Journal of Animal Health and Production
Research Article
Journal of Animal Health and Production 1 (4): 36 – 37Prevalence of Gastrointestinal Parasites in Free Range Cattle; a Case Study in Haa District, Bhutan
Golo Tshering, Nedup Dorji*
*Corresponding author:[email protected]; [email protected]
ARTICLE CITATION:
Tshering G and Dorji N (2013). Prevalence of gastrointestinal parasites in free range cattle; a case study in haa district, Bhutan. J Anim Health Prod. 1 (4): 36 – 37.
Received: 2013–09–25, Revised: 2013–10–31, Accepted: 2013–11–01
The electronic version of this article is the complete one and can be found online at (http://nexusacademicpublishers.com/table_contents_detail/11/120/html) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
This study was conducted to examine the gastrointestinal parasites in 480 cattle heads (120 of each breed and age). Fresh faecal samples of cattle were collected directly from rectum and a few samples were picked from the ground just after defecation. These faecal samples were processed by standard floatation method to identify endoparasite species. Microscopic examination revealed that about 211 samples (43.96%) were infected with gastrointestinal parasites. Among parasitic infectioned samples, helminth (54.98 %), protozoa (41.23 %) and mixed (3.79 %) were examined. Balantidium was the most common organism in faecal samples of all age groups. In addition, Strongyle, Ascaris and Coccidia were also present. This study suggests examination of endoparasites in cattle in different seasons and regions of the country. This approach will initiate proper control strategies to minimize parasitic infections.
INTRODUCTION
Parasite infestation is one of the most common problem affecting cattle of all ages and breeds (Rafiullah et al., 2011; Awraris et al., 2012). Internal parasites interfere with nutrition, growth and the production of the cattle (Pilarczyk et al., 2009; Awraris et al., 2012; Khan et al., 2013). The gastrointestinal parasitic infection is the major cause of stunted growth in calves and may lead to death. They pose considerable economic losses.
Among endoparasites, only a few species are accounted for the major problems in grazing livestock (Rafiullah et al., 2011). The free range rearing system provides an ideal condition for setting gastrointestinal parasites in Bhutan. Periods of greatest infection risk occur when ruminant are allowed for open grazing. The farmers mainly depend on dairy to sustain their livelihood and they practice open grazing. Therefore, the objective of the present study was to survey the prevalence of internal parasite in different cattle breed of Bhutan.
MATERIALS AND METHODS
Study Sites and Sample Size
Fresh faecal samples were collected from cattle of different age and breeds from mid December 2012 to March 2013 in Katsho, Eusa and Samar blocks (Haa district) to determine the endoparasites prevalence in the cattle. A total of 480 cattle including improve young (n = 120), improved adult (n = 120), native young (n = 120) and native adult (n = 120) were collected using simple circular random sampling method.
Samples were obtained directly from animal rectum as per Kanyari et al. (2010) method and a few from the ground when fresh and clean. Faecal samples were placed into vial containing 10 % formalin. The vials were labeled and placed into cool box and transported for the laboratory examination. The fecal flotation method as described by Karki (2008) was used to identify the parasite eggs in the faecal sample.
Data Analysis
The parasite eggs prevalence on individual animals was entered in Ms–excel spreadsheet and descriptive statistic of SPSS 16 was used to determine the prevalence of endoparasites.
RESULTS AND DISCUSSION
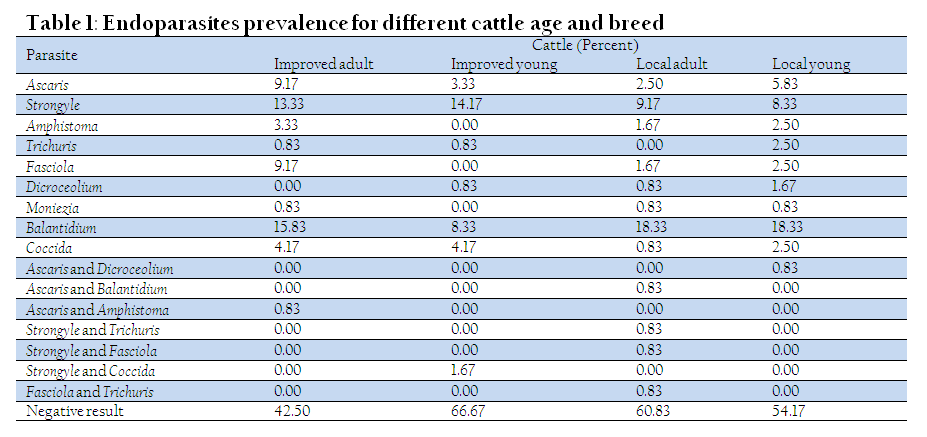
About 43.96% of the total faecal samples were positive for endoparasites which was lower than Nigerian cattle of 47.00 % kept at Research farm (Biu et al., 2009). Our study further revealed that about 26 % of the samples were positive for helminthes which includes mixed infections and was slightly lower than Madhyapur Thimi Municipality lactating cow of 26.56% (Sapkota et al., 2006). The flotation method revealed about 54.98 %, 41.23 % and 3.79 % of the total infected cattle have helminthes, protozoa and mixed, respectively in our study. This greater helminthic infection in our study was in consistent with Fatima et al. (2012) report for Kashmirian cattle.
