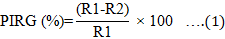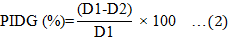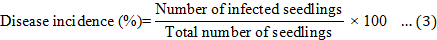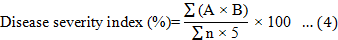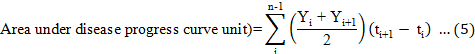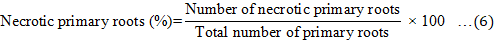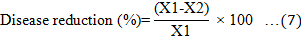Trichoderma Species’ In Vitro and In Planta Inhibition against Ganoderma boninense, a Causative Agent of Basal Stem Rot in Oil Palm (Elaeis guineensis)
Trichoderma Species’ In Vitro and In Planta Inhibition against Ganoderma boninense, a Causative Agent of Basal Stem Rot in Oil Palm (Elaeis guineensis)
Jumiati Asis1, Nur Aainaa Hasbullah1, Mohamadu Boyie Jalloh1, Noor Khairani Mohamad Basri1, Palanivell Perumal2, Peter Mojiun2 and Mohd. Rashid Mohd. Rakib1*
1Faculty of Sustainable Agriculture, Universiti Malaysia Sabah, 90000 Sandakan, Sabah, Malaysia; 2Eco Management Unit, Wilmar Plantations Sdn. Bhd. (formerly known as PPB Oil Palms Berhad), 90000 Sandakan, Sabah, Malaysia.
Abstract | Trichoderma species are well-known biological control agents (BCAs) that have significant antagonistic activity against various fungal phytopathogens. On the other hand, Ganoderma boninense has been identified as the phytopathogen causing basal stem rot (BSR), a devastating disease in oil palm crop (Elaeis guineensis). In this study, the in vitro and in planta inhibition of G. boninense using Trichoderma isolates were evaluated. A total of 20 Trichoderma isolates were collected. Among the isolates, T4RH and T8R were selected for further in planta experiments as they showed significant inhibitory activity against G. boninense via in vitro dual-culture and dual-plate assays. Isolates T4RH and T8R were identified as Trichoderma virens and Trichoderma asperellum by the internal transcribed spacer gene sequences. The in-planta experiment was conducted by inoculation of 3-month-old oil palm seedlings with rubber wood block inoculum. Single and mixture conidial suspensions 1×106 of T4RH and T8R were applied to the Ganoderma-inoculated plants. It was found that T4RH, T8R, and mixed Trichoderma treatments have significantly lower disease incidence, disease severity index, area under disease progress curve, and percentage of necrotic primary roots as compared to the positive control. The disease reduction was up to 57.70% and 43.86% when plants were treated with T4RH and T8R, respectively. Additionally, the Trichoderma treatment recorded significantly higher chlorophyll content, plant height, bole diameter, and number of fronds as compared to the positive control. The findings from this study suggested that T. virens T4RH and T. asperellum T8R have the potential to be used as promising BCAs against G. boninense.
Received | February 22, 2024; Accepted | October 11, 2024; Published | November 01, 2024
*Correspondence | Mohd. Rashid Mohd. Rakib, Faculty of Sustainable Agriculture, University Malaysia Sabah, 90000 Sandakan, Sabah, Malaysia; Email: [email protected]
Citation | Asis, J., N.A. Hasbullah, M.B. Jalloh, N.K.M. Basri, P. Perumal, P. Mojiun and M.R.M. Rakib. 2024. Trichoderma species in vitro and in planta inhibition against Ganoderma boninense, a causative agent of basal stem rot in oil palm (Elaeis guineensis). Sarhad Journal of Agriculture, 40(Special issue 1): 173-185.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40/s1.173.185
Keywords | Biocontrol, Dual culture assay, Pathology, Trichoderma virens, Trichoderma asperellum, Volatile compounds
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The African oil palm (Elaeis guineensis Jacq.) is an oil-producing plant that is mainly cultivated in tropical regions, particularly in Indonesia and Malaysia, that accounts for 60% and 25% of the world’s vegetable oil supply, respectively (USDA, 2022). The region’s oil palm faces a severe threat from basal stem rot (BSR), also called Ganoderma disease, which is primarily caused by Ganoderma boninense, a destructive disease caused by the phytopathogenic basidiomycete fungus (Rees et al., 2012; Rakib et al., 2014). Oil palm trees that are infected usually experience decay at the base of the stem, leading to a collapse, and eventual death (Hushiarian et al., 2013; Alam et al., 2015). The disease is estimated to cause economic losses as high as 68% due to the loss of productive oil palm trees and the expenses associated with controlling the disease (Assis et al., 2021). Although BSR in oil palm was discovered more than 50 years ago, it remains a major threat to the sustainability of the industry despite numerous efforts to find prophylactic and curative treatments for the disease.
However, it is strongly recommended that integrated disease management be implemented to minimise economic losses due to the disease. This can be achieved through various disease control strategies, including biological control agents (BCAs), chemical treatments, and cultural practices (Susanto et al., 2005; Lim et al., 2013). In this regard, BCAs are considered a sustainable solution for addressing plant disease management as they are non-toxic and environmentally friendly (Cornejo et al., 2020; Javeed et al., 2021).
The genus Trichoderma is mentioned in most of the literature on the biological control of plant diseases. Its usefulness has been known since 1930 (Weindling, 1934). Today, modern technologies use Trichoderma for biological control of various diseases. According to Zin and Badaluddin (2020), Trichoderma species that are extensively utilised as BCAs in bio-fungicides include T. asperellum, T. harzianum, and T. atrovitide, and to a lesser extent are T. virens, T. afroharzianum, T. gamsii, and T. polysporum. For numerous years, Trichoderma species have been used as natural enemies to combat plant diseases because of their capability to exert antagonistic behavior (Harman, 2006).
Antagonistic activity is based on both direct and indirect processes, such as rivalry for resources and space, mycoparasitic activity, antibiosis, and the development of systemic resistance to combat pathogens and promote plant growth. They act at the soil, leaf, and root levels by producing and releasing various compounds that can induce local or systemic resistance to abiotic and biotic stress factors in plants (Zaidi et al., 2014; Birkenbihl et al., 2017). The ecological success of these fungi is the result of a combination of mechanisms that the plants activate (Harman et al., 2004).
