Journal of Animal Health and Production
Research Article
Determination of Cefepime Residues in Different Rabbit Tissues using High Performance Liquid Chromatography
Mai H. Helal1*, Ahmed A. Said1, Gamal El-Din A. Shams1, Magdy F. Abu El-Ftouh2
1Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt, 44511; 2Forensic and Toxicology medicine Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt, 44511.
Abstract | Drug Residues in meat have serious effects on human health (e.g., increase antimicrobial resistance, carcinogenicity, mutagenicity, and hypersensitivity) which urge the control of veterinary drug residues to ensure consumers’ health. Thus, this work was designed to determine cefepime residues quantitatively in different tissues of the rabbits (liver, kidneys and muscles) and to determine the withdrawal time of cefepime following intra-muscular (I/M) dosing of the drug using high performance liquid chromatography (HPLC). Twenty four healthy male rabbit (growing white New Zealand) were used and randomly allocated into two equal groups, 12 rabbits/each; group 1 (negative control) was I/M injected with sterile normal saline and group 2 was I/M injected with cefepime hydrochloride (75 mg/kg BW) once daily for 5 successive days. Rabbits were sacrificed on 1st, 3rd, 7th and 14th day post antibiotic injection and liver, kidney and breast muscles samples were collected for detection of residue by HPLC. Results showed that days of experiment and tissue type has an effect on cefepime residual level as there is highly significant interaction (p =0.0001). The highest cefepime residual level was detected in kidney followed by liver while the lowest level was recorded in muscle samples. Cefepime remained within the detectable limit in the breast muscle (on the 3rd day) and in the kidney and liver tissues (on the 7th day) following the last I/M injection of the drug. While on the 14th day after last dose, the cefepime residues were disappear in all tested tissues. Cefepime administration caused non-marked changes of tested biochemical indices (liver, and kidney function tests) of rabbit then reached to safe levels during the withdrawal period at other time points. Therefore, it is recommended to pay attention to the proper withdrawal time (14 day) before slaughtering of cefepime-treated rabbits to make sure that rabbits tissues are free from cefepime residues and become safe for consumers.
Keywords | Cefepime, HPLC, Residues, Rabbits, Withdrawal time.
Received | November 03, 2020; Accepted | December 01, 2020; Published | December 27, 2020
*Correspondence | Mai H Helal, Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt, 44511; Email: memahamdy90@gmail.com
Citation | Helal MH, Said AA, Shams GDA, El-Ftouh MFA (2020). Determination of cefepime residues in different rabbit tissues using high performance liquid chromatography. J. Anim. Health Prod. 9(s1): 77-83.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/9.s1.77.83
ISSN | 2308-2801
Copyright © 2020 Helal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Several broad spectrum antibiotics were widely used for treatment and prophylaxsis of some dangerous bacterial infection in rabbits (El-Nawawy et al., 2006). Cefepime is one of fourth-generation parenteral cephalosporin antibiotic that is used for treatment of pneumonia, febrile neutropenia, urinary tract infections. Cefepime monotherapy gives both an excellent microbiological clearance and a good clinical response (El-Dars et al., 2019).
Antimicrobial drug residues are detected by chemical, biological and immunological methods. The use of high performance liquid chromatography (HPLC) is an effective, accurate, rapid and sensitive technique for the analysis of cefepime residues in the tissues (Orti et al., 2000). Intensive uncontrolled use of antimicrobials drugs in the rabbit industry especially if the withdrawal times are neglected leads to harmful effects on human health (Tajick and Shohreh, 2006). The possible human hazards health problems related to antibiotic residues have been reported including; allergic/toxic reactions, chronic toxic effects as; (carcinogenicity, reproductive and teratogenic effects) which may occur due to the prolonged exposure to low levels of antibiotic residues in addition to the development of antibiotic-resistant bacteria in the treated animal (Speer et al., 1992). Antibiotics resistance exist a serious human health hazard due to the risk of the cross resistance to other pathogens, which may affect human health (Reig and Toldrá, 2008).
