Journal of Animal Health and Production
Research Article
Antiparasiticidal Potential of Aqueous Leaves Extract of Moringa Oleifera against Ichthyophthirius multifiliis Infestation on Clarias gariepinus
Ikele B. Chika1*, Okpasuo J. Onyekachi2, Ikele F. Chioma3, Ating Emmanuel4, Obiezue N. Rose2, Mgbenka O. Bernard1
1Fishery and Aquaculture Unit,Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, Enugu State, Nigeria; 2Parasitology and Public Health Unit,Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, Enugu State, Nigeria; 3Department of Plant Science and Biotechnology, University of Nigeria, Nsukka; 4Department of Oceanography and Fishery Science, School of Nautical Studies, Maritime Academy of Nigeria, Oron.
Abstract | Ichthyophthiriasis hampers aquaculture production and alter the host physiology and growth. Currently due to the negative impact of chemotherapy on human health and environment, there is an urgent need to discover both effective and safe antiparasiticide against Ichthyophthirius multifiliis (Ich). Aqueous leaves extract of Moringaoleifera (ALEMO) seems at present to be very effective and cheap in controlling I.multifiliis. In this study, anti-ich efficiency of aqueous leaves extract of Moringa oleifera on Clarias gariepinus was investigated for 1h. Before and after infestation, gills and skin below the dorsal fin were observed microscopically for I. multifiliis abundance. After ALEMO exposure, ALEMO-treated fish showed decreased infestation of I. multifiliis in all investigated tissues than the unexposed negative control fish. The minimum concentrations of ALEMO showed a positive anti-protozoal efficacy in eliminating approximately 99% of the infective stage, theront. The viability of the infective theronts pretreated for 1h showed a slight re-infestation, prevalence and intensity with I. multifiliis against the non-treated group which indicated that I. multifiliis are less susceptible to herbal therapy, Moringa oleifera. According to the results, 4,500mg/l ALEMO significantly eradicated the ectoparasite and can be a good replacement for chemical treatments against I. multifiliis.
Keywords | Ichthyophthirius multifiliis, Theronts, Moringa oleifera, antiparasitic potentials, Clarias gariepinus
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | February 11, 2019; Accepted | April 28, 2019; Published | June 21, 2020
*Correspondence | Ikele B Chika, Fishery and Aquaculture Unit, Department of Zoology and Environmental Biology, University of Nigeria, Nsukka, Enugu State, Nigeria; Email: [email protected]
Citation | Chika IB, Onyekachi OJ, Chioma IF, Emmanuel A, Rose ON, Bernard MO (2020). Antiparasiticidal potential of aqueous leaves extract of moringa oleifera against ichthyophthirius multifiliisinfestation on clarias gariepinus.. J. Anim. Health Prod. 8(3): 113-121.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.3.113.121
ISSN | 2308-2801
Copyright © 2020 Chika et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The African catfish, Clarias gariepinus is the most important cultured fish species in aquaculture. However, most aquaculturists are constrained with massive fry and fingerlings mortalities, especially in culture systems due to the invasion of parasites especially, Ichthyophthirius multifiliis (Ich). The protozoan parasite, I. multifiliis (Foquet) is known to cause increased mortality in aquaculture worldwide, resulting in economic losses for the fish farmers (Jorgensen and Buchman, 2007). It is the causal agent of white spot disease and the most detrimental parasites in ornamental fish culture and other types of aquaculture around the world (Meinelt et al., 2009). It also damages the gills and skin of the fishes; mortality results from tissue damage and resulting respiratory compromise and interference with osmoregulation (Sudova et al., 2010). The life cycle of the parasites includes three stages: the infective theronts, the parasitic trophont and the reproductive tomont.
The use of chemicals for treatment of fish diseases has become a great concern due to restriction on it over environmental pollution and persistent residues e.g. malachite green. Meanwhile, other chemotherapeutics, such as formalin (Rowland et al., 2009), copper sulphate (Straus, 1993, Straus et al., 2009; Rowland et al., 2009), and potassium permanganate (Straus et al., 2010; Straus and Griffin, 2001) have been evaluated for their anti-parasitic efficacy in I. multifiliis. Most of the chemotherapeutant lack the potential of eliminating the parasite or are not safe for both human health and environment; therefore, the need for non-chemical alternative parasiticides is of prime importance. A plethora of other non-chemical treatments has been investigated for effectiveness in the control of free swimming stages of I. multifiliis (Gholipour et al., 2012; Ling et al., 2012; Yi et al., 2012; Zhang et al., 2013). However, there is limited knowledge about Moringa oleifera leaves extract as a herbal anti-parasiticide in the treatment and control of Ich parasites and its life key stage ‘Theront’. The aim of this study is to establish a treatment strategy against I. multifiliis in Clarias gariepinus using aqueous leaves extract of Moringa oleifera. The purpose of the present research was to determine the efficacy of aqueous extract of Moringa oleifera as non-chemical alternatives to control Ichthyophthirius multifiliis in African catfish, Clarias gariepinus Juveniles.
