Journal of Animal Health and Production
Research Article
Effect of Different Antioxidant Sources Added to Buffalo Semen Extender During Cryopreservation on Freezability and Fertility of Buffalo Spermatozoa
Wael M. Wafa*, Hamdy A. El-Nagar, Yaser S. Hussein, Ayman M. Saeed
Animal Production Research Institute, Agricultural Research Center, Dokki, Giza, Egypt.
Abstract | There are several types of antioxidants which can be use in buffalo semen preservation. The current study was performed to study the influence of antioxidants viz., Coenzyme Q10 (CoQ10), L-Carnitine (LC), or N-Acetyl-Cysteine (NAC) added to the extender of buffalo semen on freezability and fertility of spermatozoa. Semen of Egyptian buffalo bulls (n=5) were pooled and extended with tris (1:10) without addition of antioxidant (E1), or with antioxidants i.e., 0.03 mM CoQ10 (E2), 3.0 mM LC (E3), and 1.0 mM NAC (E4). Then the diluted semen was frozen at -196ºC in liquid nitrogen. Results showed that the progressive motility, livability, membrane integrity, and head-head agglutination percentages increased, while sperm abnormality and acrosomal damage decreased by all antioxidant supplements (P<0.05), being the best for E2. Pregnancy rate was higher (P<0.05) for buffalo cows inseminated with semen of E2 and E4 (81.8% for each) versus 72.7 and 63.6% for E3 and E1, respectively. In conclusion, tris-egg yolk extender supplemented with 0.03 mM CoQ10 can improve sperm characteristics of cryopreserved semen and increase pregnancy rate of inseminated buffalo cows.
Keywords | Buffalo, Semen, Antioxidants, Cryopreservation, Pregnancy
Received | May 17, 2021; Accepted | May 21, 2021; Published | July 01, 2021
*Correspondence | Wael M Wafa, Animal Production Research Institute, Agricultural Research Center, Dokki, Giza, Egypt; Email: [email protected]
Citation | Wafa WM, El-Nagar HA, Hussein YS, Saeed AM (2021). Effect of different antioxidant sources added to buffalo semen extender during cryopreservation on freezability and fertility of buffalo spermatozoa. J. Anim. Health Prod. 9(3): 222-228.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.3.222.228
ISSN | 2308-2801
Copyright © 2021 Wafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
During the semen cryopreservation process, the characteristics of mammalian spermatozoa decreased because of the subject of spermatozoa to different stresses like the cold shock which may lead to damage of the mitochondria, plasma membrane, and acrosome membrane of sperm cells (Smith et al., 2011; Perteghella et al., 2017; Zhang et al., 2019). In bull semen, the abundance of unsaturated fatty acids in the sperm plasma membrane causes its exposure to oxidation, leading to damage of the sperm membrane, DNA fragmentation, and reduced enzyme activity (Kadirvel et al., 2009; El-Regalaty, 2017), consequently reduces motility and livability of spermatozoa (Asadpour et al., 2012; Gualtieri et al., 2014). Comparing to cattle, the buffalo semen is highly affected by cryopreservation damage during freeze-thawing processes in terms of reducing its fertilization ability (Andrabi, 2009), and decreasing the conception rate of buffaloes (Guthrie and Welch, 2012).
The antioxidants are compounds that could decrease the harmful effects of oxidative stress on spermatozoa during the sperm preservation process. Antioxidants have a scavenging role for reactive oxygen species (ROS) that produced from lipid peroxidation (LPO) by improving the viability and fertilizability potential of buffalo spermatozoa (Lone et al., 2018).
Coenzyme Q10 (CoQ10) is a redox lipid that is naturally distributed in the membrane of mitochondria in all cells and the blood of mammalians (Pindaru et al., 2015), and its high concentration provide an antioxidant role by a direct action against LPO and DNA fragmentation of cryopreserved human spermatozoa (Talevi et al., 2013). Several studies indicated that CoQ can stimulate cell growth, inhibit apoptosis, and control the channels of its membrane (Crane, 2002). Most of the CoQ10 concentration in sperm was found in mitochondria located in the mid-piece of sperm (Mancini et al., 2005). It is used in extenders of bovine semen during cryopreservation to enhance sperm function (Hussein, 2018).
As a natural essential amino acid, L-Carnitine (LC) is synthesized from methionine and lysine. It has an important role in energy production in the matrix of cell mitochondria and it is needed for long-chain fatty acids oxidation in cell mitochondria (Foster, 2004). In males, LC was found with high concentration in epididymis (Matalliotakis et al., 2000) and secreted in seminal plasma. The LC has a critical role on sperm energy metabolism (Ng et al., 2004), and it could be used in semen cryopreservation to improve post-thawed human sperm quality (Banihani et al., 2014).