The efficacy of Trichoderma species has been evaluated in both individual and combined, and laboratory and nursery trials, demonstrating its effectiveness in disease reduction. However, the performance of a BCA is often insufficient when only a single BCA or organism is used (Harshita et al., 2018). Traditionally, mixed fungal cultures have been avoided as populations are difficult to predict, although a compatible mixed culture can have synergies and significantly increase the efficacy of the product. Nowadays, more emphasis is placed on the use of combined biological control agents with multiple mechanisms that could increase the success of biological control (Collinge et al., 2022).
Despite this, the study was conducted to evaluate the antagonistic activity of local Trichoderma isolates against G. boninense, and the efficacy of selected local single, and mixed Trichoderma isolates in controlling the infection of G. boninense, in planta on oil palm seedlings.
Materials and Methods
Isolation of Trichoderma species
The feather roots of healthy oil palm trees collected randomly from a local oil palm estate located in Sandakan, Sabah, Malaysia, yielded Trichoderma species that were isolated from the rhizosphere. A total of 20 Trichoderma isolates were recovered using potato dextrose agar (PDA), following the isolation procedure described by Siddique et al. (2009). The pure culture of Trichoderma isolates was identified based on the characteristics described by Baca et al. (2022), including the formation of greenish to yellowish conidia, and concentric rings, with rapid mycelial growth and loosely arranged conidia.
In vitro antagonistic activities of Trichoderma species against Ganoderma boninense
Dual-culture assay: All the Trichoderma isolates were screened for their inhibitory effects on the growth of Ganoderma via a dual-culture assay (Rahman et al., 2009). The isolate of Ganoderma boninense (G4) utilised in this research was acquired from a pure culture maintained in the Faculty of Sustainable Agriculture, Universiti Malaysia Sabah (Malaysia), and identified previously as Ganoderma boninense based on internal transcribed spacer (ITS) gene sequences. Plugs of Trichoderma isolate and G. boninense, each 8 mm in diameter, were placed 2 cm from the periphery of a Petri dish with a 9 cm diameter, on opposite sides. A plate inoculated with G. boninense, without Trichoderma as the antagonist was served as the control. The plates were incubated at 28 °C temperature for seven days in darkness. The experiment was conducted in three replicates and arranged in a completely randomised design (CRD). The radial growth of G. boninense was measured, and the percentage inhibition of radial growth (PIRG) was calculated using Equation 1 (Bivi et al., 2010).
Where R1 is the radial growth of G. boninense in control and R2 is the radial growth of G. boninense challenged with Trichoderma isolate.
Volatile organic compounds (VOCs) dual-plate assays
The VOCs assay was carried out to assess the inhibiting effect of Trichoderma isolates on G. boninense through their production of volatile compounds (Vila et al., 2017). The dual-plate assay was carried out by Rajani et al. (2021). Each Trichoderma isolate was infected with a mycelial plug (8 mm in diameter) in the centre of a Petri dish containing PDA (9 cm in diameter), and the dish was then incubated at 28 °C for seven days under dark conditions. Subsequently, a comparable-sized mycelial plug of G. boninense was inoculated onto a different PDA plate, positioned in an inverted manner above the Trichoderma isolates that were seven days old, and sealed using parafilm. The plates without Trichoderma isolate served as a control. The plates were incubated at 28 °C for seven days in darkness. The experiment was performed in a CRD arrangement with three replications. The diameter growth of G. boninense was measured, and the percentage inhibition of the diameter growth (PIDG) was calculated using Equation 2 (Ting et al., 2010).
Where D1 is the diameter growth of G. boninense in the control plate, and D2 is the diameter growth of G. boninense with Trichoderma isolates.
Molecular identification of the potential Trichoderma isolates
The Trichoderma isolates that showed potential as BCA candidates based on the in vitro assays were identified based on the ITS gene according to the protocol described by Darlis et al. (2023), which include the genomic deoxyribonucleic acid (gDNA) extraction, polymerase chain reaction (PCR), and purification and sequencing of the PCR products. The sequence was employed to identify homologous sequences through the Basic Local Alignment Search Tool (BLAST) hosted on the National Center for Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/).
In planta antagonistic activities of Trichoderma species against Ganoderma boninense
Among the isolates, T4RH and T8R were selected for further in planta experiments based on their significance in inhibition against G. boninense via in vitro dual-culture and VOCs dual-plate assays. The efficacy of both Trichoderma isolates in suppressing G. boninense infection in oil palm seedlings (in planta) was evaluated singly and in combination with untreated negative and positive controls. A 3-month-old (dura × pisifera) oil palm seedling was inoculated with a 6×6×6 cm Ganoderma-colonised rubber wood block inoculum prepared as described by Nusaibah et al. (2016). The inoculation was performed by placing the inoculum in a planting hole in a polyethylene planting bag (30×38 cm) containing a soil mixture (non-sterile soil and organic matter in a 2:1 ratio) before transplanting the seedling on top of the inoculum (Rakib et al., 2015). Trichoderma’ conidia suspension prepared according to the protocol described by Jinantana and Sariah (1998) was immediately applied after the inoculation. All five treatments in this experiment are summarised in Table 1. Each treatment has three replications, and each replicate consisted of 10 seedlings, which resulted in 150 experimental units. All experimental units were arranged in a completely randomised design (CRD) in a nursery facility at the Malaysian Palm Oil Board (MPOB) Research Station, Lahad Datu, Sabah. The experiment was conducted for nine months. The seedlings were watered twice a day, and basal fertilizer was applied monthly according to the recommended practices (MPOB, 2016).