Therefore, this study was designed to i) determine cefepime residues and withdrawal time from different rabbit tissues (liver, kidneys, and breast muscles) and ii) estimate the residual effect of cefepime on liver and kidney (via evaluation of some biochemical parameters) following 5 days of cefepime intramuscular administration at the doses of 75mg/kg using HPLC technique.
Material and Methods
Drug
Cefepime hydrochloride powder (Maxipime 1 g Bristol Myer, Squibb, New York NY, USA) was reconstituted with sterile free water. The recommended intra-muscular (I/M) dose in rabbit is 75 mg/kg body weight once daily for five consecutive days (El-Dars et al., 2019).
Experimental design
The study was approved by the Committee of Animal Welfare and Research Ethics Faculty of Veterinary Medicine, Zagazig University, Egypt. A total of twenty-four growing white New Zealand male rabbits, were obtained from Faculty of Veterinary Medicine, Zagazig University. They were fed on balanced commercial ration free from any medication, and the water was provided ad-libitum. They were kept under clean condition during the experimental period. They were randomly allocated into two equal groups; group 1 (negative control, n=12) was injected I/M with sterile normal saline for 5 successive days and group 2 (n=12) was injected I/M with cefepime hydrochloride (75 mg/kg BW) once daily for 5 successive days.
Sampling and biochemical analysis
Three rabbits were sacrificed at different time point (1st, 3rd, 7th and 14th days) and blood was collected for serum preparation. Sera used for biochemical studies using various diagnostic kits (Roch Diagnostics, GmbH, USA). Alanine aminotransferase (ALT), serum aspartate-aminotransferase (AST), and serum alkaline phosphatase (ALP) was measured by Reitman and Frankel (1957). Total protein and albumin was measured according to the method developed by (Buzanovskii, 2017). Blood urea and creatinine were determined according to the method of (Henry, 1974).
Liver, kidney and breast muscles were collected and each tissue was subsequently sliced into five parts for detecting cefepime residues and withdrawal time with HPLC (Chen-Hao and Yun, 2007).
Detection of cefepime residues by high performance liquid chromatography
Solid phase extraction: Extraction and determination of drug residues Liver, kidney and breast muscles was performed as described previously (Chen-Hao and Yun, 2007). Ten mL extract was passed through the SampliQ OPT cartridge (Varian, Les Ulis, France) at a speed of 1 mL/min then the entire effluent was discarded and the cartridge was dried under negative pressure below 2.0 kPa for 3 minutes. Finally, cefepime was eluted with 10 mL of 10 mmol/L oxalic acid in methanol, collected and dried under nitrogen below 40 °C. The resulting residue was dissolved and made to a constant volume of 0.5 mL using the methanol/10 mmol/L TFA solution (1/19) then filtered through a 0.45-μm filter membrane (p/n 5185-5836) and analyzed (Chen-Hao and Yun, 2007).
Liquid chromatography operating conditions: Flow rate, 1.5 mL/min; column (Agilent ZORBAX SB-C8 250 mm × 4.6 mm, 5 µm) temperature, 30°C; injection volume, 100 µL and detector (Series 1200 UV Vis detector) wavelength, 350nm were adjusted for HPLC analysis. Moreover the mobile phase; Methanol-acetonitrile-10 mmol/L TFA solution, gradient elution and its gradient.
Quantification of residues: Quantification of the antibiotic residues in the samples was obtained and calculated from the area under curves extrapolated automatically by the HPLC 2D Chemstation Software (Hewlett-Packard, Les Ulis, France).
Standard curve: A reference standard, cefepime powder, was obtained from Sigma-Aldrich Co, USA.
