Journal of Animal Health and Production
Mini Review
The Potential of ß-Glucan from Saccharomyces cerevisiae Cell Wall as Anti Cholesterol
Audri Dwi Gumirang Sudiana1, Hendi Kuswendi2, Vycke Yunivita Kusumah Dewi1, Roostita Lobo Balia1*
1Faculty of Medicine, Padjadjaran University, Jl. Raya Bandung-Sumedang Km.21 Jatinangor, Indonesia 45363; 2Faculty of Agro-Industrial Technology, Padjadjaran University, Jl. Raya Bandung-Sumedang Km.21 Jatinangor, Indonesia 45363.
Abstract | Hypercholesterolemia may cause diabetes in animals and often happens to beloved pets such as Labrador retrievers, Terrier cairns, and Beagles. Treatments for hypercholesterolemia commonly causes side effects. β-glucan is a biologically-derived polysaccharide from sources like extracted Saccharomyces cerevisiae. Saccharomyces cerevisiae is a valid natural/herbal alternative in reducing cholesterol levels. The safety of β-glucan from S. cerevisiae is clear, as it has been considered Generally Recognized As Safe (GRAS) by the Food and Drug Administration (FDA). Extracted Saccharomyces cerevisiae may produce up to 85% β-glucan. β-glucan may be developed for treatment of hypercholesterolemia as it has no side effects.
Keywords | β-glucan, Saccharomyces cerevisiae, anti-cholesterol
Received | November 27, 2020; Accepted | December 02, 2020; Published | January 15, 2021
*Correspondence | Roostita Lobo Balia, Faculty of Medicine, Padjadjaran University, Jl. Raya Bandung-Sumedang Km.21 Jatinangor, Indonesia 45363; Email: [email protected]
Citation | Sudiana ADG, Kuswendi H, Dewi VYK, Balia RL (2021). The potential of ß-glucan from saccharomyces cerevisiae cell wall as anti cholesterol. J. Anim. Health Prod. 9(1): 72-77.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.1.72.77
ISSN | 2308-2801
Copyright © 2021 Balia et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cholesterol is an important component as it functions as a precursor for steroid hormone synthesis, bile acid, and vitamin D in humans and in animals (Mumpuni et al., 2011). The presence of cholesterol in the body helps in building cell walls and phospholipid synthesis, a cell membrane component (Fitriarini et al., 2012).
Excess cholesterol with high serum levels for prolonged period of time can cause cholesterol plaque build-up in arteries, thickening and hardening of blood vessel walls which cause obstructions in the blood stream (Damayanti, 2013). Hypercholesterolemia in animals may trigger the development of diabetes (Manens et al., 2012).
Biological compounds are known to produce a reduction in cholesterol level without causing dangerous side effects, one of them being β-glucan (Ardhani et al., 2017; Golomb et al., 2010; Talati et al., 2009). Microorganism-based materials may be used as an alternative when plant-derived materials that are very reliant on environmental factors and unsupportive weather factors are unavailable. In addition, microorganisms are also easier to control and require less land area, making it more preferable. Microorganisms can also be grown in modified media, its productivity increased, and the extraction process is easier.
β-Glucan is a food fiber with a high molecular weight and viscosity so that it has positive effects in lipid and carbohydrate metabolism (Nazare et al., 2007). β-Glucan acts as an anti-cholesterol by preventing destruction of Low Density Lipoprotein cholesterol through oxidative processes, as an antioxidant by bonding with free radicals (Lee et al., 2001; Miura et al., 2003; Józefowski et al., 2012; Utama et al., 2020).
β-glucan may be produced from several sources such as cereals, algae, bacteria, molds, and currently often produced from the yeast S. cerevisiae. S. cerevisiae is a known producer of β-glucan. β-Glucan extracted from S.cereviseae has a higher purity compared to β-glucan extracted from molds as they contain more mycelia, unlike β-glucan derived from S. cerevisiae, because S. cerevisiae contain protein (Young., 2004).
S. cerevisiae also produces the enzyme zymase that is able to break down polysaccharide bonds, sucrose, and fructose into glucose. S. cerevisiae has a cell wall structure containing glucose and functions as glycoprotein (Febriyanti et al., 2016; Siwicki et al., 2015). The articles aimed to elaborating the data of the potential of β-glucan from S. cerevisiae as an anti-cholesterol.