Materials and Methods
Experimental Protocol
A total number of 300 apparently healthy parasite free post-juvenile catfish (Clarias gariepinus) hatched from fish eggs in the wet fisheries laboratory, Department of Zoology and Environmental Biology, University of Nigeria, Enugu State, Nsukka and reared for two months to the experimental size were obtained and used for the study. The mean weight of the fish was 102.41±11.51 g and the standard length was 15.4±0.91 cm. Fish were kept in 120L tanks containing aerated municipal water. Experiments were conducted in triplicate. A total of 210 Clarias gariepinus were distributed in 21 tanks each containing 10 fish each. The water temperature was 22.3°C-24.2°C, pH 6.7-7.2 and dissolved oxygen 5.7mg/L-6.9mg/L with proper aeration. The fish were fed 5% BW/day with commercial feed (copens).
Fresh leaves of Moringa oleifera, were collected from trees of the Natural Horticultural Research Institute (NHRI) of Idi-Ishin, Ibadan, Nigeria. Thereafter, the leaves were dried under room temperature and later ground into powdery form and sieved using 0.3μ muslin cloth. They were concentrated in water, shaken vigorously and left for 24h after which the solution was filtered with muslin cloth and the filtrate concentrated to dryness using rotary evaporator. Concentrations of aqueous leaves extract of Moringa oleifera (ALEMO) for the dip treatments (60minutes) were: Group A Non infested (Normal control), Group B Infested not treated (Negative control), Group C: Infested treated with standard drug (Fish cure) (Positive control) Group D Infested treated with 1,500mg/L aqueous extracts of Moringa oleifera leaves (ALEMO), Group E Infested treated with 2,500mg/L (ALEMO), Group F Infested treated with 3,500mh/L (ALEMO), Group G Infested treated with 4,500mg/L (ALEMO).
Parasite and Parasite Challenge
The catfish infected with mature trophonts were collected from the wild (Anambra River, Anambra State, Nigeria), used as parasite source and anaesthetized with 150mg/l tricaine methanesulphonate (MSS-22, Sigma). I. multifiliis was isolated from the infected fish and the trophont were transferred into beakers containing 20 500ml of dechlorinated tap water and allowed to settle for 4h. The trophont were gently rinsed with dechlorinated tap water to remove organic matter and were incubated in the dark chamber to allow for mitotic division; the cells encysted into tomonts and finally free swimming theronts emerged within 48h (Figure 1). Furthermore, groups B-G excluding the control were challenged with isolated infective theronts from the cultured water samples which were kept for 24 – 48 h. The theronts were quantified under a binocular microscope (Olympus-CH, Japan) using a Sedgewick-Rafter cell to detect the number of viable theronts that were produced.
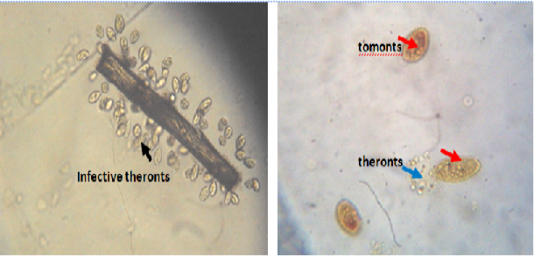
Figure 1: Infective theronts (black arrow) attached to a substrate and tomonts actively releasing theronts. Lugol iodine stain, mag. X40.
Approximately 44,000 theronts were administered to each group B-G (in order to secure the same infection pressure and environmental conditions for all fish groups). During the challenge test, the fish were kept in darkness and were allowed to remain for a period of 5-10 at 24°C. Active white spots were seen on the body of the fish within this period, in other to validate infection by Ich. The fish (6 fish per group) were euthanized by brain incision using a scalpel, the excised gill was cut through the operculum and gill arch to obtain three gill archs containing the filaments and the lamellae. They were further placed on a slide and three drops of water added and covered with cover slip and examined under a binocular Olympus CH microscope magnification X40 for presence of parasites. The number of parasites on three randomly selected zones was counted and estimate of total number of parasites recorded. However, the percentage of theronts that successfully established infection (% PEI) in individual fish was quantified.