N-Acetyl-Cysteine (NAC) is a chemical compound certified for medicinal use (Fischer and Ganellin, 2006). It is described as an antioxidant and free radical scavenger (Dodd et al., 2008) that prevents oxidative stress, because it participates in glutathione (GSH) synthesis (Rushworth and Megson, 2014).
The present work was performed to investigate the influence of supplementation of semen buffalo-extender with antioxidants Co Q10, LC, or NAC on freezability and fertilizing ability of spermatozoa.
Materials and methods
Ethical Statement
The ethical policies have been adhered to and the appropriate ethical review committee approval have been received. EU standards for the protection of animals used for scientific purposes and feed legislation (2010/63/EU) were followed in this study.
Animals and collection of semen
In this study, Egyptian buffalo bulls (n=5) ranged from 400 to 450 kg live body weight and averaged 4 years of age were used as semen donors. These animals were chosen from herd of El-Gemmezah Animal Production Experimental Station, Animal Production Research Institute (APRI), Egypt. The bulls were fed on ration composed of 4 kg concentrate fed mixture, 3 kg clover hay and 4 kg rice straw according to NRC (2001).
During a semen collection of eight weeks, semen was taken at 8 a.m., twice/week, and an artificial vagina (IMV, France) was used.
The collected ejaculates were immediately transported at water bath (37oC) to the Animal Physiology laboratory for evaluating and cryopreservation. Ejaculates with or more than 70% mass motility were used in this study. Semen mass motility was determined as a percentage of spermatozoa wave motion in drop of semen placed on glass slide.
Experimental semen extenders
All ejaculates were pooled and extended in Tris-extender with four levels of supplementation. The base extender used in this experiment was Tris-egg yolk (TEY). It contained Tris (0.325 g), citric acid (1.675 g), glucose (0.75 g), and antibiotics (streptomycin, 0.005 g and lincomycin, 0.25 g) in 100 ml distilled water. Fresh egg yolk (10% v:v) and glycerol (7%) were added to 83 ml of TEY. Semen was diluted with different supplementations at a rate of 1:10 with spermatozoa concentration of 20x106 per 0.25 ml. The Tris-extenders used were without supplementation (E1), while E2 was supplemented with 0.03 mM CoQ10 (C9538: Sigma Aldrich Co. St. Louis, Mo, USA), E3 with 3.0 mM LC (Roche Diagnostics GmbH, Mannheim, Germany), and E4 with 1.0 mM NAC (A7250: Sigma Aldrich Co. St. Louis, MO, USA). Levels of antioxidant supplementation were chosen according to Saeed et al. (2016) and Hussein (2018) in bovine semen.
Cryopreservation of semen
The medium-sized French straws (0.25 ml) were used to load the semen extended with extenders of different supplements. The straws were sealed with polyvinyl alcohol powder, and cooled (5oC) for 4 h as an equilibration period, then all straws were exposed for 10 min to vapor of liquid nitrogen (LN, -196oC), and then the straws were plunged into LN for a storage period of 4 weeks.
Evaluation of semen
The percentages of individual motility (Salisbury et al., 1978), livability and abnormality (Barbas, and Mascarenhas, 2009), acrosomal damage (Chowdhury et al., 2014), and membrane integrity of spermatozoa estimated by hypo-osmotic swelling test (HOS-t) were determined in post-thawed semen. Spermatozoa exhibited curled tails in response to HOS-t (50 mOsm/l) for 30 min were used to calculate the percentage of membrane integrity according to El-Sherbieny (2004). The percentage of the agglutination of head to head was also determined according to Senger and Saacke (1976).
Table 1: Sperm characteristics in post-thawed buffalo semen as affected by type of antioxidants.
| Treatment groups | Sperm characteristics (%) | |||||
| Progressive motility | Sperm livability | Sperm abnormality | Acrosomal damage | Membrane integrity | HHA | |
| E1 |
48.33±1.06c |
54.58±1.16c |
35.75±1.51a |
34.33±1.04a |
56.83±0.90b |
46.08±0.43d |
| E2 |
60.75±1.51a |
65.50±1.13a |
24.33±1.09b |
26.17±1.16b |
65.25±0.87a |
59.75±0.39a |
| E3 |
55.00±1.15b |
61.83±0.98b |
26.17±1.05b |
26.92±1.07b |
64.42±0.91a |
54.42±0.34c |
| E4 |
58.92±1.68a |
64.33±1.02ab |
25.75±1.07b |
24.75±0.95b |
66.33±0.98a |
57.08±0.31b |
Means in the same column for each factor with different superscripts differ significantly (P<0.05).
E1: Control; E2: Coenzyme Q10 (0.03 mM); E3: L-Carnitine (3.0 mM); E4: N-Acetyl-cysteine (1.0 mM).
HHA: Head to head agglutination.