Table 1: Description of treatments.
|
Code |
Treatment |
Description |
|
T1 |
Untreated (negative control) |
Non-inoculated seedlings, and without any treatment. |
|
T2 |
Untreated (positive control) |
Ganoderma-inoculated seedlings, and without any treatment. |
|
T3 |
Trichoderma virens |
Ganoderma-inoculated seedlings and treated with locally isolated T. virens. Conidial suspension of 106 was applied once by drenching 100 mL onto the soil after transplanting. |
|
T4 |
Trichoderma asperellum |
Ganoderma-inoculated seedlings and treated with locally isolated T. asperellum. Conidial suspension of 106 was applied once by drenching 100 mL onto the soil after transplanting. |
|
T5 |
T. virens and T. asperellum |
Ganoderma-inoculated seedlings and treated with a mixture of locally isolated T. virens and T. asperellum. Conidial suspension of 106 was applied once by drenching 100 mL onto the soil after transplanting. |
Table 2: Classes of diseases in oil palm seedlings and their associated symptoms can be identified based on a numerical value.
|
Class |
Symptoms |
|
0 |
An oil palm seedling that is healthy, with green leaves and no signs of fungal growth on any part of the plant. |
|
1 |
The presence of 1-3 chlorotic leaves on the oil palm seedling, and there is no fungal mass development on any part of the plant. |
|
2 |
The fungal mass may be present with or without leaves showing chlorosis (yellowing). |
|
3 |
The oil palm seedling may exhibit more than 3 yellowish (chlorotic) leaves and dead (necrotic) leaves, which may or may not have fungal mass on any part of the plant. |
|
4 |
Severe chlorosis or necrosis is present in at least 50% of the total leaf count, with or without fungal mass. |
|
5 |
Oil Palm seedling that are dead, with or without a fungal mass. |
Source: Rakib et al. (2015).
Data collection
Disease signs and symptoms of the Ganoderma infection were assessed at monthly intervals over a 9-month period. Disease incidence (DI), disease severity index (DSI) based on the disease class described in Table 2, the area under disease progress curve (AUDPC), and the percentage of necrotic primary roots were recorded at the end of the 9-month experiment and calculated according to Rakib et al. (2015). The DI, DSI, AUDPC, percentage of necrotic primary roots, and percentage of disease reduction were calculated using Equations 3 to 7. In addition, the oil palm seedlings physiological data were recorded. The plant height, bole or stem diameter, number of fronds, and chlorophyll content were recorded at the end of the nursery trials. The chlorophyll content was recorded using a single photon avalanche diode (SPAD) chlorophyll device (Rakib et al., 2019).
Where A is the disease class (0 to 5), B is the number of seedlings showing the disease class per treatment, n is the total number of replications, and 5 is the constant representing the highest class of assessment.
Where n is the number of assessment times, Y is the disease incidence, and t is the observation time.
Where; X1 is the AUDPC of positive control (T2), and X2 is the AUDPC of the treated seedlings.
Statistical analysis
All the data were subjected to a one-way analysis of variance (ANOVA), and the differences among the means were determined for significance at p ≤ 0.05 level using Turkey’s test. The statistical analysis was performed using SPSS statistical software (version 26).
Results and Discussion
In vitro antagonistic activities of Trichoderma species against Ganoderma boninense
All 20 Trichoderma isolates were successfully isolated from the rhizosphere of feather roots of healthy oil palms. Generally, the mycelium of Trichoderma species appeared transparent (hyaline) or white on PDA, and the reverse usually with a pale yellowish colour. The colony primarily appeared green and yellow, with rapid growth, similar to those described by Baca et al. (2022). It was found that all Trichoderma isolates inhibited the mycelial growth of G. boninense, with a PIRG (%) value greater than 60% as shown in Table 4. Notably, two Trichoderma isolates, T4RH and T8R had the highest PIRG values of 92.08% and 91.09%, respectively. Both Trichoderma isolates are shown in Figure 1, which varied in terms of their appearance on PDA. The isolate T4RH has dense mycelia with greenish-to-yellowish conidia, while T8R has hyaline mycelium with greenish conidia. Molecular identification confirmed the identity of the isolate T4RH as Trichoderma virens, and T8R as Trichoderma asperellum as summarised in Table 3. Similar findings were reported by Shamala (2013), where Trichoderma isolates inhibited the mycelial growth of G. boninense by more than 60% and, recorded the highest PIRG values for T. virens and T. asperellum. This finding also corresponds to various studies that have reported that Trichoderma species can suppress the growth of a range of phytopathogens, including Rhizoctonia solani, Alternaria alternata, Curvularia lunata, Fusarium oxysporum, G. boninense, Sclerotium. rolfill, Sclerotinia sclerotiorum, and Sclerotium cepivorum (Shamala, 2013; John et al., 2015; Hirpara et al., 2017).
Trichoderma species have shown promising effectiveness in controlling fungal phytopathogens through direct mechanisms like antibiosis and mycoparasitism, as well as indirect mechanisms such as competing for nutrients, enhancing plant defense responses, and promoting plant growth (Dukare et al., 2022). The strong effectiveness of Trichoderma isolates against the pathogenic fungal strains responsible for BSR in oil palm was confirmed in the current study. All Trichoderma isolates inhibited G. boninense, grew at a faster rate, and showed varying levels of inhibition, likely attributable to genetic variability (Debbi et al., 2018). The cause of these antagonistic behaviors may be mycoparasitism and competition for space and nutrients, as demonstrated by the dual culture assay. Mycoparasitism is one of the mechanisms of Trichoderma sp. to protect plants against pathogen attack, which involves hyphal interaction and parasitism. The different hyperparasitic potentials of various isolates of Trichoderma have been reported (Prasad and Rageswaram, 1999; Sankar and Sharma, 2001; Pan and Bhagat, 2007). This is because the individual species or isolates produce different amounts of hydrolytic enzymes when they attack the mycelium of the pathogen. The ability of Trichoderma bioagents to break down the hyphae of phytopathogenic fungi may be attributed to various external enzymes, including chitinases, and cellulases. These enzymes aid in the penetration of Trichoderma bioagents into the hyphae of phytopathogens (Bhat, 2017; Rajani et al., 2021). Trichoderma species exhibit highly competitive biological activities due to their rapid colonization rates, which in turn reduce the presence of competing microbes (Bizos et al., 2020). In addition, the proliferation of G. boninense might be inhibited by the metabolites generated by Trichoderma species, as they are known for synthesizing various antibiotics such as trichodernin and herzianolides using secondary metabolites, which are crucial in the antibiosis mechanism (Küçük and Kıvanç, 2004; Khan et al., 2020; Tomah et al., 2020).