%PEI= 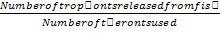 x
x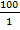
The fish were handled in accordance with the regulatory guidelines of the University of Nigeria animal care committee (UNN-ACC, Protocol No. 0764/2013).
Antiprotozoal Activity of Aqueous Leaves Extract of M. Oleifera on the Survival Of Theronts After 24 H
Approximately 900 theronts in 1ml of culture water containing theronts (due to the difficulty in counting the theronts) were dispensed in 15 250ml beakers (three replicates each) (A-F). Different concentrations of dry aqueous leaves extract of M. oleifera was added in each beaker (100ml); 0.01g/100ml, 0.02g/100ml, 0.03g/100ml, 0.05g/100ml and 0.08 g/100ml and 0.0g/100ml (negative control), respectively. They were allowed to stand for 24h after which the number dead and alive theronts were enumerated using sadgewick-rafter cells under binocular microscope and recorded.
Assessment of Morphological Alterations of Infected Theronts Following Post-Treatment with Aqueous Leaves of M. oleifera
Live or dead theronts were enumerated with binoculars microscope at the end of the antiprotozoal activities of Moringa oleifera to ascertain or check any possible morphological alteration in the infective stage of the parasite (theronts). Theronts were photographed with a motic camera installed in the same binocular microscope (Olympus-CH, Japan) at x40 magnification.
Evaluation of Infection Potential/Or Viability of Theronts (Infective Stage) Following 1H Pre-Treatment with Aqueous Leaves Extract of M. oleifera.
Approximately 7000 theronts were exposed to 0.5g/100ml of aqueous leaves extract of Moringa oleifera in a 250ml beaker. The theronts which survived were painstakingly isolated with a plastic pipette transferred immediately to a 100mm plastic petri dish. However, fifteen uninfected/disinfected fish were separated into two plastic containers (A-B) with 5 fish per replicate to ascertain whether the theronts can still be viable in infecting the healthy Ich-free fish for 14 days. The water temperature was maintained at 22oC - 25oC. However, at the end of the infection cycle, each fish in each container was removed, the skin scrapped into slide with addition of 2-3 drops of water, covered with cover slip and viewed under the binoculars microscope. The number of each stage of the parasite found (trophont or theront) as well as white spot on the body of the fish and in the water was noted.
Determination of Infective Prevalence and Intensity of Theronts Pre-Treated for 1h with Aqueous Extract of M. oleifera.
Infective prevalence was estimated using the formula:
Infective prevalence (%) =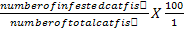
Infective intensity (%) = 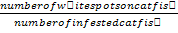
Statistical Analysis
The Statistical Package for Social Sciences (SPSS) version 16 was used. Mean values were analyzed for significant differences (P <0.05) using the analysis of variance (ANOVA). Differences between means was partitioned using the Duncan New Multiple Range test.
Results
Parasite
The trophont burdens on the experimental fish were determined at one time points, 1h after initial infestation, when only the triplicate set of tanks (C - G) received treatment. The level of decrease in the number of trophonts present in the body smear was concentration and time dependent (Table 1). It was also observed that trophonts present in body smear during pre-treatment were significantly (P<0.05) higher in all the experimental groups. The lowest number of trophonts in the body smear of the infested fish was seen in group F (Table 3). However, at the end of the post-treatment, the number of trophonts in the negative treatment increased significantly (P<0.05) compared to the ALEMO treated groups. The data indicated that there were significant differences in I. multifiliis infestation of the skin between the standard drug and ALEMO groups at the end of the dip treatment. Moreover, the trophont decreased in a concentration and time dependent manner.
The number of trophonts present in the gill after treatment with ALEMO and Fish cure (standard drug), showed no significant differences (P>0.05) whereas the negative control had highest trophonts infestation in the gill. Meanwhile, it was observed that the number of infestation in the gills of the negative control was statistically significant (P<0.05). The infestation with I. multifiliis decreased in the ALEMO group, while the infestation in the ALEMO-exposed group D, E and F had a mixed trend of infestation reduction potency as shown in Table 2. The curative potency of aqueous leaves extract of M. oleifera in the trophonts infestation present in the body and gill of the fish were significantly different at (P<0.05). The curative potentials of the ALEMO treated groups and standard drug in eliminating the adult parasite was significant. The standard drug demonstrated a significant efficacy by reducing the parasitic infestation by 99.32%. However, the ALEMO treated groups showed similar efficacy by reducing the parasitic infestation in a dip bath treatment for 1h. Similar findings were also observed in the gills of the infested fish where the positive control group reduced the parasitic infestation by 88.33% (Table 3).