Table 2: Enzyme and total antioxidant activity in post-thawed buffalo semen as affected by type of antioxidants.
| Treatment groups | Enzyme activity (IU/l) | Total antioxidant activity (mmol/l) | ||
| AST | ALT | LDH | ||
| E1 |
35.83±1.26a |
26.84±1.11a |
299.92±7.34a |
1.40±0.39d |
| E2 |
21.82±0.83b |
18.17±0.95c |
231.58±5.39c |
3.02±0.11a |
| E3 |
22.50±1.01b |
19.25±1.09bc |
242.00±4.26c |
2.16±0.05c |
| E4 |
23.92±0.92b |
21.16±0.84b |
257.42±4.15b |
2.58±0.96b |
Means in the same column for each factor with different superscripts differ significantly (P<0.05).
AST: Aspartate transaminase. ALT: Alanine transaminase. LDH: Lactic dehydrogenase.
E1: Control; E2: Coenzyme Q10 (0.03 mM); E3: L-Carnitine (3.0 mM); E4: N-Acetyl-cysteine (1.0 mM).
Seminal plasma enzymes and total antioxidant activity
Activity of aspartate (AST) and alanine (ALT) transaminases (Schmidt and Schmidt, 1963), and lactic dehydrogenase (LDH, Howell et al., 1979) was estimated in the seminal plasma of semen after thawing. As well, total antioxidant activity (TAA) was determined (Koracevic et al., 2001). Enzymes and TAA activities were determined using spectrophotometer (Jenway, 6405UV/Vis, England) by commercial kits (Salucea, Netherlands).
Fertility trial
Total of 54 sexually mature buffaloes were intra muscularly injected with Estrumate (3 ml/animal, PGF2α, Essex Animal Health Friesoythe, Germany) for estrous synchronization. Estrus was observed within 24-72 h after the 1st or 2nd Estrumate injection. A total of 44 animals were synchronized out of 54 treated buffaloes came in heat were used for fertility trail. These buffaloes were randomly allotted to 4 groups (11 animals in each) and were artificially inseminated with semen extended with different extenders (four extenders). On the day of artificial insemination (AI), semen straws were thawed using filled plastic AI gun close to the cervix. All inseminations were done by the same inseminator. About 45-50 days post-AI, all inseminated animals were palpated via the rectum for pregnancy diagnosis.
Statistical analysis
The obtained data were processed by version 20 of the IBM SPSS analysis program (2011) using the repeated measurement model with one-way ANOVA to study the effect of supplementation on different sperm characteristics. The results were presented as means ± SE. Multiple range Test of Duncan (1955) was used to test the differences, being significant among means at P<0.05. Pregnancy rate of buffaloes in different treatment groups were analyzed using the x2 test.
Results
Sperm characteristics post-thawing
After thawing, sperm characteristics, including progressive motility, livability, abnormality, acrosome damage, membrane integrity, and head-head agglutination percentages were better (P<0.05) with all types of antioxidants than in control, being the best for extender with CoQ10, followed by NAC and finally LC (Table 1).
Activity of enzymes and total antioxidants in seminal plasma post-thawing
After thawing, seminal activity of AST, ALT, and LDH were lower (P<0.05) with all types of antioxidants than with control, particularly, in with E2. However, total antioxidant levels increased (P<0.05) in the seminal plasma of all types of antioxidants than in control, being the highest in E2 (Table 2).
Fertility rate
Pregnancy rate of buffalo-cows inseminated with post-thawed semen with the experimental extenders (Figure 1) showed that semen extended with both 0.03 mM CoQ10 and 1.0 mM of NAC supplementation had higher pregnancy rate (9/11, 81.8% for each) than those inseminated by semen supplemented with 3 mM of LC (8/11, 72.7%). Finally, the lowest pregnancy rate was recorded for the control group, being 63.6% (7/11).
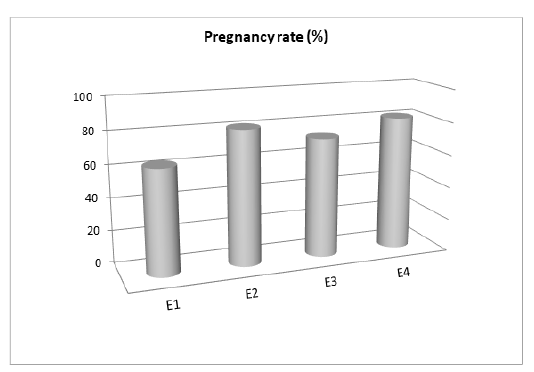
Figure 1: Effect of supplemented extender with different antioxidants on pregnancy rate. E1: Control; E2: CoQ10 (0.03 mM); E3: LC (3.0 mM); E4: NAC (1.0 mM).
