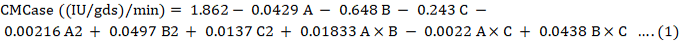Utilization of Bombyx ceiba Seed Pods: A Novel Substrate for Cellulase Production through Solid State Fermentation using Response Surface Methodology
Utilization of Bombyx ceiba Seed Pods: A Novel Substrate for Cellulase Production through Solid State Fermentation using Response Surface Methodology
Sobia Nazir1, Muhammad Irfan1*, Muhammad Nadeem2, Hafiz Abdullah Shakir3*, Muhammad Khan3, Shaukat Ali4, Quratulain Syed2
1Department of Biotechnology, University of Sargodha, Sargodha, Pakistan
2Food and Biotechnology Research Center, Pakistan Council of Scientific and Industrial Research Laboratories Complex, Ferozepur Road Lahore, Pakistan
3Department of Zoology, University of the Punjab, New Campus, Lahore, Pakistan
4Department of Zoology, Government College University, Lahore, Pakistan
Abstract | In this work, Trichoderma viride was used for cellulase production in solid state fermentation (SSF) using seed pods of Bombyx ceiba as substrate. Three parameters were used such as inoculum size (1, 5.5 and 10%), medium volume (1, 3 and 5ml) and incubation period (3, 4, 5 days) to observed the maximum activity of cellulase using Box-Bhenken design of response surface methodology. Maximum activity of CMCase (0.700 IU/gds/min) and FPase (0.260 IU/gds/min) was observed on 4th day with inoculum size of 1% and medium volume of 1ml (for 5g substrate). The proposed model was highly significant as depicted by ANOVA. The results recommended that seed pods of Bombyx ceiba play important role in cellulase production, which is useful for the food industry, textile industry and ethanol production.
Article History
Received: April 17, 2019
Revised: Npvember 20, 2019
Accepted: November 29, 2019
Published: December 24, 2019
Authors’ Contributions
SN conducted the research. MI conceived the sstudy design. SN and MI drafted the manuscript. MN analysed the data whilw MAS interpreted it. MK and SA reviewed the literature. QS reviewed the article.
Keywords
Cellulase, Trichoderma sp., Solid state fermentation, Bombyx ceiba, Response surface methodology
Corresponding author: Muhammad Irfan and Hafiz Abdullah Shakir, [email protected]; [email protected]; [email protected]
To cite this article: Nazir, S., Irfan, M., Nadeem, M., Shakir, H.A., Khan, M., Ali, S. and Syed, Q., 2019. Utilization of Bombyx ceiba seed pods: a novel substrate for cellulase production through solid state fermentation using response surface methodology. Punjab Univ. J. Zool., 34(2): 213-219. https://dx.doi.org/10.17582/journal.pujz/2019.34.2.213.219
Introduction
Cell wall of plant is composed of cellulose which is the chief component. When its composite structure is splited, it gives such components that are used by microorganisms. Due to its crystalline structure, it is difficult to degrade (Bras et al., 2011). Hydrolytic enzymes are peculiar for the acquisition of many other compounds and cellulase is one of the enzymes which digest the cellulose. There are three types of cellulase enzyme, which break the components of cellulose (Ozioko et al., 2013). Endo-1, 4-β-glucanase simply called endoglucanase, split randomly building block β-1, 4-glucosidic concatenation inside the long chains of glucose. The endoglucanases are frequently anatomized by glutinousness deductions in carboxymethyl cellulose (CMC) solution. Exo-1, 4-β-glucanase simply called exoglucanases, divide the open ends of cellulose element to free glucose.1, 4-β-Glucosidase catalyzed the dissolved pulp and polymers of glucose along degree of polymerization (DP) till six to prepare sugar molecules in the fluid stage. Cellulase can be obtained from bacteria, fungi and other termites. Fungi are proven to be best source for cellulase production. Contagious species from the sort Trichoderma are rendered by quick development and plenteous creation of conidial spores (Błaszczyk et al., 2014).