Table 1: Changes in the trophont number in the body smear of Clarias gariepinus infested with Ichthyophthirius multifiliis before and after dip treatment (1h) with aqueous leaves extract of Moringa oleifera and standard drug (fish cure).
| Experimental groups |
Mean number of trophonts before treatment |
Mean Number of trophonts after treatment |
| A (Healthy control) | - | - |
| B (Infested not treated) |
84.33±43.9c1 |
258.00±44.53b2 |
| C (Standard drug) |
47.67±5.13bc1 |
1.67±0.57a2 |
| D(1,500mg/L) |
82.33±44.16c1 |
24.67±21.13a2 |
| E (2,500mg/L) |
55.00±12.77bc1 |
17.00±2.65a2 |
| F (3,500mg/L) |
45.33±13.61ab1 |
27.00±11.14a2 |
| G (4,500mg/L) |
40.67±14.15abc1 |
14.33±2.52a2 |
Mean value with the same alphabets as superscript in a column are not significantly different (P>0.05). Mean values with different numbers as superscript in a row are significantly different (P<0.05).
Table 2: Changes in the trophont number in the gill of Clarias gariepinus infested with Ichthyophthirius multifiliis before and after dip treatment (1h) with aqueous leaves extract of Moringa oleifera and standard drug (fish cure).
| Experimental groups |
Trophonts present |
Trophont present |
| before treatment | after treatment | |
| A (Healthy control) | - | - |
| B (infested not treated) |
9.10±4.58ab1 |
20.33±10.50b2 |
| C (standard drug) |
4.33±5.86ab1 |
2.00±2.00a2 |
| D (1,500mg/L) |
9.33±8.32ab1 |
5.33±3.51a2 |
| E (2,500mg/L) |
12.67±1.53b1 |
8.10±2.65a2 |
| F (3,500mg/L) |
12.67±1.53b1 |
4.67±5.03a2 |
| G (4,500mg/L) |
12.00±4.58b1 |
2.10±2.00a2 |
Mean value with the same alphabets as superscript in a column are not significantly different (P>0.05). Mean values with different alphabets as superscript in a column are significantly different (P<0.05).
Table 3: Changes in the curative potential of aqueous leaves extract of Moringa oleifera and standard drug (fish cure) in eliminating the adult parasite trophonts from the body smear of the infested fish, after dip treatment
| Experimental groups |
Total Percentage (%) curative of trophonts present in the gill of infested fish |
Percentage (%) curative of trophonts alive and present in the body of the infested fish. |
| Group B(infested not treated) |
0.00±0.00a2 |
0.00±0.00a1 |
| Group C(standard drug) |
83.33±20.82b1 |
99.32±0.33c2 |
| Group D(1,500mg/L) extract |
66.17±23.80b1 |
92.02±9.26b2
|
| Group E (2,500mg/L) extract |
54.83±21.68b1 |
93.16±2.19bc2 |
| Group F (3,500mg/L)extract |
79.03±25.96b1 |
89.77±2.63b1 |
| Group G (4,500mg/L) extract |
83.33±20.82b1 |
94.17±2.14bc2 |
Mean value with the same alphabets as superscript in a column are not significantly different (P>0.05). Mean values with different numbers as superscript in a row are significantly different (P<0.05).