Table 3: Identity of the isolates T4RH and T8R based on the similarity between sample sequences and the sequences in GenBank.
|
Isolate code |
Accession number (NCBI) |
Identity |
ITS fragment length |
Homology |
BLAST bit score |
|
T4RH |
KJ739790.1 |
Trichoderma virens |
615 base pairs |
99.84% |
1104 |
|
T8R |
MF774876.1 |
Trichoderma asperellum |
603 base pairs |
100% |
1088 |
Table 4: Percentage inhibition of radial growth (PIRG) and Percentage inhibition of diameter growth (PIDG) of Ganoderma boninense based on in vitro dual-culture and volatile organic compounds (VOCs) dual-plate assays.
|
Trichoderma isolates |
PIRG in dual-culture assay (%) |
PIDG in dual-plate assay (%) |
|
T3R |
87.13 ±1.98abcd |
66.07 ±6.48abc |
|
T5R |
82.18 ±1.71abcdef |
50.89 ±6.58bc |
|
T6R |
85.15 ±2.97abcdef |
53.57 ±5.70abc |
|
T8R |
91.09 ±1.71ab |
42.41 ±9.87c |
|
T11R |
86.14 ±2.62abcde |
79.91 ±2.05a |
|
T12R |
78.22 ±0.99cdef |
46.88 ±5.26bc |
|
T15R |
75.25 ±2.62f |
53.57 ±1.61abc |
|
T1RH |
83.17 ±0.99abcdef |
52.68 ±1.18abc |
|
T3RH |
88.12 ±1.71abc |
50.89 ±5.15bc |
|
T4RH |
92.08 ±2.62a |
75.45 ±1.95ab |
|
T5RH |
81.19 ±0.99bcdef |
70.09 ±7.35abc |
|
T7RH |
84.16 ±0.99abcdef |
41.52 ±7.82c |
|
T9RH |
82.18 ±1.71abcdef |
45.09 ±5.41c |
|
T10RH |
85.15 ±1.71abcdef |
58.48 ±5.41abc |
|
T11RH |
77.23 ±0.99def |
63.84 ±6.14abc |
|
T3S |
64.36 ±3.43g |
63.84 ±4.31abc |
|
TSF |
76.24 ±2.97ef |
66.96 ±1.95abc |
|
G18R |
86.14 ±0.99abcde |
42.41 ±7.73c |
|
TFPL |
81.19 ±0.99bcdef |
41.52 ±3.13c |
|
TThai |
90.10 ±2.62ab |
57.59 ±0.45abc |
Note: Means (± Standard error) followed by different letters within the column were significantly different at p ≤ 0.05 by Tukey’s test.
The inhibitory potential of Trichoderma isolates against G. boninense was also evaluated using a volatile compounds (VOCs) assay. The dual-plate assay was used to evaluate the effect of volatile antifungal compounds released by Trichoderma species (Vila et al., 2017). The study discovered that production of volatile compounds effectively by Trichoderma inhibited the growth of G. boninense. Isolate T4RH demonstrated the highest inhibition of G. boninense at 75.45%, whereas isolate T8R showed a lower inhibition at 45.4%. Previous research has reported comparable findings, demonstrating that the growth of various phytopathogens was inhibited by the volatile compounds generated by Trichoderma species (Shamala, 2013; Marques et al., 2018; Baiyee et al., 2019).
This production is an antibiosis mechanism within the Trichoderma species. The production released by Trichoderma species can benefit the host plant by exerting an antifungal effect and providing protection against plant pathogens. The production of volatile compounds such as pyrones and sesquiterpenes has been described as a possible mechanism by Trichoderma sp. (Reino et al., 2008). In addition, the VOCs phenlethyl alcohol (PEA) have been reported to be produced by several Trichoderma species, such as T. alroviride, T. harzianum, T. spirale, and T. virens, which exhibit antifungal abilities against various plant pathogens (Stoppacher et al., 2010; Siddiquee et al., 2012; Liu et al., 2014; Baiyee et al., 2019). PEA also has strong antifungal activity against G. boninense (Angel et al., 2016). Siddiquee et al. (2012) reported a few other fungal volatile metabolites with antifungal efficacy against pathogenic fungi including aromatic metabolites, pyrones, terpenes, butenolides, and polyketides. The metabolites could inhibit the production of ergosterol (the primary sterol in fungi) and also impede the synthesis of macromolecules, resulting in toxicity to plant pathogens (Ghannoum and Rice, 1999).
This study discovered that different Trichoderma isolates may produce various amounts and toxicity of VOCs, resulting in varying levels of pathogen inhibition. Even while both T. virens T4RH and T. asperellum T8R have demonstrated the potential ability to inhibit the growth of G. boninense G4 in vitro, the results are insufficient to conclude that these isolates are suitable BCAs for application in the field. Therefore, both T4RH and T8R were selected for further examination in planta to gain comprehensive and conclusive data as screening methods for selecting potential BCAs.
In planta antagonistic activities of Trichoderma species against Ganoderma boninense
The results of the in plant trials in the nursery showed that treatment with the local Trichoderma isolates T3, T4, and T5 significantly reduced the signs and symptoms of disease in seedlings infected with G. boninense compared to the untreated positive seedlings (T2). The untreated negative control (T1) showed no signs and symptoms of disease. The application of a single Trichoderma species, either T. virens or T. asperellum, showed similar efficacy in planta inhibition of G. boninense infection. Compared to the positive control, both T. virens and T. asperellum significantly reduced DI, DSI, AUDPC, and the percentage of necrotic primary roots by 73.33–76.67%, 30–34%, 41.67–55.00 unit, and 41.05–42.81%, respectively, when applied singly. While mixed applications of T. virens and T. asperellum have lower efficacy than T. virens alone. This was evident in the decrease of DI and DSI by 13.34% and 8%, respectively, as shown in Figure 2.
This finding is consistent with the work of You et al. (2016), who attributed the ability of Trichoderma to control plant diseases to various mechanisms, including competition, mycoparasitism, the formation of a restrictive structure, antibiosis, secondary metabolite formation, as well as the induction of resistance in plants. The antagonistic mechanisms are not mutually exclusive, and a specific mechanism may belong to multiple categories. Additionally, Trichoderma species have been reported to have rapid growth, thus colonizing the substrate faster, and reducing the activity of other fungi by occupying and depleting the substrate (Martin and Loper, 1999; Bizos et al., 2020). The beneficial microorganisms fight against infections at infection sites, reducing the chances for pathogens to proliferate, and produce secondary metabolites on the plant surface, and allowing them to parasitise directly. Moreover, Trichoderma species are known to produce various enzymes, such as chitinases, proteases, and cellulases, that can degrade pathogen cell walls and reduce pathogen virulence (Musa et al., 2017). Some strains of Trichoderma can also produce secondary metabolites, such as peptaibols, gliotoxin, harzianic acid, and trichokonins, which exhibit antifungal activity against a wide range of plant pathogens (Harman et al., 2004). Furthermore, Trichoderma sp. can induce plant systemic resistance which is a long-lasting and broad-spectrum resistance against a variety of pathogens. This resistance is achieved by activating the plant’s defense mechanisms, such as the production of phytoalexins, pathogenesis-related proteins, and lignin (Harman et al., 2004).