Organisms in the family Trichoderma have been known since from 1920s for the capacity to go about as biocontrol agents in contrast to plant microbe. Up to this point, the important components for control have been thought to be those basically following up on the pathogens and included mycoparasitism, antibiosis, and rivalry for assets and space. Late advances show that the impacts of fungus on plants, including incited systemic or confined resistance, are likewise critical. The cellulase production is obtained using SSF. The SSF is very significant because it is less costly. It is characterized as the maturation prepare in which microbes develop on strong substantial devoid of the absence of clear fluid. Idea of utilizing strong base material is most likely most seasoned strategy utilized for the production of enzymes to work for welfare of mankind (Bhargav et al., 2008).
Response surface methodology (RSM) was introduced in 1950. The objective to use response surface methodology is to enhance yield which is impacted by a few autonomous factors. Main purpose of RSM is to optimize process parameters thus reducing the production cost (Jiménez-Contreras et al., 2008). RSM is more efficient process of optimization as compared to other methods as it requires less experiment for calculation of numerous variables and their interaction (Kavitha et al., 2016). Cellulase has many applications like in food industry, paper and pulp industries, biofuel industry, and textile industry. The present study investigated the potential utilization of seed pods of Bombyx ceiba as carbon source in solid state fermentation by Trichoderma viride for cellulase production through response surface methodology.
Materials and Methods
Microorganism
Trichoderma viride was obtained from Fermentation Biotechnology Laboratory, PCSIR Labs. Complex Ferozepur road Lahore, and was used for the production of cellulase. The strain was maintained on PDA (potato dextrose agar) slants and revived biweekly.
Substrate
The substrate used in this study was seed pods of Bombyx ceiba. The substrate was washed and sun dried followed by oven drying at 70oC till constant weight. After that it was ground to fine powder (2mm) and sealed in airtight container for further processing.
Inoculum preparation
Inoculum was prepared by adding sterilized distilled water into the 5-days old slant of Trichoderma viride. With inoculating loop, the spores were mixed and one ml (2 x 108) of spore suspension was used as an inoculum.
Fermentation techniques.
Trichoderm viride strain was used to produce cellulase in SSF. Five gram powder of seed pods of Bombyx ceiba was added in media (g/l, (NH4)2SO4 10. CaCl2 0.5, MgSO4 0.5, KH2PO4 4) with ratio of 1, 3 and 5ml. After routine sterilization and inoculation, incubation was carried out at 30˚C for 3, 4 and 5 days.
Extraction of enzyme
After incubation, samples were taken and citrate buffer was added with the ratio of 1:10 (solid: liquid ratio). In 2g of substrate, 20ml of citrate buffer was added. Samples were placed on shaker for 2 h with shaking speed of 150rpm. After shaking it was filtered and residue was discard and then it was centrifuged and further analysis purpose (Irfan et al., 2010).
Enzyme assay
CMCase and FPase activity was measured as described by Irfan et al. (2011). Glucose was used as standard and one enzyme unit is quantity of enzyme required to produce one micromole of glucose per milliliter per minute under standard assay condition.
Experimental design
Box-Bhenken design of RSM was used to carry out enzyme production. The variables and their levels used were presented in Table 1.
Table 1: Coded and actual level of the three independent variables for cellulase production.
|
Independent Variable |
Code |
Code and actual factor level |
||
|
-1 |
0 |
+ 1 |
||
|
Volume of media (ml) |
A |
1 |
3 |
5 |
|
Inoculum size (ml) |
B |
1 |
5.5 |
10 |
|
Incubation period (days) |
C |
3 |
4 |
5 |
Results and Discussion
Cellulase was produced using seed pods of Bombyx ceiba through SSF using BBD. Proximate analysis of substrate indicated that seed pods comprised of 34.0% cellulose, 25.6% hemicellulose and 23.7% lignin. Fifteen experiments were run with different variables i.e. inoculum
Table 2: Results of BBD showing observed and predicted response for cellulase activity.