β-GLUCAN FROM S. cerevisiae CELL WALLS
β-glucan is a polysaccharide containing D-glucose monomers that bond with β-glycosidic bonds, and according to the Food Drug and Administration (FDA) of United States of America, it is categorized under GRAS (Generally Recognized As Safe), safe for human consumption without toxicity or side effects (Zhu et al., 2016; Ardhani et al., 2017; Leonid., 1998). β-glucan are generally formed by D-glucose molecule chains in positions (1,3) and mostly have branches in positions (1,4) or (1,6) (Appeldoorn et al., 2002).
S. cerevisiae is a form of yeast that plays a big role in fermentation of food products. FDA approved this yeast as GRAS, and has authorized it safe for consumption without side effects. Many researchers are interested in S.cereviseae and its biological potentials, including as a producer of β-glucan (Pengkumsri et al., 2016), (Leentjens et al., 2014).
The safety of S. cerevisiae has made it a subject of numerous studies aiming to develop its potential, including as a source of β-glucan. (Liu et al., 2017). β-glucan extracted from S. cerevisiae has better yield compared to those extracted from molds, which contain higher number of mycelia compared to β-glucans (Kustyawati et al., 2016).
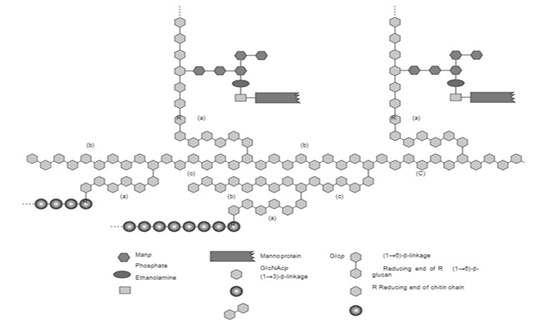
The structure of the S.cereviseae cell wall is mostly comprised of glucan polysaccharides with β-1,3-glucan and β-1,6-glucan bonds (Zeković et al., 2005). Its other components include mannoprotein located in the outer cell wall and which binds to β-(1,6)-glucan (Stone., 2009).
β-glucan is the main component of the S. cerevisiae cell wall (Figure 1) (Kusmiati et al., 2016). The dry weight of S. cerevisiae cell wall is about 10% – 25% of its total cell biomass, wherein that dry weight of the cell wall contains 35% - 40% mannoprotein, 5% - 10% β-1,6 glucan, more than half or 50% - 55% is made up of β-1,3 glucan, 2% - 14% lipid, and 1% - 2% chitin (Kapteyn et al., 1996), (Appeldoorn et al., 2002). The type of β-glucan in the S. cerevisiae cell wall is β-(1,3)-glucan with β-(1,6)-glucan branches. The β-(1,3)-glucan and chitin complex is the main constituent of the inner cell wall, β-(1,6)-glucan connects the inner and outer cell wall components that bound with mannoprotein on the outer surface of the cell wall (Stone., 2009).
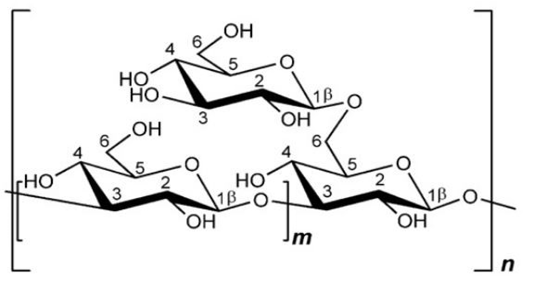
β-glucan from S. cerevisiae consists of the main chain β-(1,3) with β branches bonded in positions O-6 (Figure 2). β-(1,3;1,6)-glucan isolated from S. cerevisiae is not water-soluble, as it has low branching values, and is bonded with chitin and several other polysaccharides, making it insoluble in high-temperature alkaline solutions (Synytsya., 2013). The average polymerization of β-(1,3)-glucan yeast is about 1.500 degrees with a molecular mass of 240 kDa, whereas the average polymerization of β-(1,6)-glucan is about 150 degrees with a molecular mass of 24 kDa (Nie et al., 2018).