The relatively lower efficacy reported in this study in inhibiting G. boninense infection by the mixed application of two Trichoderma species is shown in Figure 1. This is consistent with a study conducted by Shamala (2013), which was associated with interspecific competition between the two isolates. Behavioral or chemical mechanisms that restrict one organism’s access to the substrate are known as interference competition, and they can result in mycelial interactions within or between different species (Wicklow, 1992). The non-synergistic effect of T. virens and T. asperellum did not result in significant disease suppression due to the strong antagonism between the isolates. Although mixed inoculation of biological control agents cannot be completely disregarded, it is suggested that a combination of compatible isolates or a mixture of species plays a crucial role in studying the effect of single or mixed Trichoderma isolates. The use of compatible isolates or a mixture of species may be necessary to achieve the desired effect in controlling diseases in crops.
As shown in Table 5, the suppression of G. boninense infection by Trichoderma species (in T3, T4, and T5) has been attributed to significantly higher chlorophyll content, plant height, bole diameter, and number of fronds compared with untreated positive control (T2). However, no significant difference was found among different Trichoderma treatments. The highest levels of chlorophyll content (60.83%), plant height (118.33 cm), and palm diameter (19.50 cm), were
Table 5: Chlorophyll content, plant height, bole diameter, and number of fronds of oil palm seedlings after nine months of inoculated with Ganoderma boninense.
|
Treatment |
Chlorophyll content (SPAD value) |
Plant height (cm) |
Bole diameter (cm) |
Number of fronds |
|
T1: Untreated (Negative control) |
60.83±0.64a |
118.33±1.59a |
19.50±0.21a |
7±0.33a |
|
T2: Untreated (Positive control) |
36.26±2.09c |
83.33±0.67c |
14.43±0.59c |
5±0.33c |
|
T3: T. virens |
51.37±1.67b |
101.77±1.19b |
17.37±0.52b |
6±0.58ab |
|
T4: T. asperellum |
51.72±1.62b |
98.82±1.69b |
16.27±0.20b |
6±0.58ab |
|
T5: T. virens and T. asperellum |
49.50±0.37b |
96.00±3.06b |
15.83±0.24bc |
6±0.33ab |
Note: Means (± standard error) followed by different letters within the column were significantly different at p≤ 0.05 by Tukey’s test.
recorded in the uninfected and untreated seedlings (T1). The relatively better physiological characteristics of Trichoderma-treated oil palm seedlings may be related to several factors, including direct colonisation, increased positive interaction with the plant, and increased nutrient uptake by the plant due to reduced activity of the pathogen (Harman, 2000). Studies have shown that oil palm seedlings inoculated with T. virens and T. asperellum have increased leaf and root biomass compared to the control (Shamala, 2013). The capability Trichoderma species to secrete plant growth-promoting hormones like auxins, gibberellins, and cytokinins may attributed to this phenomenon, as these substances promotes plant growth and development (Cornejo et al., 2009). In addition, Trichoderma can also indirectly promote plant growth by inducing systemic resistance to pathogens. The induction of systemic resistance can increase the plant’s defenses against pathogenic attacks, resulting in healthier and more vigorous plants (Benhamou, 1996; Yu et al., 2022). The production of volatile organic compounds and hydrolytic enzymes by Trichoderma can also stimulate plant growth by enhancing nutrient acquisition and root growth (Hermosa et al., 2013).
It is noteworthy that the isolated T. virens and T. asperellum were able to increase plant growth of oil palm seedlings in terms of height, stem diameter, number of fronds, and chlorophyll content in the present work. These findings agreed with those reported in other studies (Nusaibah et al., 2016; Shamala, 2013; Muniroh et al., 2019). T. virens and T. asperellum can produce IAA, the most active auxin (Li et al., 2018; Muniroh et al., 2019; Inayati et al., 2021). IAA is a phytohormone that promotes plant development and growth both directly and indirectly, primarily through the modulation of other phytohormones, such as gibberellic acid (Stewart and Hill, 2014). Directly, IAA enhances root system development by increasing the number of lateral and adventitious roots, improving nutrient uptake, and stimulating root exudation, thereby providing additional resources for soil microbes to interact with the roots (Gamalero and Glick, 2011). In addition to improving the root system, IAA even in lower concentrations can directly enhance growth by promoting cell division and elongation (Brummell and Hall, 1987), and it has also been reported to enhance the fitness of plant-microbe interactions (Patten and Glick, 2002). This might contribute to an increase in plant performance in nursery trials. Therefore, it would be very useful to use these Trichoderma strains, which have been shown to be effective in controlling Trichoderma disease and promoting plant growth.
Conclusions and Recommendations
In conclusion, this study has identified two Trichoderma species, namely T. virens (T4RH) and T. asperellum (T8R), as potential BCAs against G. boninense. The promising results of in vitro assays revealed that both T. virens and T. asperellum exhibited high levels of inhibition against G. boninense. Moreover, in planta assessment of the local Trichoderma isolates showed significant suppression of G. boninense infection. The outcomes of this study suggest that T. virens T4RH and T. asperellum T8R can be considered effective BCAs to manage G. boninense infection. These findings contribute to the advancement of sustainable and environmentally friendly strategies for mitigating the detrimental effects of G. boninense on oil palm cultivation. However, further analyses, characterisation, and validation assays are necessary to substantiate and confirm the potential of the local Trichoderma isolates as effective BCAs against G. boninense.
Acknowledgments
Special thanks go to the Universiti Malaysia Sabah (UMS) and Wilmar Plantations Sdn. Bhd. (formerly known as PPB Oil Palms Berhad) for their support in granting research funding through the Research Collaboration Grant (GKP0026-2019). The author also would like to thank the Malaysian Palm Oil Board (MPOB) Lahad Datu Research Station for allowing to use of their research facility to conduct the nursery trial and the Sabah Agriculture Department for providing oil palm planting material.