|
Run # |
A |
B |
C |
CMCase activity (IU/gds/min) |
FPase activity (IU/gds/min) |
||||
|
Observed |
Predicted |
Residual |
Observed |
Predicted |
Residual |
||||
|
1 |
5.5 |
3 |
4 |
0.100 |
0.090 |
0.0100 |
0.180 |
0.140 |
0.0000 |
|
2 |
1 |
3 |
5 |
0.090 |
0.148 |
-0.058 |
0.160 |
0.160 |
-0.005 |
|
3 |
5.5 |
5 |
5 |
0.190 |
0.227 |
-0.037 |
0.150 |
0.135 |
0.0100 |
|
4 |
5.5 |
5 |
3 |
0.070 |
0.080 |
-0.010 |
0.160 |
0.055 |
-0.000 |
|
5 |
10 |
1 |
4 |
0.040 |
0.136 |
-0.096 |
0.140 |
0.135 |
0.0050 |
|
6 |
10 |
3 |
5 |
0.030 |
-0.05 |
0.0862 |
0.050 |
0.055 |
-0.005 |
|
7 |
5.5 |
3 |
4 |
0.060 |
0.090 |
-0.030 |
0.180 |
0.180 |
0.0000 |
|
8 |
1 |
5 |
4 |
0.120 |
0.023 |
0.0962 |
0.250 |
0.255 |
-0.005 |
|
9 |
10 |
3 |
3 |
0.050 |
-0.008 |
0.0587 |
0.090 |
0.085 |
0.0050 |
|
10 |
1 |
3 |
3 |
0.070 |
0.156 |
-0.086 |
0.185 |
0.185 |
0.0050 |
|
11 |
5.5 |
3 |
4 |
0.110 |
0.090 |
0.0200 |
0.180 |
0.180 |
0.0000 |
|
12 |
1 |
1 |
4 |
0.700 |
0.651 |
0.0487 |
0.260 |
0.255 |
0.0050 |
|
13 |
10 |
5 |
4 |
0.120 |
0.168 |
-0.048 |
0.160 |
0.165 |
-0.005 |
|
14 |
5.5 |
1 |
3 |
0.590 |
0.552 |
0.0375 |
0.140 |
0.150 |
-0.010 |
|
15 |
5.5 |
1 |
5 |
0.360 |
0.350 |
0.0100 |
0.120 |
0.120 |
0.0000 |
size (A), solid to liquid ratio (B) and time period (C).Highest value for CMCase was 0.19U/ml when inoculum size was 5.5%, solid to liquid ratio 5ml and time period was 5 days (Table 2). Highest value for FPase was 0.250 when inoculum size was 1%, solid to liquid ratio of 5ml and time period of 4 days. Result for CMCase and FPase was calculated by polynomial equation of regression as shown in Equation 1 and 2. There was very close resemblance between observed and predicted values (Fig. 1) indicating the accuracy of the model.
Statistical significance of data was evaluated by applying ANOVA for CMCase and FPase (Table 3). The proposed model for FPase production was found highly significant with P value of 0.000 having F-value of 54.57 while for CMCase the P and F-value was 0.03 and 6.13 respectively. The correlation (R2 value) in model that the proposed model exhibited 98.99% and 91.69% accurately for FPase and CMCase, respectively. Coded coefficients for CMCase and FPase are shown in Table 4. Significant response for coefficient was found to be mainly dependent upon T value and P value. If P value is lesser then 0.05 and T value more than 0.05, then the model will be significant.
Figure 2 demonstrated contour plots for CMCase and FPase production from T. viride in SSF. These plots revealed that each variable had significant impact on enzyme production. Different color patterns in these plots reflect different enzyme values at various concentrations.
Desirability chart for CMCase and FPase are shown in Figure 3. These charts revealed that the enzyme values predicted in this chart was verified by repeating various experiments.
In this study inoculum size has been tested for maximum cellulase production by T. viride under SSF when inoculum size ranges between 5-15%. Inoculum size of
2.0×106 for Purpureocillium lilacinum has been reported for maximum cellulase production in submerged fermentation (Srilakshmi et al., 2017). The highest cellulase yield was obtained by T. viride FBL1 in SSF using 5 % inoculum size (Irfan et al., 2010).
