Unveiling the Safety of Cassia alata Leaf Extract: A Subchronic Oral Toxicity in Rats
Amaq Fadholly1,5*, Siti Sa’diah1,6, Diah Nugrahani Pristihadi1, Eva Harlina2, Maharani Kartika Ramadhan3, Yumna Annisa Kurnia Budiharjo4, Cyntia Rafi Adinugroho4, Afifah Alawiyah Rahmah4, Nahla Fifi Azizah4
1Division of Pharmacology and Toxicology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia; 2Division of Pathology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia; 3Doctoral Program in Animal Biosciences, Department of Biology, Faculty of Mathematics and Natural Sciences, IPB University, Bogor, Indonesia; 4Bachelor Program in Veterinary Medicine, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia; 5Laboratory Animal Management Unit, School of Veterinary Medicine and Biomedical Sciences, IPB University, Indonesia; 6Tropical Biopharmaca Research Center, IPB University, Indonesia.
*Correspondence | Amaq Fadholly, Division of Pharmacology and Toxicology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia; Email: amaqfadholly@apps.ipb.ac.id
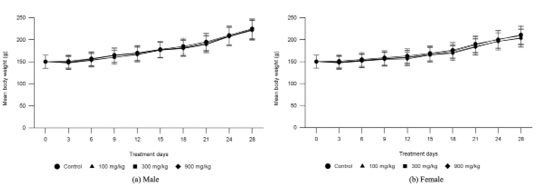
Figure 1:
Mean body weight changes in male (a) and female (b) rats treated with CALE at dose levels of 0 (control), 100, 300, and 900 mg/kg/day for 28 days.
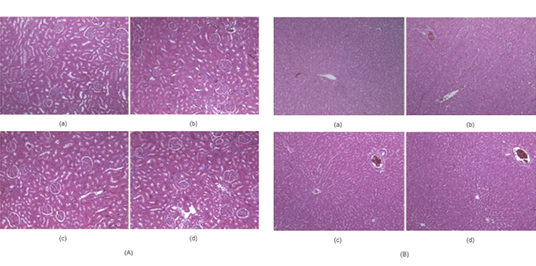
Figure 2:
Representative images of histopathologic changes in kidneys of male rats (A) and liver of female rats (B) in each dose group for 28 days of CALE administration. (a): control; (b): 100 mg/kg/day; (c): 300 mg/kg/day; (d): 900 mg/kg/day.