additionally, Dortmans et al. (2010) reported that the viral replication complex was associated with the minimal pathogenicity of the pigeon paramyxovirus type 1 in chickens despite of its virulent cleavage site motif.
Conclusion
The NDV-VIId genotype induced mild disease conditions with no mortality in quails; however, NDV-VIb led to a highly pathogenic disease inducing severe morbidity in quails. The minimal pathogenicity in NDV-VIId infected quails was due to limited virus replication although it contains multiple basic amino acids at fusion protein cleavage site.
Acknowledgments
We are thankful to Dr. Ola Hassanin, Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt for providing us with the velogenic NDV VIId (NDV/chicken/Egypt/1/2015) under the accession number of KX231852.
Authors’ Contributions
AAHA, FA and AA designed and carried out the experiment. YM assisted in the propagation and titration of viruses. FA and AA analyzed the experimental data and wrote the first draft of the manuscript. GK assisted in the experimental work. All authors reviewed and approved the final manuscript.
Conflict of Interest
The authors declare that they have no competing interests related to the experimental study or publication.
References