Impact of time period on cellulase production was checked by inoculating the substrate with fungi and incubated for 3, 4 and 5 days of fermentation period. In our study, 4th day of fermentation period was found best for maximum cellulase production in solid state fermentation. Similar findings of 4th day was also reported for cellulase production by new mutant strain of T. reesei in SSF (Darabzadeh et al., 2018). Yadav et al. (2016) isolated fungi from soil which have capability to produce maximum cellulase after 6th day of fermentation period. Purpureocillium lilacinum yielded highest cellulase production after 7 days of fermentation period (Srilakshmi et al., 2017). Pachauri et al. (2017) reported maximum cellulase production using 5g pretreated sugarcane bagasse from T. koningii after 10 days of incubation time. T. stipitatus MTCC 12687 had capability to secrete maximum cellulases on 5th day of incubation period (Bharti et al., 2018). Irfan et al. (2016) described optimum fermentation period of 3rd day for endoglucanase production by T. harzianium in submerged fermentation. Moosavi-Nasab and Majdi-Nasab (2008) worked on cellulase production by T. reesei using sugar beet pulp as substrate and maximum activity (0.46 IU/ml) was obtained on 4-6 days. Singhania et al. (2006) obtained maximum cellulase activity of 154.58U/gds from T. reesei NRRL 11460 after 72h in SSF strategy.
Table 3: Analysis of variance for CMCase and FPase.
|
CMCase (IU/gds/min) |
Sources |
DF |
Adj SS |
Adj MS |
F value |
P value |
|
Model |
9 |
0.545725 |
0.060636 |
6.13 |
0.030 |
|
|
Linear |
3 |
0.246975 |
0.082325 |
8.32 |
0.022 |
|
|
A |
1 |
0.068450 |
0.068450 |
6.92 |
0.047 |
|
|
B |
1 |
0.177013 |
0.177013 |
17.89 |
0.008 |
|
|
C |
1 |
0.001513 |
0.177013 |
0.15 |
0.712 |
|
|
Square |
3 |
0.158825 |
0.052942 |
5.35 |
0.051 |
|
|
A2 |
1 |
0.007067 |
0.007067 |
0.71 |
0.437 |
|
|
B2 |
1 |
0.145852 |
0.145852 |
14.74 |
0.012 |
|
|
C2 |
1 |
0.000698 |
0.000698 |
0.07 |
0.801 |
|
|
2 Way interaction |
3 |
0.139925 |
0.046642 |
4.71 |
0.064 |
|
|
A*B |
1 |
0.108900 |
0.108900 |
11.01 |
0.021 |
|
|
A*C |
1 |
0.000400 |
0.000400 |
0.04 |
0.849 |
|
|
B*C |
1 |
0.030625 |
0.030625 |
3.09 |
0.139 |
|
|
Error |
5 |
0.049475 |
0.009895 |
22.89 |
0.042 |
|
|
Lack of fit |
3 |
0.048075 |
0.016025 |
|||
|
Pure error |
2 |
0.001400 |
0.000700 |
|||
|
Total |
14 |
0.595200 |
||||
|
FPase (IU/gds/min) |
Model |
9 |
0.039293 |
0.004366 |
54.57 |
0.000 |
|
Linear |
3 |
0.023750 |
0.007917 |
98.63 |
0.000 |
|
|
A |
1 |
0.022050 |
0.022050 |
275.63 |
0.000 |
|
|
B |
1 |
0.000450 |
0.000450 |
5.63 |
0.064 |
|
|
C |
1 |
0.001250 |
0.001250 |
15.63 |
0.011 |
|
|
Square |
3 |
0.015268 |
0.005089 |
63.62 |
0.000 |
|
|
A2 |
1 |
0.000006 |
0.000006 |
0.07 |
0.799 |
|
|
B2 |
1 |
0.001667 |
0.001667 |
20.84 |
0.006 |
|
|
C2 |
1 |
0.012744 |
0.012744 |
159.30 |
0.000 |
|
|
2 Way interaction |
3 |
0.000275 |
0.000092 |
1.15 |
0.416 |
|
|
A*B |
1 |
30.00027 |
0.000225 |
2.81 |
0.154 |
|
|
A*C |
1 |
0.000025 |
0.000025 |
0.31 |
0.600 |
|
|
B*C |
1 |
0.000025 |
0.000025 |
0.31 |
0.600 |
|
|
Error |
5 |
0.000400 |
0.00080 |
* |
* |
|
|
Lack of fit |
3 |
0.000400 |
0.000133 |
|||
|
Pure error |
2 |
0.000000 |
0.000000 |
|||
|
Total |
14 |
0.039693 |
Table 4: Coded coefficients for CMCase and FPase production.