Novelty Statement
Local isolates of Trichoderma species from Sabah, Malaysia, were identified as potential biological control agents against Ganoderma boninense, a phytopathogen causing basal stem rot in oil palm. These potential BCAs could be mass-produced to further developed as a commercial product.
Author’s Contribution
Nur Aainaa Hasbullah, Mohamadu Boyie Jalloh, Palanivell Perumal, Peter Mojiun, and Mohd. Rashid Mohd. Rakib: Conceptualised the ideas.
Jumiati Asis, Nur Aainaa Hasbullah, Mohamadu Boyie Jalloh, and Mohd. Rashid Mohd. Rakib: Designed the methodology.
Jumiati Asis: Wrote the original draft, performed the statistical analysis, investigation
Mohd. Rashid Mohd. Rakib, Palanivell Perumal and Peter Mojiun: Provided the research materials, reviewed and edited the draft.
Jumiati Asis, Noor Khairani Mohamad Basri: Reviewed and edited the draft.
Mohd. Rashid Mohd. Rakib and Nur Aainaa Hasbullah: supervised the research.
Mohd. Rashid Mohd. Rakib: Administered the project, acquired research funds.
All authors read and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Alam, A.F., A.C. Er and H. Begum. 2015. Malaysian oil palm industry: Prospect and problem. J. Food, Agric. Environ., 13(2): 143-148.
Angel, L.P.L., M.T. Yusof, I.S. Ismail, B.T.Y. Ping, I.N.A. Mohamed Azni, N.H. Kamarudin and S. Sundram. 2016. An in vitro study of the antifungal activity of Trichoderma virens 7b and a profile of its non-polar antifungal components released against Ganoderma boninense. J. Microbiol., 54(11): 732-744. https://doi.org/10.1007/s12275-016-6304-4
Assis, K., C.K. Phin, I.A. Seman, D. Gabda and H.C. Mun. 2021. Estimating the yield loss of oil palm due to Ganoderma basal stem rot disease by using bayesian model averaging. J. Oil Palm Res., 33(1): 46-55.
Baca, M.A.M, C.U. García, S.P. Álvarez, M.A.F. Córdova, C.M.E. Bonilla, M.A.M. Tapia and E.S. Chávezd. 2022. Morphological and molecular characterization of a new autochthonous Trichoderma sp. isolate and its biocontrol efficacy against Alternaria sp. Saudi. J. Biol. Sci., 29(4): 2620-2625. https://doi.org/10.1016/j.sjbs.2021.12.052
Baiyee, B., C. Pornsuriya, S.I. Ito and A. Sunpapao. 2019. Trichoderma spirale T76-1 displays biocontrol activity against leaf spot on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol. Cont., 129: 195-200. https://doi.org/10.1016/j.biocontrol.2018.10.018
Benhamou, N., 1996. Elicitor-induced plant defense pathways. Trends. Plant Sci., 1(9): 233-240. https://doi.org/10.1016/S1360-1385(96)86901-0
Bhat, K.A., 2017. A new agar plate assisted slide culture technique to study mycoparasitism of Trichoderma sp. on Rhizoctonia solani and Fusarium oxysporium. Int. J. Curr. Microbiol. Appl. Sci., 6(8): 3176-3180. https://doi.org/10.20546/ijcmas.2017.608.378
Bio-Pesticide Data Base. 2021. Bio-Pesticide Database, University of Hertfordshire, Hatfield, Hertfordshire, UK (http://sitem.herts.ac.uk/aeru/bpdb/). Retrieved on Sep 20, 2022.
Birkenbihl, R.P., S. Liu and I.E. Somssich. 2017. Transcriptional events defining plant immune responses. Curr. Opin. Plant Biol., 38: 1-9. https://doi.org/10.1016/j.pbi.2017.04.004
Bivi, M.R., M.S.N. Farhana, A. Khairulmazmi and A. Idris. 2010. Control of Ganoderma boninense: A causal agent of basal stem rot disease in oil palm with endophyte bacteria in vitro. Int. J. Agric. Biol., 12(6): 833-839.
Bizos, G., E.M. Papatheodorou, T. Chatzistathis, N. Ntalli, V.G. Aschonitis and N. Monokrousos. 2020. The role of microbial inoculants on plant protection, growth stimulation, and crop productivity of the olive tree (Olea europea L.). Plants, 9(6): 743. https://doi.org/10.3390/plants9060743
Brummell, D.A. and J.L. Hall. 1987. Rapid cellular responses to auxin and the regulation of growth. Plant, Cell Environ., 10(7): 523-543. https://doi.org/10.1111/j.1365-3040.1987.tb01833.x
Collinge, D.B., D.F. Jensen, M. Rabiey, S. Sarrocco, M.W. Shaw and R.H. Shaw. 2022. Biological control of plant diseases. What has been achieved and what is the direction? Plant Pathol., 71: 1024-1047. https://doi.org/10.1111/ppa.13555
Cornejo, H.A.C., L.M. Rodríguez, C.C. Penagos and J.L. Bucio. 2009. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol., 149(3): 1579-1592. https://doi.org/10.1104/pp.108.130369
Cornejo, H.A.C., L.M. Rodríguez, E. del-Val and J. Larsen. 2020. Interactions of Trichoderma with plants, insects, and plant pathogen microorganisms: chemical and molecular bases. Co-evolution of secondary metabolites. 1sted. Springer, Mexico.