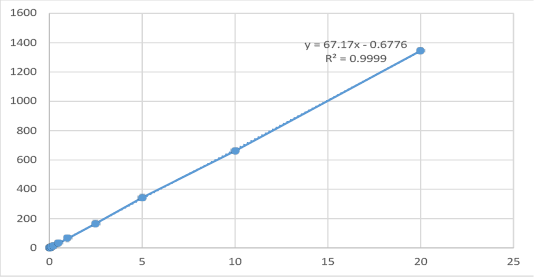
Spiked samples of cefepime: Cefepime standard prepared at concentrations of 0.025, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5, 10 and 20 µg/gm and homogenized with 5 g of control rabbit muscles then treated according to the abovementioned extraction procedure. The calibration curve was calculated by linear regression equation method as y = 67.17x- 0.6776 where y symbol indicated area under peak and x symbol indicated concentrations of cefepime.
Statistical analysis
Data were screened and Shapiro-Wilk test was applied to test normality assumption, Levene’s test also was run to evaluate homogeneity of variance. Differences in total protein and the other liver function enzymes were tested by one-way ANOVA. While Two-way ANOVA was run to test differences in the concentration of cefepime in different tissues at different times of experiment. The significant results followed by Tukey’s honesty significant test to compare between means. Results reported as mean ± SE. P < 0.05 statistically denotes statistical significance. All results were performed by SPSS version 25 ((Armonk, NY: IBM Corp)).
Results
The results obtained from the High performance liquid chromatography analysis indicated that the corresponding peak responses (area under peak) of cefepime standard concentrations of 0.025, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5,10 and 20 μg/gm was 1.033, 2.822, 6.102, 16.300, 32.426, 65.492, 166.240, 343.930, 661.650, 1355.400 as illustrated in Table (1) and Figure (1). Linearity existed within range of 0.025, and 20 μg/gm with a correlation coefficient (r2=0.99993). The recovery percentage of spiked samples ranged from 95-98%. The limit of detection for cefepime was 0.0025 μg/ml, while, LOQ (limit of quantification) was 0.01 μg/ml.
Table 1: The concentrations of cefepime spiked tissues (μg/gm) and their corresponding peak response automatically using HPLC.
| RT |
Level
|
Concentration (µg/gm) | Area | Found concentration | Recovery % |
| 4.108 | 1 | 0.025 | 1.033 | 0.025473 | 101.8907 |
| 2 | 0.05 | 2.822 | 0.052107 | 104.2132 | |
| 3 | 0.1 | 6.102 | 0.100938 | 100.9379 | |
| 4 | 0.25 | 16.3 | 0.252762 | 101.1047 | |
| 5 | 0.5 | 32.426 | 0.492839 | 98.56781 | |
| 6 | 1 | 65.492 | 0.985112 | 98.51124 | |
| 7 | 2.5 | 166.24 | 2.485008 | 99.40033 | |
| 8 | 5 | 343.93 | 5.130386 | 102.6077 | |
| 9 | 10 | 661.65 | 9.860473 | 98.60473 | |
| 10 | 20 | 1345.4 | 20.03987 | 100.1993 |
Cefepime distribution in rabbit tissues (liver, kidney, and breast muscles) was represented in Table (2). The typical chromatogram of cefepime is shown in Figure (2,3,4, and 5). The days of experiment showed a great effect on level of cefipime regardless type of tissue from which they recorded, the results were highly significant (p=0.0002), the highest level was recorded on first day and the value gets lower.
Table 2: The concentrations of cefepime in tissues of slaughtered rabbits at different time points (1st, 3rd, 7th and 14th days) following the last dose of cefepime 75 mg/kg once daily for 5 consecutive days using HPLC. (Mean ± SE), (n=3).