Antiprotozoal Activity of Aqueous Leaves Extract of Moringa Oleifera Leaves on the Key Life Stage (Theront) of Ichthyophthirius Multifiliis
Infective stage of the parasite was exposed to varied concentration of aqueous leaves extract of M. oleifera extract (B-G). The number of theronts surviving each concentration of the extract displayed a clear dose response, survival decreasing with the increasing concentration of the extract. The number of theronts surviving each concentration, given as the mean number of theronts that survived at the end of 24h of exposure was concentration and time dependent. The percentage number of theronts that survived during the antiprotozoal activity of M. oleifera on the infective stage of the parasite, given as the mean percentage followed by the standard deviation was concentration and time dependent. The number of theronts that survived the ALEMO-exposure were significantly different (P<0.05). The level of mortality that occurred in theronts exposed to graded concentration of aqueous leaves extract of M. oleifera at the end of 24h was highest in the group F (Table 4). The mortality of the theronts was concentration depen-
Table 4: Antiprotozoalactivity of aqueous leaves extract of Moringa oleifera on the key life stage (theront) of Ichthyophthirius multifiliis
| Experimental groups |
Mean number of theronts per replicate ~ 900 theronts per ml |
Mean number of theronts dead at the end of 24h |
Mean number of theronts survived |
Percentage dead (%) | Percentage survived (%) |
| A (0.0g/ml) of extract |
819.10±16.09a |
4.00±4.34a |
896.00±4.35d |
0.43±0.49a |
99.52±0.45b |
| B (0.01g/100ml) |
855.67±16.04b |
47.0±9.84a |
853.0±9.85d |
5.17±1.09a |
94.767±1.14b |
| C (0.02g/100ml) |
857.33±4.73b |
128.33±49.36ab |
771.67±49.36ab |
14.23±5.45ab |
85.73±5.48b |
| D (0.03g/100ml) |
856.67±8.96b |
263.33±145.21b |
636.87±145.211c |
29.26±16.13b |
70.70±16.15ab |
| E (0.05g/100ml) |
853.33±18.88b |
554.0±97.26c |
346.00±97.26b |
61.52±10.79c |
38.43±10.80a |
| F (0.08g/100ml) |
845.0±8.54b |
778.67±60.13d |
121.33±60.14a |
86.50±6.66d |
37.45±42.56a |
Mean value with the same alphabets as superscript in a column are not significantly different (P>0.05). Mean values with different alphabets as superscript in a column are significantly different (P<0.05).
Table 5: Changes in the viability of infective theronts pretreated with 0.5g/L of Moringa oleifera leaves for 1h in infectivity potential in Healthy Clarias gariepinus for 14 days.
| Experimental groups |
Number of trophonts in the body smear |
Number of white spots observed |
Number of trophonts in the gill |
| A (Negative control) | 76.80±30.14 | 607.800±289.62 | 9.40±4.16 |
| B (0.5g/L) pretreated group | 15.80±9.63 | 131.200±49.33 |
1.40±1.34 |
Table 6: Infective prevalence and intensity of theronts pretreated for 1h with aqueous leaves extract of Moringa oleifera (mean±SD of 3 tanks)
| Treatment groups |
Number of theronts per replicate |
No of fish per replicate n=15 |
Number of fish infested per replicate |
Infestive Prevalence (%) |
Infestive Intensity |
| A(non-extract treated) |
≈ 7,000 |
5 5 5 |
5 5 5 |
100 100 100 (100±0.00)a |
120.8 184.2 174.8 (159.67±34.14)a |
|
B (0.5g/L of the extract) |
≈ 7,000 |
5 5 5 |
2 3 1 |
13.3 20 60 (31.10±25.25)b |
105.5 47 94 (82.17±30.99)b |
Infective prevalence (%) = number of infected catfish/number of total catfish X 100; infective intensity=number of white spots on catfish body/number of infected catfish; values marked by different letters with the same column are significantly different (P<0.05).
dent and it differed significantly at P>0.05. More so, no significant death was recorded in the group A (Negative control) (Table 4). Meanwhile, the LC50 of the extract was estimated at 95% confidence limit for concentration as 0.247g/ml (Figure 2). Meanwhile, the model explains the data at 70.0% i.e. the model is efficient.
Morphological Alteration of Theronts Exposed to Different Concentrations of Aqueous Leaves Extract of Moringa oleifera
The untreated live theronts (negative control) were dolichomorphic having a fusiform shape and well defined cilia as shown Figure 3. The number of theronts that survived during the antiprotozoal activity especially in group F was very sluggish and appeared dead. The dead theronts became spherical in shape (Figure 4), with loss of cilia after exposure to the extract. During the deformation process, the theronts were alive at the early stage and rotated at the same location and the treated theronts lost their swimming ability especially the cilia and died with a swollen body configuration/or deformation.
Viability of Infectivity of Theronts After Pre-Treatment with Aqueous Leaves Extract of Moringa Oleifera for 1H and Exposed to Clarias Gariepinus for 14 Days
The infectivity of the theronts on C. gariepinus was lost and it decreased appreciably after live theronts were treated with ALEMO for 1h. Meanwhile, group B pretreated with 0.5g/100ml of the extract for 1h, and allowed to reinfect normal healthy fish lost its infestation. Not all the pretreated theronts were able to complete its life cycle on the skin of the healthy fish by producing trophonts (Figure 5 and 6). The number of trophonts that emerged in group B was significantly reduced compared to the negative control (Table 5). However, the emergence of white spots between the infested fish in group A was higher compared to the pretreated group B. However, the number of trophonts present in the gill of fish exposed in group A and B differed significantly (P<0.05). It can be deduced that the viability of the infective theronts when pretreated might be reduced significantly by not completing its life cycle in the skin and gill of fish.