Darlis, D., M.B. Jalloh, C.F.S. Chin, N.K.M. Basri, N.A. Besar, K. Ahmad and M.R.M. Rakib. 2023. Exploring the potential of Bornean polypore fungi as biological control agents against pathogenic Ganoderma boninense causing basal stem rot in oil palm. Sci. Rep., 13: 10316. https://doi.org/10.1038/s41598-023-37507-0
Debbi, A., H. Boureghda, E. Monte and R. Hermosa. 2018. Distribution and genetic variability of Fusarium oxysporum associated with tomato diseases in Algeria and a biocontrol strategy with indigenous Trichoderma spp. Front. Microbiol., 9: 282. https://doi.org/10.3389/fmicb.2018.00282
Dukare, A.S., R.K. Singh, R.K. Jangra and B. Bhushan. 2022. Non-fungicides-based promising technologies for managing post-production Penicillium induced spoilage in horticultural commodities: A comprehensive review. Food Rev. Int., 38(3): 227-267. https://doi.org/10.1080/87559129.2020.1727497
Gamalero, E. and B.R. Glick. 2011. Mechanisms used by plant growth-promoting bacteria. Bacteria in agrobiology: Plant nutrient management. 1st edn. Springer, Italy. https://doi.org/10.1007/978-3-642-21061-7_2
Ghannoum, M.A. and L.B. Rice. 1999. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev., 12(4): 501-517. https://doi.org/10.1128/CMR.12.4.501
Harman, G.E., 2000. Myths and dogmas of biocontrol changes in perceptions derived from research on Trichoderma harzinum T-22. Plant Dis., 84(4): 377-393. https://doi.org/10.1094/PDIS.2000.84.4.377
Harman, G.E., 2006. Overview of mechanisms and uses of Trichoderma spp. Phytopathology, 96(2): 190-194. https://doi.org/10.1094/PHYTO-96-0190
Harman, G.E., C.R. Howell, A. Viterbo, I. Chet and M. Lorito. 2004. Trichoderma species opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol., 2(1): 43-56. https://doi.org/10.1038/nrmicro797
Harshita, A., J.B. Sinha, S. Khan, A. Trivedi and G. Rao. 2018. Compatibility of fungal and bacterial bio-agents and their antagonistic effect against Fusarium oxysporum f. Sp. Lycopersici. Int. J. Curr. Microbiol. Appl. Sci., 7(7): 2305-2316. https://doi.org/10.20546/ijcmas.2018.707.269
Hermosa, R., M.B. Rubio, R.E. Cardoza, C. Nicolás, E. Monte and S. Gutiérrez. 2013. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol., 16(2): 69-80.
Hirpara, D.G., H.P. Gajera, H.Z. Hirpara and B.A Golakiya. 2017. Antipathy of Trichoderma against Sclerotium rolfsii Sacc.: Evaluation of cell wall-degrading enzymatic activities and molecular diversity analysis of antagonists. Microb. Physiol., 27(1): 22-28. https://doi.org/10.1159/000452997
Hushiarian, R., N.A. Yusof and S.W. Dutse. 2013. Detection and control of Ganoderma boninense: Strategies and perspectives. Springer Plus, 2: 1-12. https://doi.org/10.1186/2193-1801-2-555
Inayati, A., L. Setyowati, L.Q. Aini and E. Yusnawan. 2021. Plant growth promoter produced by Trichoderma virens and its effect on mungbean (Vigna radiata (L.) Wilczek) seedling. IOP Conf. Ser. Earth Environ. Sci., 803: 012013. https://doi.org/10.1088/1755-1315/803/1/012013
Javeed, M.T., T. Farooq, A.S. Al-Hazmi, M.D. Hussain and A.U. Rehman. 2021. Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J. Invertebr. Pathol., 183: 107626. https://doi.org/10.1016/j.jip.2021.107626
Jinantana, J. and M. Sariah. 1998. Potential for biological control of Sclerotium foot rot of chilli Trichoderma spp. Pertanika J. Trop. Agric. Sci., 21(1): 1-10.
John, N.S., I.P. Anjanadevi, V.S. Nath, S.A. Sankar, M.L. Jeeva, K.S. John and R.S. Misra. 2015. Characterization of Trichoderma isolates against Sclerotium rolfsii, the collar rot pathogen of Amorphophallus. A polyphasic approach. Biol. Cont., 90: 164-172. https://doi.org/10.1016/j.biocontrol.2015.07.001
Khan, R.A.A., S. Najeeb, S. Hussain, B. Xie and Y. Li. 2020. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms, 8(6): 817. https://doi.org/10.3390/microorganisms8060817
Küçük, C. and M. Kıvanç. 2004. In vitro antifungal activity of strains of Trichoderma harzianum. Turk. J. Biol., 28(2): 111-115.
Li, Y.T., S.G. Hwang, Y.M. Huang and C.H. Huang. 2018. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot., 110: 275-282. https://doi.org/10.1016/j.cropro.2017.03.021
Lim, K.H., S.S. Lim, F. Parish and R. Suharto. 2013. RSPO manual on best management practices (BMPs) for existing oil palm cultivation on peat. 1st ed. RSPO, KL, Malaysia.
Liu, P., Y. Cheng, M. Yang, Y. Liu, K. Chen, C.A. Long and X. Deng. 2014. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol., 14(1): 1-14. https://doi.org/10.1186/s12866-014-0242-2
Malaysian Palm Oil Board (MPOB). 2016. MPOB code of practice - code of good nursery practice for oil palm nurseries. 2nd ed. MPOB, Malaysia.
Marques, E., I. Martins and S.C.M.D. Mello. 2018. Antifungal potential of crude extracts of Trichoderma spp. Biota Neotrop., 18(1): 1676-0611. https://doi.org/10.1590/1676-0611-bn-2017-0418
Martin, F.N. and J.E. Loper. 1999. Soilborne plant diseases caused by Pythium spp.: Ecology, epidemiology, and prospects for biological control. Crit. Rev. Plant Sci., 18(2): 111-181. https://doi.org/10.1016/S0735-2689(99)00389-5
Muniroh, M.S., S.A Nusaibah, G. Vadamalai and Y. Siddique. 2019. Proficiency of biocontrol agents as plant growth promoters and hydrolytic enzyme producers in Ganoderma boninense infected oil palm seedlings. Curr. Plant Biol., 20: 100116. https://doi.org/10.1016/j.cpb.2019.100116
Musa, H., M.A. Hassan, M.S. Isyaku, J. Halidu and A.S. Suleiman. 2017. Antagonistic potential of Trichoderma species against Ganoderma disease of oil palm. Niger. J. Agric. Food Environ., 13(2): 60-67.
Nusaibah, S.A., A.S.N. Akmar, A.S. Idris, M. Sariah and Z.M. Pauzi. 2016. Involvement of metabolites in early defense mechanism of oil palm (Elaeis guineensis Jacq.) against Ganoderma disease. Plant Physiol. Biochem., 109: 156-165. https://doi.org/10.1016/j.plaphy.2016.09.014
Pan, S. and S. Bhagat, 2007. Antagonistic potential of Trichoderma and Gliocladium spp. from West Bengal. J. Myco. Plant Pathol., 37(2): 235-239.