| Parameters | Cefepime residual level |
| Main effect (Tissue) | |
| kidney |
2082.33 ± 508.33a |
| Liver |
1251.00 ± 370.3ab |
| Breast muscle |
267.89 ± 86.97b |
| P- value | 0.007 |
| Main effect (Days) | |
| Day 1 |
2426.44 ± 502.13a |
| Day 3 |
921.78 ± 192.01b |
| Day 7 |
253.00 ± 103.89b |
| P- value | 0.0002 |
|
Simple effect Kidney Day1 Day3 Day7 P- value Liver Day1 Day3 Day7 P- value Breast muscle Day1 Day3 Day7 P- value |
4055 ± 0.58a 1527.00 ± 0.58b 665.00± 0.58c < 0.001 2630.33 ± 0.87a 1028.67± 0.33b 94.00 ± 0.58c < 0.001 594 ± 0.58a 209.67± 0.33b 00.00 ± 0.00c < 0.001 |
| Interaction (Tissue × days) | |
| Kidney × Day1 |
4055 ± 0.58a |
| Kidney × Day3 |
1527.00 ± 0.58c |
| Kidney × Day7 |
665.00± 0.58e |
| liver × Day1 |
2630.33 ± 0.87b |
| liver × Day3 |
1028.67± 0.33d |
| liver × Day7 |
94.00 ± 0.58h |
| Breast muscle × Day1 |
594 ± 0.58f |
| Breast muscle × Day3 |
209.67± 0.33g |
| Breast muscle × Day7 |
00.00 ± 0.00i |
| P- value | 0.0001 |
abcdefghi Means with different superscripts within column were statistically different at P < 0.05 according to Tukey’s test.
Note: At 14th day cefepime was completely disappeared in all tissues.
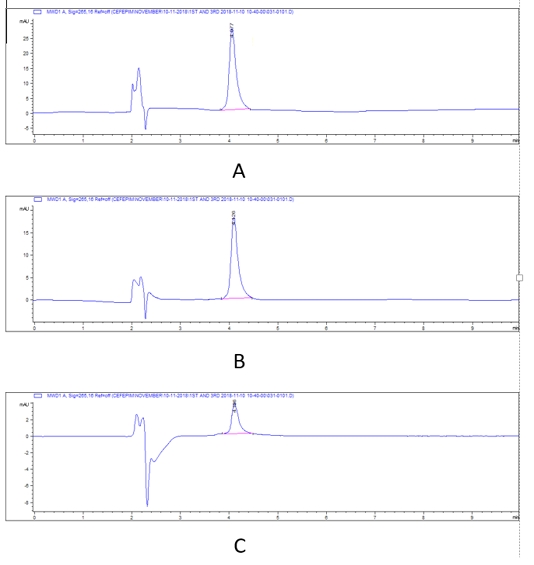
Figure 2: HPLC chromatograms of cefepime residual level in rabbit tissues in 1st day post I/M cefepime treatment. (A) cefepime residue in kidney, (B). cefepime residue in liver ,(C). cefepime residue in breast muscle.
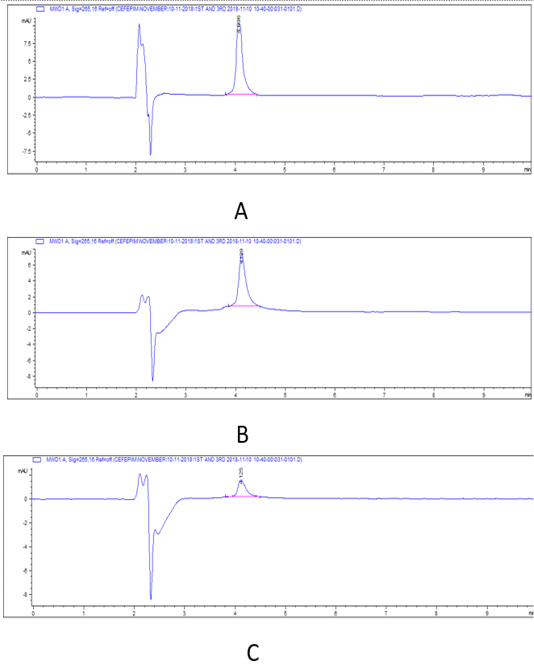
Figure 3: HPLC chromatograms of cefepime residual level in rabbit tissues on 3rd day post I/M cefepime treatment. (A) cefepime residue in kidney, (B). cefepime residue in liver ,(C). cefepime residue in breast muscle.