Among helminth infection, nematodes (72.41 %) was highest followed by trematodes (25.00 %) and cestodes (2.59 %). Gastrointestinal nematodes observed were Strongyles (46.55 %), Ascaris (21.55 %) and Trichuris (4.31 %) in our study. The higher percent of Strongyles in cattle was also documented in Kenya (Biu et al., 2009) and Nigeria (Kanyari et al., 2010). The only cestode detected in our study was Moniezia however, in to Tandon et al. (2005) Moniezia, Anoplocephala and Taeniid were found. The feaceal having trematodes in our study areas includes liver infested Fasciola (13.79 %) and Dicroceolium (3.45 %) and rumen infested Amphistoma (7.76 %) but in Wamrong village only Fasciola was detected (Tandon et al., 2005). Moreover, Fasciola predominate any trematodes species and is supporting to the claim made by Kanyari et al. (2010) in Kenyan cattle and incidence of Fasciola is determined by the presence of snail (Tandon et al., 2005; Rafiullah et al., 2011). The prevalence of protozoa infections were Balantidium (34.60%) and Coccida (6.64%) in our investigation. Several studies have reported that Coccidia is a common parasites in animals because of favourable climatic conditions (Pilarczyk et al., 20).
Overall the order of endoparasites descends: improved adult (57.50 %) > native young (45.83 %) > native adult (39.17 %) > improved young (33.33 %). The adult cattle presented higher infection rate than the young animals agreeing Fatima et al. (2012). This could be attributed accessible to contaminated pasture by the adult (Fatima et al., 20). The lower infection rate in improved young may be due to restriction of free–grazing by the owner as reported by Bilal et al. (2009) and Rafiullah et al. (2011). Parasite such as Strongyle, Ascaris and Coccidia were identified in the feacel sample for all groups of cattle (Table 1). It is noteworthy to state that Balantidium (protozoan) incidence was highest for all categorized cattle contradicting with Sharma (2011) who reported for Strongyle. The highest prevalence of Balantidium could be due to presence of pig in the grazing areas. The prevalence of gastrointestinal parasites depend type of pasture, environmental conditions and age of animal (Bilal et al., 2009; Biu et al., 2009). However, many eggs and intermediate stages of gastrointestinal parasites such as Fasciola and Dicroceolium may survive in unfavourable condition for several months. The other reasons could be because of contamination by infected dogs and horses in grazing areas (Tandon et al., 2005)
CONCLUSION
The results of this study revealed the prevalence of various internal parasites in cattle with higher infection rate in improved adult breed than native. There is a need to create farmer awareness about prevention of parasitic infestations. The animal should be de–wormed at regular interval with an appropriate antihelminth. Proper husbandry practices should be exercised to improve overall hygiene of the grazing sites.
ACKNOWLEDGEMENTS
We would like to thank technician for the laboratory assistance. Further the authors would thank the Department of Livestock for providing financial support for the project. We also thank the reviewer for the valuable comments and suggestions.
REFERENCES
Awraris T, Bogale B and Chanie M (2012). Occurrence of gastro intestinal nematodes of cattle in and around gondar Town, Amhara regional state, Ethiopia. ActaParasitologica Globalis. 3 (2): 28–33.
Bilal MQ, Hameed A and Ahmad T (2009). Prevalence of gastrointestinal parasites in Buffalo and cow calves in rural areas of Toba Tek Singh, Pakistan. J. Anim. & Plant Sci. 19(2): 67–70.
Biu AA, Maimunatu A, Salamatu AF and Agbadu ET (2009). A faecal survey of gastrointestinal parasites of ruminants on the University of Maiduguri Research Farm. Int. J. Biomed. and Hlth Sci. 5 (4): 175–179.
Fatima M, Chishti MZ, Ahmad F and Lone BA (2012). Epidemiological study of Fasciolosis in cattle of Kashmir valley. Adv. Biol. Res. 6 (3): 106–109.
Kanyari PWN, Kagira JM and Mhoma JRL (2010). Prevalence of endoparasites in cattle within urban and peri–urban areas of Lak Victoria basin, Kenya with special reference to zoonotic potential. Sci. Parasitol. 11(4): 171–178.
Karki K (2008). Protocol for parasite egg identification in faecal samples in parasitology unit. Available at http://www.docstoc.com/docs/893810/Protocol-for-parasite-egg-identification-in-faecal-samples. Retrieved on 31 November 2013.
Khan JM, Hussain A, Bukhsh A and Shumaila M (2013). Identification of Ostertagia Ostertagi in cattle with persistent diarrhea. Res. j. vet. Pract. 1 (2): 16–17.
Pilarczyk B, Balicka–Ramisz A, Kozak W and Ramisz A (2009). Occurrence of endoparasites in heifers imported to Poland from the Netherland. Archiv Tierzucht. 52(3): 265–271.
Rafiullah, Turi AA, Sajid A, Shah SR, Ahmad S and Shahid M (2011). Prevalence of gastrointestinal tract parasites in cattle of Khyber Pakhtunkhwa. ARPN J. Agri. and Biol. Sci. 6(9): 9–15.
Sapkota RC, Shrestha S, Bashyal S, Adhikari S and Poudel S (2006). Prevalence of helminth parasites in lactating cattle in Madhyapur Thimi municipality of Bhaktapur. G. F. J. Himalayan College of Agri. Sci. and Tech. 4(2): 169–175.
Sharma DB (2011). Handbook on livestock and poultry diseases of Bhutan. KMT Press, Phuntsholing, 225 – 237 .
Tandon V, Kar PK, Das B, Sharma B and Dorjee J (2005). Preliminary survey of gastro–intestinal helminth infection in herbivorous livestock of mountainous regions of Bhutan and Arunachal Pradesh. Zoos' Print J. 20(5): 1867–1868
http://dx.doi.org/10.11609/JoTT.ZPJ.1227.1867-8