β-glucan from S.cereviseae or plant cell walls has a high molecular weight and branches containing more than 250,000 glucose (Robinson, 1995). β-1,3-glucan has a polymerization degree of about 1500 with a molecular weight of 240,000 and fiber length of 660nm (Lipke and Ovalle, 1998). Administration of β-glucan from S. cerevisiae ex
Table 1: The production of β-glucan from Saccharomyces cerevisiae
| Source | Production Process | Production Time | Amount of β-glucan | Sources |
|
S. cerevisiae strains HII31 |
NaOH/HCI extraction
|
72 hour
|
41.69 % |
|
|
S. cerevisiae strains TISTR5003 |
36.31 % |
|||
|
S. cerevisiae strains TISTR5024 |
38.48 % | |||
|
S. cerevisiae strains TISTR5051 |
39.42% | |||
|
S. cerevisiae strains TISTR5059 |
41.24% | |||
|
S. cerevisiae strains TISTR5191 |
38.58% | |||
|
S. cerevisiae strains TISTR5197 |
38.07% | |||
|
S. cerevisiae strains TISTR5278 |
40.07% | |||
|
S. cerevisiae strains TISTR5328 |
38.53% | |||
|
S. cerevisiae strains TISTR5623 |
40.60% | |||
| Saccharomyces cerevisiae | 0.25 M KOH and 1.2 M KCl extraction | ND | 85% |
|
| Saccharomyces cerevisiae |
NaOH 0.75 M
|
72 hour
|
56.48% |
|
| Saccharomyces cerevisiae | 85-90% |
traction has a larger effect in the body’s immune system compared to β-glucan sourced from wheat (Novak et al., 2008; Vetvicka et al., 2010), (Vetvicka et al., 2006).
Mechanism of β-glucan formation on the cell walls of yeast is through glucose metabolism. Glucose is converted into glucose-6-phosphate, which then produces glucose-1-phosphate because of the enzyme phosphoglucomutase, and is then converted back into uridine diphosphate glucose (UDP-glucose). This UDP-glucose is a component that makes up the yeast cell wall, including β-glucan (Appeldoorn et al., 2002; Diez, 2016). β-glucan in the S. cerevisiae cell wall (Figure 1) works as supporting framework for the cell wall to strengthen the cell’s structure, also as a food reserve for the cell (Nguyen et al., 1998). Various studies have shown that S. cerevisiae is one of many yeasts with potential to produce β-glucan (Table 1).
Another study (Odabasi et al., 2006) reported that S. cerevisiae that is extracted contains a supernatant β-glucan up to 2.336 pg/ml. β-glucan with structure β-(1,3)-glucan and β-(1,6)-glucan from S. cerevisiae, may produce immunomodulators with positive effects on an animal’s immune system (Yalçın et al., 2012; Ortuňo et al., 2002)
β-Glucan as an Anti-Cholesterol
β-glucan acts as anti-hypercholesterol through mechanisms of reducing cholesterol absorption in the intestines by binding, disturbing enterohepatic circulations, causing an increase in bile acid production from cholesterol, therefore reducing cholesterol levels in the liver and its food fiber components increase excretion of bile acid or neutral lipids, through increased catabolism of Low Density Lipoprotein, and reduces fat absorption (Ferdiansyah., 2018; Mursito et al., 2011). β-glucan regulate 7-alpha-hydroxylase activity, which in turn regulates excretion of bile acid and reduce fat absorption (Yang et al., 2003; Andersson et al., 2002). In other mechanisms, β-glucan form a gel layer in the gastrointestinal tract especially in the small intestine; as in lower concentrations, β-glucan may dissolve water and has high viscosity (Nazare et al., 2007). This increased viscosity delays gastric emptying and reduce glucose and lipid absorption in the small intestine (Lund et al., 1989; Wurscht et al., 1997; Babicek et al., 2007). High viscosity of the intestine may reduce reabsorption of bile acid, increasing excretion of bile acid, causing increased bile acid synthesis and increased Low Density Lipoprotein absorption in the liver (El-Arab et al., 2009).
Table 2: Results of In Vivo Studies of β-glucan as an Anti-cholesterol
| Strain | Result | Sources | |
| S. cerevisiae |
By consuming 15 grams of β-glucan every day for 7 weeks, may reduce cholesterol levels up to 8% in obese and hypercholesterolemia patients |
||
|
S. cerevisiae |
β-glucan extracted from S. cerevisiae may reduce total cholesterol level up to nearly normal levels with dosages 10 mg 33.71% and 32.79%. Whereas for triglyceride levels with dosages 30 mg β-glucan 19.45% and 10 mg β-glucan 64.43% in blood plasma with reasonable decrease |
||
| S. cerevisiae |
Consumption of β-glucan from S. cerevisiae isolated from bread yeast for 21 days with a dose of 1 mg/kgBW/day may reduce cholesterol levels in mice blood up 67%. |
||
Reduction of cholesterol may also happen as a secondary mechanism from fermentation of β-glucan by large intestinal microflora. Fermentation of β-glucan results in the formation of Short-Chain Fatty Acids (SCFA), mainly acetate, propionate, and butyrate. Propionates are proven to significantly inhibit cholesterol synthesis in the human body by inhibiting HMG-Coenzyme A reductase enzyme activity (Chaiyasut et al., 2018; Ide et al., 1977). Inhibition of the enzymes hydroxylase and reductase which converts HMG-Coenzyme A into mevalonate, will inhibit cholesterol production. This is due to the process of cholesterol production in the liver starting from formation of HMG (Hydroxymethylglutaryl)-Coenzyme A, mevalonate, squalene, lanosterol, until cholesterol is formed (Amaral et al., 1992; Robbins et al., 1995).