Patten, C.L. and B.R. Glick. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol., 68(8): 3795-3801. https://doi.org/10.1128/AEM.68.8.3795-3801.2002
Prasad, R.D. and R. Rangeshwaran, 1999. Granular formulation of Trichoderma and Gliocladium spp. in biocontrol of Rhizoctonia solani of chickpea. J. Mycol. Plant Pathol., 29(20): 222-226.
Rahman, M.A., M.F. Begum and M.F. Alam. 2009. Screening of Trichoderma isolates as a biological control agent against Ceratocystis paradoxa causing pineapple disease of sugarcane. Mycobiol., 37(4): 277-285. https://doi.org/10.4489/MYCO.2009.37.4.277
Rajani, P., C. Rajasekaran, M.M. Vasabthakumari, S.B. Olsson, D. Ravikanth and R.U Shaanker. 2021. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbial Res., 242: 126595. https://doi.org/10.1016/j.micres.2020.126595
Rakib, M.R.M., A.H. Borhan and A.N. Jawahir. 2019. The relationship between SPAD chlorophyll and disease severity index in Ganoderma-infected oil palm seedlings. J. Bangladesh Agric. Univ., 17(3): 355-358. https://doi.org/10.3329/jbau.v17i3.43211
Rakib, M.R.M., C.F.J. Bong, A. Khairulmazmi and A.S. Idris. 2014. Genetic and morphological diversity of Ganoderma species isolated from infected oil palms (Elaeis guineensis). Int. J. Agric. Biol., 16(4): 691-699.
Rakib, M.R.M., C.F.J. Bong, A. Khairulmazmi and A.S. Idris. 2015. Aggressiveness of Ganoderma boninense and G. zonatum isolated from upper-and basal stem rot of oil palm (Elaeis guineensis) in Malaysia. J. Oil Palm Res., 27(3): 229-240.
Rees, R.W., J. Flood, Y. Hasan, M.A. Wills and R.M. Cooper. 2012. Ganoderma boninense basidiospores in oil palm plantations: evaluation of their possible role in stem rots of Elaeis guineensis. Plant Pathol., 61(3): 567-578. https://doi.org/10.1111/j.1365-3059.2011.02533.x
Reino, J.L., R.F. Guerrero, R. Hernandez-Galan and I.G. Collado. 2008. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev., 7: 89-123. https://doi.org/10.1007/s11101-006-9032-2
Sankar, P. and R. Sharma. 2001. Management of charcoal rot of maize with Trichoderma viride. Indian Phytopath., 55(3): 390-391.
Shamala, S., 2013. The effects of Trichoderma in surface mulches supplemented with conidial drenches in the disease development of Ganoderma basal stem rot in oil palm. J. Oil Palm Res., 25(3): 314-325.
Siddiquee, S., B.E. Cheong, K. Taslima, H. Kausar and M.M. Hasan. 2012. Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC-MS using three different capillary columns. J. Chromatogr. Sci., 50(4): 358-367. https://doi.org/10.1093/chromsci/bms012
Siddiquee, S., U.K. Yusuf, K. Hossain and M.S. Jahan. 2009. In vitro studies on the potential Trichoderma harzianum for antagonistic properties against Ganoderma boninense. J. Food Agric. Environ., 7: 970-976.
Stewart, A. and R. Hill. 2014. Applications of Trichoderma in plant growth promotion. Chapter 31: 415-428. In: Biotechnology and biology of Trichoderma. Elsevier, New Zealand. https://doi.org/10.1016/B978-0-444-59576-8.00031-X
Stoppacher, N., B. Kluger, S. Zeilinger, R. Krska and R. Schuhmacher. 2010. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods, 81(2): 187-193. https://doi.org/10.1016/j.mimet.2010.03.011
Susanto, A., P.S. Sudharto and R.Y. Purba. 2005. Enhancing biological control of basal stem rot disease (Ganoderma boninense) in oil palm plantations. Mycopathologia, 159(1): 153-157. https://doi.org/10.1007/s11046-004-4438-0
Ting, A.S.Y., S.W. Mah and C.S. Tee. 2010. Identification of volatile metabolites from fungal endophytes with biocontrol potential towards Fusarium oxysporum F. sp. cubense race 4. Am. J. Agric. Biol. Sci., 5(2): 177-182. https://doi.org/10.3844/ajabssp.2010.177.182
Tomah, A.A., I.S. Abd Alamer, B. Li and J.Z. Zhang. 2020. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biol. Cont., 145: 104261. https://doi.org/10.1016/j.biocontrol.2020.104261
United States Department of Agriculture (USDA). 2022. Oilseeds: World markets and Trade. Washington, United States (www.usda.gov). Retrieved on Sep 20, 2023.
Vila, A.G., N. Teixidó, A.D. Francesco, J. Usall, L. Ugolini, R. Torres and M. Mari. 2017. Antifungal effect of volatile organic compounds produced by Bacillus amyloliquefaciens CPA-8 against fruit pathogen decays of cherry. Food Microbiol., 64: 219-225. https://doi.org/10.1016/j.fm.2017.01.006
Weindling, R., 1934. Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology, 24(11): 1153-1179.
Wicklow, D.T., 1992. Interference competition. The fungal community: Its organization and role in the ecosystem: 2nd ed. USA.
You, J., J. Zhang, M. Wu, L. Yang, W. Chen and G. Li. 2016. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biol. Cont., 101: 31-38. https://doi.org/10.1016/j.biocontrol.2016.06.006
Yu, Y., Y. Gui, Z. Li, C. Jiang, J. Guo and D. Niu. 2022. Induced systemic resistance for improving plant immunity by beneficial microbes. Plants, 11(3): 386. https://doi.org/10.3390/plants11030386
Zaidi, N.W., M.H. Dar, S. Singh and U.S. Singh. 2014. Trichoderma species as abiotic stress relievers in plants. In: Biotechnology and Biology of Trichoderma. Elsevier, India. Chapter 38: 515-525. https://doi.org/10.1016/B978-0-444-59576-8.00038-2
Zin, N.A. and N.A. Badaluddin. 2020. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci., 65(2): 168-178. https://doi.org/10.1016/j.aoas.2020.09.003
To share on other social networks, click on any share button. What are these?