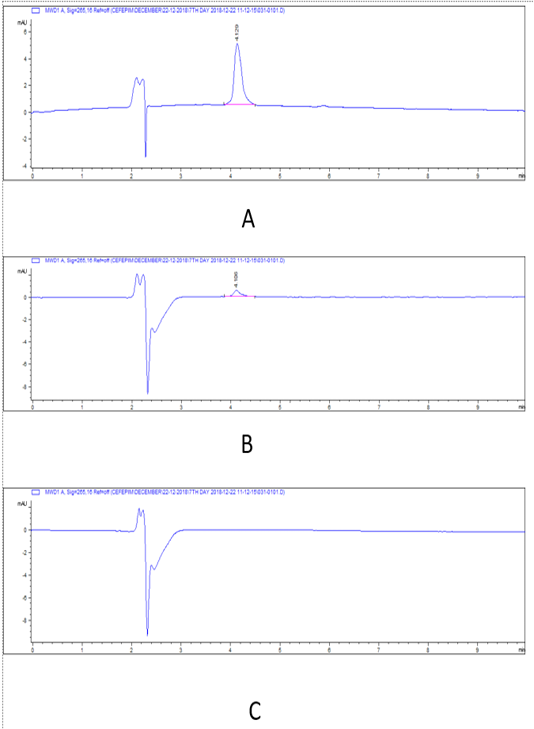
Figure 4: HPLC chromatograms of cefepime residual level in rabbit tissues at 7th day post I/M cefepime treatment. (A) cefepime residue in kidney, (B). cefepime residue in liver, (C). cefepime residue in breast muscle.
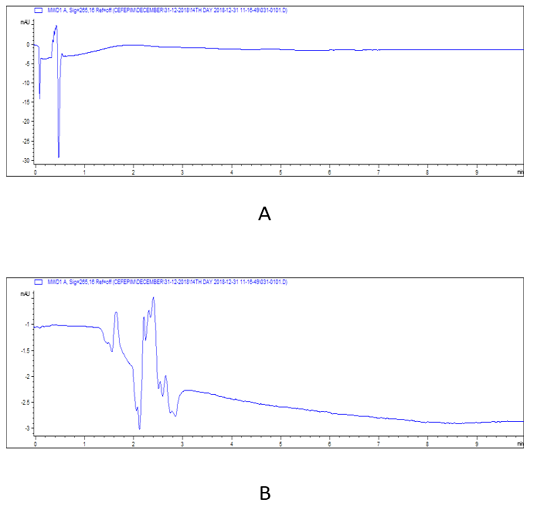
Figure 5: HPLC chromatograms of cefepime residual level in rabbit tissues at 14th day post I/M cefepime treatment. (A) cefepime residue in kidney, (B). cefepime residue in liver.
There was a widespread distribution of the cefepime in the tested tissues (p=0.007). The mean concentration of cefepime residues were 4055, 2630.33, and 594 μg/kg on the 1st day post I/M cefepime treatment in kidney, liver, and breast muscles, respectively and on the 3rd day post I/M cefepime treatment, the residues were decreased in all organs to be 1527, 1028.67, and 209.67 μg/kg for kidney, liver, and breast muscles, respectively. Moreover, on the 7th day after last dose, the residues continuous to be minimized in kidney and liver (665, and 94 μg/kg), while cefepime residues were disappear in breast muscles. Also, on the 14th day after last dose, the cefepime residues were disappear in all tested tissues. From the obtained results it was found that the highest cefepime residual level was detected in kidney followed by liver while the lowest level was recorded in muscle samples. Two-way ANOVA results showed that the effect of interaction between type of tissue and days of experiment markedly affect the concentration of. The highest concentration of cefepime (p < 0.001) was detected in kidney on the 1st day of experiment then as the days of experiments increased the concentration decreased. The results also showed that the highest concentration was in kidney then liver and the lowest was in breast muscle at the 1st day. The concentration at kidney was also higher than that in liver and breast muscle at 3rd, and 7th days. From the obtained results it was found that the highest cefepime residual level was detected in kidney followed by liver while the lowest level was recorded in muscle samples. There was a highly marked alternation (p=0.0001) in detection values of cefepime residues in tissues (kidney and that of liver, and muscle) and along the investigation periods.