|
CMCase (IU/gds/min) |
Terms |
Effect |
Coef |
SE Coef |
T value |
P value |
VIF |
|
Constant |
0.900 |
0.0574 |
1.57 |
0.178 |
|||
|
A(%) |
-0.1850 |
-0.0925 |
0.0352 |
-2.63 |
0.047 |
1.00 |
|
|
B |
-0.2975 |
-0.1487 |
0.0352 |
-4.23 |
0.008 |
1.00 |
|
|
C |
-0.0275 |
-0.0137 |
0.0352 |
-0.39 |
0.712 |
1.00 |
|
|
A2(%) |
-0.0875 |
-0.0437 |
0.0518 |
-0.85 |
0.437 |
1.01 |
|
|
B2 |
0.3975 |
0.1987 |
0.0518 |
3.84 |
0.012 |
1.01 |
|
|
C2 |
0.0275 |
0.0137 |
0.0518 |
0.27 |
0.801 |
1.01 |
|
|
A*B |
0.3300 |
0.1650 |
0.0497 |
3.32 |
0.021 |
1.00 |
|
|
A*C |
-0.0200 |
-0.0100 |
0.0497 |
-0.20 |
0.849 |
1.00 |
|
|
B*C |
0.1750 |
0.0875 |
0.0497 |
1.76 |
0.139 |
1.00 |
|
|
FPase (IU/gds/min) |
Constant |
0.18000 |
0.00516 |
34.86 |
0.000 |
||
|
A(%) |
-0.10500 |
-0.05250 |
0.00316 |
-16.60 |
0.000 |
1.00 |
|
|
B |
0.01500 |
0.00750 |
0.00316 |
2.37 |
0.064 |
1.00 |
|
|
C |
-0.02500 |
-0.01250 |
0.00316 |
-3.95 |
0.011 |
1.00 |
|
|
A2(%) |
0.00250 |
0.00125 |
0.00465 |
0.27 |
0.799 |
1.01 |
|
|
B2 |
0.04250 |
0.02125 |
0.00465 |
4.57 |
0.006 |
1.01 |
|
|
C2 |
-0.11750 |
-0.05875 |
0.00465 |
-12.62 |
0.000 |
1.01 |
|
|
A*B |
0.01500 |
0.00750 |
0.00447 |
1.68 |
0.1541 |
1.00 |
|
|
A*C |
-0.00500 |
-0.00250 |
0.00447 |
-0.56 |
0.600 |
1.00 |
|
|
B*C |
0.00500 |
0.00250 |
0.00447 |
0.56 |
0.600 |
1.00 |
Moisture content play an important role in production of enzymes during solid state fermentation. In this study, different volumes of liquid medium were used to moisten the solid substrate for maximum cellulase production. Maximum enzyme production was obtained when medium volume of 1ml for 5g of dry substrate powder was used. T. viride and T. reesei produced maximum cellulase in SSF with solid to liquid ratio of 1:1 using empty fruit bunch as substrate (Wonoputri et al., 2018). Saini et al. (2017) reported maximum production for CMCase (11.65U/gds) and FPase (1.29U/gds) using T. reesei when solid to liquid ratio was 1:2.
Statement of conflict of interset
The Authors declare there is no conflict of interset.
References
Bhargav, S., Panda, B.P., Ali, M. and Javed, S., 2008. Solid-state fermentation: An overview. Chem. Biochem. Eng. Q., 22: 49-70.