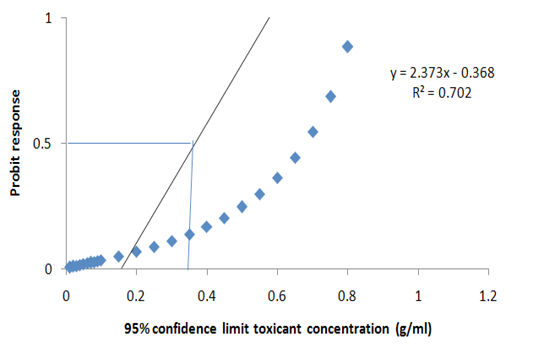
Figure 2: Probit transformed responses for minimum inhibitory concentration in the antiprotozoal activity of M. oleifera on the key life stage (theronts) of Ichthyophthirius multifiliis.
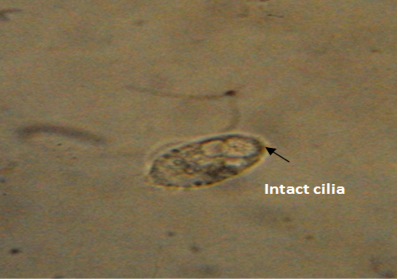
Figure 3: Normal morphology of theronts showing intact cilia (black arrow) with fusiform shape. Neutral red stain. mag. X40.
Infective Prevalence and Intensity of Theronts Pretreated for 1H with 0.5G/L of Moringa oleifera
The infective prevalence and intensity of the theronts pretreated for 1h with 0.5g/L of ALEMO decreased signific-
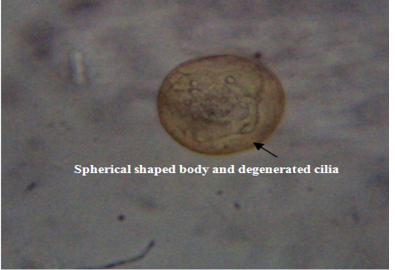
Figure 4: Deformed theronts after exposure to aqueous leaves extract of Moringa oleifera showed, distorted and degenerated cilia and spherical shaped body (black arrow). Neutral red stain. mag. X40
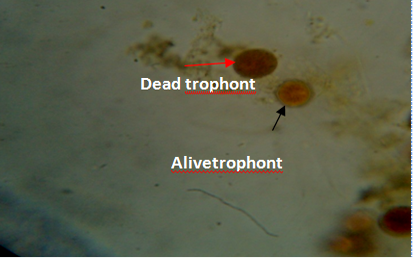
Figure 5: Gill of fish showing dead (red arrow) and live (black arrow) trophonts with intact nucleus under lugol iodine stain during treatment with aqueous leaves extract of Moringa oleifera.
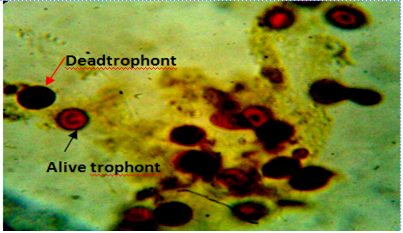
Figure 6: Body smear of fish showing dead (red arrow) and alive trophonts (white arrow) with intact nucleus under lugol iodine stain during treatment with aqueous leaves extract Moringa oleifera. Mag. 100X.
icantly (P<0.05) between the two groups (A and B). The infective prevalence of the group was the highest with mean value of 100.00±0.00 compared to the group A which had the lowest infective prevalence of 31.10±25.25. Meanwhile, the infective intensity of 159.67±34.4 was observed in the group B (negative control) in comparison with 82.17±30.99 seen in the group A. The treatments of theronts with M. oleifera at 0.5g/L showed low infectivity prevalence and intensity (Table 6).