The results of one-way ANOVA revealed non-marked change of serum AST, ALT, ALP, total protein, albumin, urea, and creatinine values on the 1st day 3rd, 7th, and 14th day post I/M injection of cefepime (75mg/kg BW) for 5 successive days when compared with control group (Figure 6, 7 and 8).
Discussion
Many countries have monitoring scheme to avoid antibiotic residues in food of animal origin to ensure food safety (Albayoumi, 2015). Therefore, the European Community was constructed maximal residual limits (MRLs) for each drug to avoid human health hazard and to ensure consumers safety. This study is expected to indirectly increase awareness of adverse effects of cefepime residues in rabbits, and could help in the reduction of cefepime residues effects (Albayoumi, 2015).
The current study showed that the highest cefepime residual level after repeated intramuscular injections was detected in kidney that had the longest drug withdrawal time followed by liver then the breast muscle. Complete disappearance of the drug was observed on the 7th day of post I/M cefepime treatment in breast muscles but it was detectable in kidney and liver. Based on the aforementioned results, cefepime was eliminated from the muscle firstly followed by liver then kidney that indicated that kidney considered the organ of accumulation and excretion for cefepime.
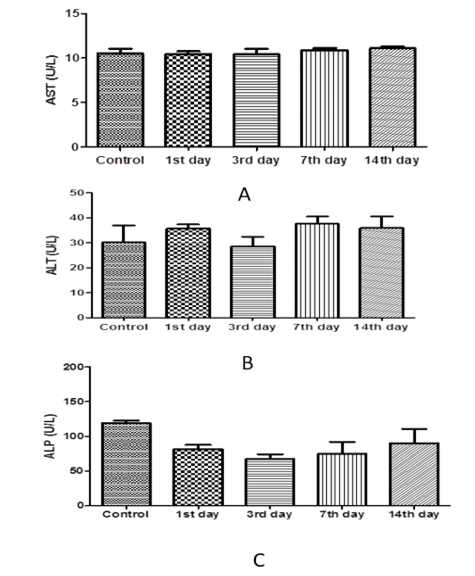
Figure 6: Effect of cefepime on serum AST, ALT and ALP concentration (U/L) on 1st,3rd,7th and 14th days post treatment. (A) AST(U/L), (B) ALT (U/L), (C) ALP(U/L).
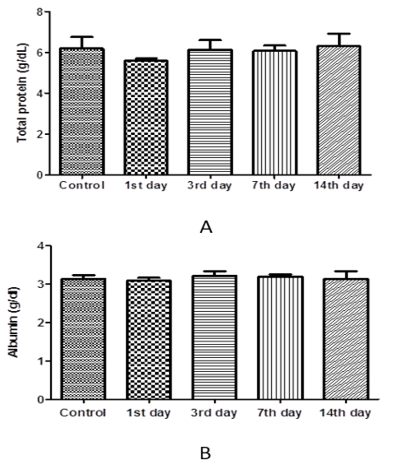
Figure 7: Effect of cefepime on serum total protein and albumin concentration (g/dl) on 1st, 3rd, 7th and 14th day post treatment. (A) Total protein (g/dl), (B) Albumin (g/dl).
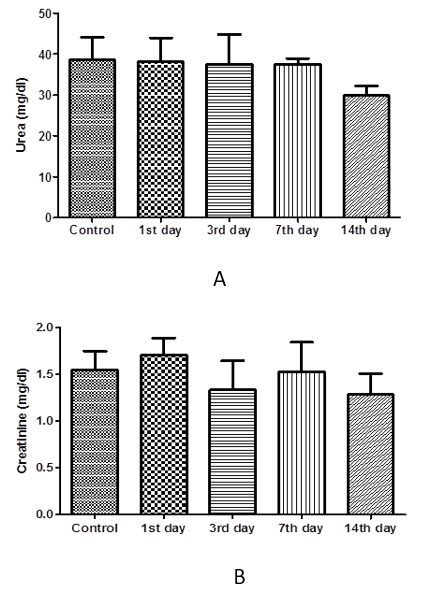
Figure 8: Effect of cefepime on urea and creatinine concentration (g/dl) on 1st, 3rd,7th and 14th day post treatment. (A) urea (mg/dl), (B) creatinine (mg/dl).