Discussion
During the cryopreservation process, with the cellular respiration of spermatozoa, a great amount of reactive oxygen species (ROS) was produce resulting in a decrease in endogenous antioxidants levels (O’Flaherty, 2014). Under these conditions, the human sperm motility and fertilizability were negatively affected in term of increasing the number of abnormal sperm cells (Sikka, 2004) and immature spermatozoa with DNA damage (Aitken et al., 2010).
The current study was conducted to reveal the fact of either antioxidants are against lipid peroxidation and oxidative stress in farm animal spermatozoa (Shoae and Zamiri, 2008; Tariq et al., 2015). Therefore, the main goal of the current study was to determine the effect of antioxidant properties of CoQ10, LC, and NAC in buffalo semen extender on maintaining the freezability and fertilizing ability. In this context, the obtained results showed clear significant improvement in all sperm parameters thawing as affected by dilution of semen in all extender types, being the best with 0.03 mM CoQ10 (E2), followed by 1.0 mM of NAC (E4) and finally 3 mM of LC (E3). Harmoniously, several studies indicated the role of CoQ10 as a bio-energetic and an antioxidant to reduce generation of ROS in sperm mitochondria then stabilizing its membrane potential, and increasing the production of ATP (Mancini and Balercia, 2011). In addition, CoQ10 preserves spermatozoa from DNA fragmentation (Talevi et al., 2013). The present results concerning improving post-thawed sperm characteristics in E3 (LC) are in agreement with Abdel-Khalek et al. (2015) in Friesian bulls. In this respect, Mazzilli et al. (1999) indicated a clear correlation between the content of LC in sperm and the motility, thus, it can be an indicator of the life span of sperm motility. Also, El-Raey et al. (2016) reported that LC significantly improved the motility and viability of post-thawed buffalo bull spermatozoa. The marked reduction in acrosomal damage in E2 (CoQ10) is in association with the decrease in the activity of AST, ALT, and LDH (El-Harairy et al., 2011; Borah et al., 2015). Semen with perfect quality can be characterized by the reduction in AST, ALT, and, LDH levels (Taha et al., 2000). Also, it was reported a significant improvement in the quality of post-thawed buffalo semen as affected by different levels of CoQ10 addition (Saeed et al., 2016). The LC stabilizes mitochondrial membranes (Liu et al., 2002) that protect spermatozoa from apoptosis via reducing the oxidative stress, and preserve the cell plasma membrane that maintains AST, ALT and LDH enzymes from leakage out of sperm (Liu et al., 2004).
The present results revealed that the addition of 0.03 mM CoQ10 or 1.0 mM of NAC to buffalo semen improved the pregnancy rate by 28.62% as compared to control semen along with an improvement rate by 14.31% with 3 mM of LC addition than controls. The remarkable increase in pregnancy rate of buffalo cows inseminated with CoQ10 (E2) was explained by Greco et al. (2005), who mentioned that antioxidants treatment for male reduced the free radical that induce sperm DNA fragmentation which may increase the pregnancy rate. The addition of LC to frozen buffalo semen improved its fertilization rate (El-Raey et al., 2016) that may be associated with the increase in ATP production in spermatozoa besides its antioxidant role that protects DNA in spermatozoa from damage (Sun et al., 1997). In addition, the NAC had an important role in GSH production and scavenge the free radicals that increase the sperm antioxidant capacity (Dattilo et al., 2016), then increasing the pregnancy rate (Barekat et al., 2016). Also, antioxidants properties of NAC can reduce the semen viscosity that important for improving the fertility and pregnancy rate (Verit et al., 2009). According to the fact, GSH production was affected by NAC treatment as a source of L-cysteine (Rushworth and Megson, 2014), it is clear to note that the significant improvement in spermatozoa characteristics in E4 (NAC) compared to control (E1) was in relation with the roles of NAC as ROS scavenger (Patel et al., 2016; Koohestanidehaghi et al., 2020) and cell apoptosis preserver (Dhouib et al., 2016).
Conclusion
In conclusion, the supplementation with natural antioxidants, CoQ10, and LC or NAC as synthetic antioxidants can improve the buffalo bull semen quality. Addition of CoQ10 to buffalo semen extender at a level of 0.03 mM showed promising results to enhance post-thawing sperm characteristics reflecting a high fertility.
Acknowledgements
The authors thank the El-Gemmezah Animal Production Experimental Station, APRI, Egypt for allowing animals used in this study.
conflict of interest
The authors declare no conflicts of interest.
authors contribution
Wafa, W.M., El-Nagar, H.A. and Hussein, Y.S., contributed to design the experimental work, performed the sample preparations for laboratory analysis and collected data, Saeed, A.M., helped in wrote the first draft of the manuscript. Wafa, W.M., conducted the statistical analyses and critically revised the manuscript.
References