Discussion
There has been a concerted effort to identify suitable replacement agents or strategies for the effective control and management of I. multifiliis infection. The continuous application of high, moderate and low concentration based on the immersion dip treatment patterns, in this present work ensued reduction in the number of I. mulifilis surviving and able to reinfect. M. oleifera is a plant that has beneficial effects on humans, animals, exhibiting antimicrobial, antioxidant potentials. Ich infestation causes direct damage to fish skin and gills and leads to fish mortality. From the forgoing, it was observed that fish exposed to over 44,000 theronts in challenge trials, had visible white spots within a short period of time. The newly established trophonts caused less damage to fish tissues which supported the findings of Xu et al. (2009). The variations discovered in the trophonts present in the body smear and gill of the fish especially in some treated groups was because, surface of fish is covered with a mucous layer which is a part of the defense mechanism against parasite and at the same time entrapped the adult parasite and made it uneasy to be knocked off/or removed from the body of the fish by the extract. In addition since infestation is the result of random encounters between the fish and the protozoal parasites, movements of water over the gills can increase the probability of parasite contact. Meanwhile, little mucous covering the secondary lamella of the gills when stressed may not be copious to entrap the parasite, therefore, can leave the gill at any time even when treatment has been administered. Erkin et al. (2012) opined that gills appeared to be more susceptible to infestation than the body surface. This coincided with the report of our present study where higher percentage curative in the body smear of the infested fish was seen when compared to the gill. The number of trophonts that were removed from the body of fish is also as a result of the fact that the trophonts evacuated the host tissue and settled within 2-6 hours on a substrate in the water to form cyst encapsulated tomonts (dividing stage). More so, parasites evicted from the tissue due to the efficacy of the treatment failed to develop into tomonts and eventually died. This corresponded to the report of the Hui et al. (2012) who reported that parasites that were knocked off from the body of the host before the scheduled time for spontaneous departure, failed to develop into tomont stage. The decreased numbers of trophont observed during the dip bath treatment were due to varied concentration of the ALEMO extract reducing the I. multifiliis adult parasite. This agreed with the report of (Ekanem et al., 2004) who reported that Gold fish (Carassius auratus) infested with Ich and treated with 200 mg/L of Mucuna pruriens after 72h and 96h reduced the adult parasite by 90% after in vivo test. At the same time they opined that 200 mg/L of Carica papaya led to 100% morality of I. mulitfiilis after 6h. From our present study, the efficacy of the graded concentration of M. oleifera depended on the duration of treatments and led to massive reduction in the eradication of the adult parasite.
There is still paucity of literature on the efficacy of M. oleifera in the treatment of Ich infested fish. But further findings on the efficacy of herbal extracts in controlling/or eradicating the I. multifiliis has been reported by (Yao et al., 2000; Gholipour et al., 2012; Zhang et al., 2013). Furthermore, Yao et al. (2010) suggested that 0.9mg/L sanguinarine eradicated I. mutifilis from the gills by 96.8% in comparison to untreated group at 250C for 48h.
The exposure duration for killing all theronts was another parameter for evaluating a potential parasiticide and was used on this study to evaluate anti-Ich efficacy rapidly eliminated theronts. Antiprotozoal activity screening of M. oleifera proved to be effective in controlling the infective stage of the parasite (theronts). The screening showed that aqueous leaves extract of M. oleifera had the highest activity against I. multifiliis theronts within 24h of exposure. Similar findings was reported by Ling et al. (2013) who opined that 1.25mg/L methanol extract of Psoralea corylifolia eradicated 100% infective theronts within 4h of exposure. However, Camacho (2010) reported short exposure (30min) to high doses of bronopol (i.e. 20, 50, 100mg/L) appeared to have marked effect on the survival of the tormont, cyst, and theront stages of I. multifiliis. Yao et al. (2000) further reported that in vitro antiparasitic efficacy tests exhibited by sanguinarine was 100% effective against I. multifiliis at a concentration of 0.7mg/L with LC50 and LC90 values of 0.437 and 0.833mg/L after 4h of exposure. In addition, prolonged, low doses of bronopol (i.e. 1mg/L) had notable effects on the survival and subsequent development of tomonts and cysts and upon the release of theronts. Similarly, a marked difference in the percentage of theronts surviving ALEMO exposure was demonstrated. Although a large number of theronts were collected for this trial (i.e. 900 theronts per group) and randomly assigned to one of the five experimental containers, it is not possible to say whether there was a skilled distribution in the size of tomonts subsequently releasing theronts (a single tomont can release between 50-300 theronts) in each population and also in the number of theronts released from each cyst. Caution should be used when comparing the number of the theronts in different treatment groups as the number of parasites release from each cyst can vary widely from 250 to 3,000 tomites (Camacho et al., 2010). The current study has shown that low concentration of M. oleifera over a short period of time can have recondite effect on the mortality and survival of theronts of I. multifiliis. Yi et al. (2012) reported that methanol extracts of Magnolia officinalis and Sophora alopecuroides displayed the highest antiprotozoal activity against theronts within 4h and LC50 values was estimated to be 2.45 and 3.43 mg/L. There is paucity of literature on the antiprotozoal efficacy of Moringa oleifera plant parts on the control of the infective stage of the I. multifiliis.