In keeping with these lines, Shams et al. (2019) found that the higher tissue levels of Ceftiofur detected in kidney followed by liver then muscle. Number of studies was conducted to investigate the residual concentrations of cefepime. Most of these studies found that the maximum antibiotic residues which observed in kidney and liver could be attributed to the role of liver and kidney in drug metabolism (detoxification, filtration and blood purification); while the lower antibiotic residues in muscle could be due to most of the cephalosporins are eliminated from the body via the kidney and bile (Swafy et al., 2015).
In our study, we found that cefepime residues in tested samples were lower than the MRL of WHO/FAO for cephalosporin group. Cefepime residues were lower than that MRL recommended by [Codex Alimentarius Commission (CAC/ MRL) (2018)] (6000, 2000 and 1000 µg/kg in kidney, liver, and muscle, respectively) at the 1st day after the treatment; this findings were supported by (Beconi-barker et al., 1995) who found that the ceftiofur residues in sheep tissues post I/M ceftiofur treatment for 5 successive days at a dose of 2.2 mg/kg were lower than the MRL defined by the FDA by 50% indicated a safe residual limit, while (El-Sayed et al., 2015) proved that treated chickens must not be slaughtered before 3rd days from the last dose of ceftiofur, also San–Martin et al. (1998) reported that the withdrawal time was 104 hour after single Intraperitoneal administration of cefquinome at dosage of 20 mg/kg in Coho Salmon fish. Moreover, (Abd El-Aty et al., 2007) found that no ceftazidime residues were observed in plasma and tissues post-72 h from intramuscular injection of 50 mg/kg, twice daily for 5 consecutive days in rabbits.
From the obtained results it was found that cefepime remained in all tissue till 3rd day post I/M treatment but not detected in muscle after this time. However, except in the kidney and liver tissues the drug residues were detected at 7th day post drug treatment. The proper withdrawal period for cefepime was 14th days to make sure that no serious residues stay in food products post-slaughter. Therefore, it is recommended that rabbits treated with ceftiofur must not be slaughtered before the 14th day post the last I/M cefepime dose in order to be safe for human consumption.
Our results revealed that treated group with cefepime had non-marked change on liver and kidney function tests within days post-administration. In contrast to our findings, cefepime at low level (45 mg /kg b.wt. for 5 days) not change liver function tests but cefepime at high level (90 and 180 mg /kg b.wt. for 5 days) increase liver function tests in rats. Also, cefepime caused decreased serum proteins level. This might be attributed to the progressive cellular and tubular dysfunction manifested histopathologically (El-Sayed et al., 2014). This was consistent with that recorded in rabbits following administration of both cefpirome (Deki et al., 1990) and after oral dosing of cefmtaline, urinary protein was detected (Kato et al., 2001).
Conclusion
Thus, it is recommended that rules should be taken to ensure observing proper withdrawal time before slaughtering of food animals to ensure human safety. This study even goes so far as to observe the highest level of cefepime residues usually in kidney and liver rather than in muscle. Cefepime I/M injection could administered without any adverse effects on functions of liver, and kidney. In addition, a monitoring policy should be implemented to ensure the conformity of rabbit meat sold in Egypt with international standards. Moreover further residual investigation will be required to determine the exact withdrawal period of cefepime from rabbit tissue.
ACKNOWLEDGEMENTS
This work was supported by Faculty of Veterinary Medicine, Zagazig University, Egypt.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHORS CONTRIBUTION
All authors contributed equally in this work.
References