Bharti, A.K., Kumar, A., Kumar, A. and Dutt, D., 2018. Exploitation of Parthenium hysterophorous biomass as low-cost substrate for cellulase and xylanase production under solid-state fermentation using Talaromyces stipitatus MTCC 12687. J. Radiat. Res. Appl. Sci., 11: 271-280. https://doi.org/10.1016/j.jrras.2018.01.003
Błaszczyk, L., Siwulski, M., Sobieralski, K., Lisiecka, J. and Jędryczka, M., 2014. Trichoderma spp.–application and prospects for use in organic farming and industry. J. Plant Prot. Res., 54: 309-317. https://doi.org/10.2478/jppr-2014-0047
Brás, J.L., Cartmell, A., Carvalho, A.L.M., Verzé, G., Bayer, E.A., Vazana, Y. and Romão, M.J., 2011. Structural insights into a unique cellulase fold and mechanism of cellulose hydrolysis. Proc. Nat. Acad. Sci., 108: 5237-5242. https://doi.org/10.1073/pnas.1015006108
Darabzadeh, N., Hamidi-Esfahani, Z. and Hejazi, P., 2018. Optimization of cellulase production under solid- state fermentation by a new mutant strain of Trichoderma reesei. Food Sci. Nutri., 00: 1–7. https://doi.org/10.1002/fsn3.852
Irfan, M., Gulsher, M., Abbas, S., Syed, Q., Nadeem, M. and Baig, S., 2011. Effect of various pretreatment conditions on enzymatic saccharification. Songklanakarin J. Sci. Technol., 33: 397-404.
Irfan, M., Asghar, U., Nadeem, M., Shakir, H.A., Qazi, J.I., Nelofer, R. and Syed, Q., 2016. Process optimization for endoglucanase production by Trichoderma harzianium in submerged fermentation. Hacettepe J. Biol. Chem., 44: 87–93. https://doi.org/10.15671/HJBC.20164417569
Irfan, M., Syed, Q., Yousaf, M., Nadeem, M., Baig, S. and Jafri, S.A., 2010. Studies on the Pretreatment of wheat straw for improve production of Carboxymethyl Cellulase by thermophilic Trichoderma viride FBL1 in Solid State fermentation. Acad. Arena, 2: 18-30.
Jiménez-Contreras, E., Torres-Salinas, D., Moreno, R., Baños, R. and López-Cózar, E., 2008. Response surface methodology and its application in evaluating scientific activity. Scientometrics, 79: 201-218. https://doi.org/10.1007/s11192-009-0413-3
Kavitha, G., Kurinjimalar, C., Sivakumar, K., Kaarthik, M., Aravind, R. and Palani, P., 2016. Optimization of polyhydroxybutyrate production utilizing wastewater as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int. J. Biol. Macromol., 93: 534–542. https://doi.org/10.1016/j.ijbiomac.2016.09.019
Moosavi-Nasab, M. and Majdi-Nasab, M., 2008. Cellulase production by Trichoderma reesei using sugar beet pulp. Iran Agric. Res., 25: 107-116.
Ozioko, P.C., Ikeyi, A.P. and Ugwu, O.P.C., 2013. Review article cellulases their substrates activity and assay methods. Experiment, 12: 778-785.
Pachauri, P., Chakradhari, Y. and Veeramani, A., 2017. Study of activators and inhibitors on cellulase production from isolated Trichoderma koningii for effective saccharification of sugarcane bagasse. Biofuels, https://doi.org/10.1080/17597269.2017.1400856
Saini, A., Aggarwal, N.K. and Yadav, A., 2017. Cost-effective cellulase production using Parthenium hysterophorus biomass as an unconventional lignocellulosic substrate. Biotechnology, 7: 12. https://doi.org/10.1007/s13205-017-0604-1
Singhania, R.R., Sukumaran, R.K., Pillai, A., Prema, P., Szakacs, G. and Pandey, A., 2006. Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Int. J. Biol. Technol., 5: 332-334.
Srilakshmi, A., Gopal, D. and Narasimha, G., 2017. Impact of bioprocess parameters on cellulase production by Purpureocillium lilacinum isolated from forest soil. Int. J. Pharma Bio. Sci., 8: 157-165. https://doi.org/10.22376/ijpbs.2017.8.1.b157-165
Wonoputri, V., Subiantoro, Kresnowati, M.T.A.P. and Purwadi, R., 2018. Solid state fermentation parameters effect on cellulase production from empty fruit bunch. Bull. Chem. React. Eng. Catal., 13: 553-559. https://doi.org/10.9767/bcrec.13.3.1964.553-559
Yadav, M.P.R., Chauhan, M.P.B., Gahlout, M. and Prajapati, H., 2016. Isolation, screening and optimization of process parameters for enhanced production of cellulase by solid state fermentation. Int. J. Adv. Res. Biol. Sci., 3: 21-27.
To share on other social networks, click on any share button. What are these?