Viability of Infective Theronts After Pre-Treatment with Aqueous Extract of M. Oleifera for 1H
Theronts are the infective stage in the life cycle of I. multifiliis. Meanwhile, tomites that are liberated into the water column quickly differentiated into free swimming infective theronts. The process of differentiation from a tomite to a theront remained unclear but is characterized by the acquisition of a pyriform to fusiform shape and the development of buccal apparatus and an apical perforatorium (Camacho, 2010). The theronts must find a host during its relatively short life-span to survive and complete its life cycle. The life span of this infective stage was shown to last a maximum of 92h under low water temperature and to be inversely proportional to the ambient water temperature (Camacho, 2010). In addition, the infectivity of the theronts in our present work showed that the Invasion of theronts into the host observed in the present study was as a result of the manner by which the theronts invaded the host integument which facilitated the excretion of sticky substances from subpellicular crystalline organelle called mucocysts. Hui et al. (2012) reported that active penetrations of the theronts in the host led to focal necrosis of the epithelial cells. It has been suggested that during penetration of the theronts, they produced hyaluronidase and other enzymes. The present study supported the findings of Ling et al. (2012) who opined that 2.50mg/L of P. corylifolia reduced the infectivity of the theronts. Reports of (Chu et al., 2010; Ling et al., 2012; Yi et al., 2012), indicated similar findings, that theronts are more sensitive to plant-active parasiticides or chemicals (Rowland et al., 2009) when compared to other stages of Ich. Zhang et al. (2013) reported low concentration of pentagally glucose at 1 or 0.5mg/L could not prevent infestation of theronts (100% prevalence), but significantly reduced infectivity intensity. Treatment with ALEMO reduced theronts infectivity potentials by the decrease in Ich infectivity prevalence and intensity observed in the study. Generally, speaking, the fish density in a pond is much lower than that in the experimental tank, however, it takes much longer time for theronts to find a fish host to invade in a pond environment in comparison with that in an experimental condition. When the searching duration for a potential host is equal to or more than 1h, the theronts will almost loose the infectivity ability by the treatment of aqueous leaves extract of M. oleifera at a concentration 0.5g/L based on the infective percentage and intensity of theronts pretreated for 1h recorded in the study. Therefore, ALEMO may be applied as alternatives to chemotherapeutics and environmental friendly drugs for controlling ichthyophthiriasis in fishes. In addition, it should be noted that effects of exposure time, concentrations may affect efficacy and effect on fish which necessitate further preliminary test before a given species of fish are treated (Rzgar et al., 2013).
Conclusion
Ichthyophthirius multifiliis or ‘white spot’ is a wide spread ciliate parasitic protozoan that is commonly encountered in most cultured fresh water fin fish species. Our present findings offered a range of treatment method options for use in commercial farms systems not only for the control of Ich infestations but also other fresh water and marine protozoan’s diseases. There is need for proper licensing of environmentally friendly non-chemotherapeutics to control parasite infection in food fish farms. In addition, the most effective management strategy to use against Ich, however, may be site/farm specific and require a combination of the different treatments considered in the current study. Finally the bath treatment demonstrated efficacy of novel non-chemical treatment Moringa oleifera at high and low concentration in eliminating and reducing the survival of free living stages especially theronts and trophonts.
Acknowledgement
The University of Nigeria provided basic infrastructures in the University facilities in The Department of Zoology and Environmental Biology. The Department of Veterinary Pathology Laboratory, University of Nigeria, Nsukka gave me access to the histopathology and cytology Laboratory. Thanks to Safety Diagnostics Services for funding part of the research.
CONFLICT OF INTEREST
There is no conflict of interest among the authors
Authors Contribution
IKELE, B, CHIKA designed and conducted the experiment, IKELE, C.Faith. identified the plant material and analysed the phytochemical constituents of the plant, Okpasuo, J, Onyekachi, analysed the data, Ating Emmanuel, partly funded the research and edited the manuscript, Obiezue, N, Rose, and Mgbenka, B. Obialor, edited the manuscript and gave technical guidelines of the research.
REFERENCES