Low Density Lipoprotein is the main cholesterol in blood, LDL cholesterols may be oxidized by free radicals (reactive oxygen). In order to prevent LDL cholesterol build-up on blood vessel walls, cholesterol must avoid oxidative processes in the body. Concentrations of cholesterol may be reduced up to 13% and LDL cholesterol plasma level up to 14% in hypercholesterolemia patients undergoing β-glucan therapy (Kirby et al., 1981). High LDL cholesterol content is also a risk factor for coronary heart disease.
Feeding mice with high-fiber diets and added β-glucan from barley significantly increased plasma glucose concentration and reduced cholesterol levels when compared to mice fed low-fiber diets such as corn, flour, and rice (41). High-fiber feed can bind bile acid, free fatty acid, and cholesterol, reducing absorption and increasing fecal excretion (Chau et al., 2005; Cho et al., 2007). Feeding β-glucan on mice may reduce total cholesterol, triglyceride, and malondialdehyde levels up to 42% (Kusmiati et al., 2016). β-glucan from yeast may reduce cholesterol levels in mice and may elicit monocyte and macrophage response (Vetvicka et al., 2009).
By consuming 3 grams/day β-glucan as a part of a low-saturated-fat diet in addition to a healthy life style, it may reduce blood cholesterol (Othman et al., 2011). Male rats from the Wistar breed given feed containing 10% β-glucan showed hypocholesterolemic activities (Yoshida et al., 2004). β-glucan produced from extraction of S. cerevisiae showed positive activity as a hypocholesterolemic effective in reducing total cholesterol, triglyceride, and repairing lipid metabolism disturbances (Waszkiewicz et al., 2009). β-Glucan exhibited a Biological Defence Mechanism (BDM) with potential to activate the body’s immune system through macrophages (Andriani., 2007). β-glucan has an easily-absorbed metabolic property (El khoury et al., 2012) and a great biological activity by reducing cholesterol levels up to 8% in patients with diabetes and obesity by consuming 15 grams/day β-glucan for seven weeks. β-glucan inside the body elicits a positive response, not causing any dangerous side effects for the body’s health (Babicek et al., 2007).
CONCLUSIONS
S. cerevisiae is a yeast categorized under GRAS by the FDA, therefore its role in the health sector may be optimized as a producer of β-glucan. Production of β-glucan from S. cerevisiae is predicted to increase in the future, considering the plentiful β-glucan contents in cell walls of S. cerevisiae and its safety. The safety results of in vivo trials has pushed β-glucan from S. cerevisiae to become the subject of numerous studies in optimizing its role as an anti-cholesterol.
Development of β-glucan from S. cerevisiae as an anti-cholesterol is met with various challenges such as β-glucan production from S. cerevisiae is still prone to contamination, lowering its purity. Even so, with the continuing advancements of technology, it is entirely possible to create and develop a technology to extract β-glucan from S. cerevisiae that will produce a heavier molecular weight β-glucan and one that is safer from contamination.
ACKNOWLEDGEMENT
Authors would like to thank the Rector of Universitas Padjadjaran for the Academic Leadership Grant and Ministry of Education and Culture of Indonesia for the research grant with the scheme of “Penelitian Dasar”.
CONCLUSIONS
S. cerevisiae is a yeast categorized under GRAS by the FDA, therefore its role in the health sector may be optimized as a producer of β-glucan. Production of β-glucan from S. cerevisiae is predicted to increase in the future, considering the plentiful β-glucan contents in cell walls of S. cerevisiae and its safety. The safety results of in vivo trials has pushed β-glucan from S. cerevisiae to become the subject of numerous studies in optimizing its role as an anti-cholesterol.
Development of β-glucan from S. cerevisiae as an anti-cholesterol is met with various challenges such as β-glucan production from S. cerevisiae is still prone to contamination, lowering its purity. Even so, with the continuing advancements of technology, it is entirely possible to create and develop a technology to extract β-glucan from S. cerevisiae that will produce a heavier molecular weight β-glucan and one that is safer from contamination.
ACKNOWLEDGEMENT
Authors would like to thank the Rector of Universitas Padjadjaran for the Academic Leadership Grant and Ministry of Education and Culture of Indonesia for the research grant with the scheme of “Penelitian Dasar”.
REFERENCES