Toxicity and Feeding Deterrence Properties of Selected Insecticides against Fall Armyworm (Spodoptera frugiperda J.E. Smith): Implications for Pest Management in Agriculture
Toxicity and Feeding Deterrence Properties of Selected Insecticides against Fall Armyworm (Spodoptera frugiperda J.E. Smith): Implications for Pest Management in Agriculture
Nimra Altaf1, Muhammad Irfan Ullah1*, Muhammad Afzal1,2, Muhammad Arshad1, Samina Khalid3, Naunain Mehmood4
1Department of Entomology, University of Sargodha, 40100, Sargodha, Pakistan
2Baba Guru Nanak University, Nankana Sahib, Pakistan.
3Department of Environmental Sciences, COMSATS University, Vehari, Pakistan
4Department of Zoology, University of Sargodha, 40100, Sargodha, Pakistan
Abstract | The Fall armyworm, Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae), is a significant agricultural pest that can cause extensive damage to maize crop. We determined the effectiveness of various insecticides against S. frugiperda and their ability to deter feeding. Radiant and voliam flexi had the highest mortality rates of 95.0% and 91.7%, respectively, at higher concentrations, while Coragen and Proclaim were moderately effective, with mortality rates ranging from 36.7% to 81.7%. The least effective insecticides were Match and Sunitol, with mortality rates ranging from 26.7% to 58.3%. Radiant (-24.57%) and Voliam flexi (-22.19%) were more efficient in terms of feeding deterrence as compared to other insecticides. These results indicate that Radiant and Voliam flexi could be useful in managing S. frugiperda infestations. This study provides essential information regarding the efficacy of commonly used insecticides against S. frugiperda, a highly detrimental agricultural pest with global prevalence.
Novelty Statement | In this study, we present a novel and promising approach to combat the devastating Fall armyworm, a notorious agricultural pest with a widespread global impact. Our study provides essential insights into the efficacy of widely employed insecticides, offering practical guidance for addressing a critical issue in the field of agriculture.
Article History
Received: June 20, 2023
Revised: October 05, 2023
Accepted: October 21, 2023
Published: November 03, 2023
Authors’ Contributions
NA performed experiments and data curation. NM and NA wrote the manuscript. MIU designed the experiment. M Afzal and MIU supervised the project. M Arshad and NM analysed the data. SK and M Afzal reviewed the final manuscript.
Keywords
Concentrations, Insecticides, Mortality, Pest control, Spodoptera frugiperda
Copyright 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding Author: Muhammad Irfan Ullah
Muhammad.irfanullah@uos.edu.pk
To cite this article: Altaf, N., Ullahm M.I., Afzal, M., Arshad, M., Khalid, S. and Mehmood, N., 2023. Toxicity and feeding deterrence properties of selected insecticides against fall armyworm (Spodoptera frugiperda J.E. Smith): implications for pest management in agriculture. Punjab Univ. J. Zool., 38(2): 163-169. https://dx.doi.org/10.17582/journal.pujz/2023.38.2.163.169
Introduction
The fall armyworm, Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae), is a major insect pest that poses a significant threat to various crops, such as maize, rice, sorghum, and cotton (Barros et al., 2010; Altaf et al., 2022). Severe damage occurs by feeding of larval stages of S. frugiperda on the leaves, stems, and reproductive parts of the host plants, resulting in significant yield losses and economic damage (Ganiger et al., 2018; Makgoba et al., 2021). The larvae cause damage by extensive tissue destruction and defoliation, particularly targeting the tender parts of the plant, such as young leaves, developing tassels, and silks, which reduces photosynthesis and nutrient uptake by the plants (De Almeida Sarmento et al., 2002) Furthermore, the feeding activity triggers several plant defense mechanisms, such as toxic secondary metabolite production and increased lignification of the cell walls, further reducing the nutritional value of the plant tissues (Divekar et al., 2022). The damage is often deteriorated by the migratory behavior of S. frugiperda larvae, which enables them to spread rapidly across large areas, causing significant crop losses and exacerbating food insecurity (Goergen et al., 2016).
S. frugiperda is responsible for significant economic losses, with estimates losses of $2.5 to $6.2 billion annually (Singh et al., 2022). In Africa, where S. frugiperda was first reported in 2016, has resulted in estimated losses of up to $6.1 billion in maize production (CABI, 2017). Similarly, in India, reports indicate losses of up to 25% in maize production due to this pest (Overton et al., 2021). The recent invasion of this new pest poses a significant threat to food security and livelihoods in many countries (Womack et al., 2020). Its presence has become widespread in more than 100 countries (He et al., 2021). The pest’s impact on global food security is a growing concern, and effective control measures are urgently needed to mitigate its damage. Hence, it is crucial to establish effective management strategies to control this pest and protect the livelihoods of farmers.
As an emergency response to combat invasive pests, many farmers tend to rely on traditional insecticides as their preferred method of control (Veres et al., 2020). Different classes of insecticides, such as pyrethroids, organophosphates, carbamates, and neonicotinoids, have been used to control S. frugiperda (Belay et al., 2012). Spinetoram has been reported to achieve over 90% control rates against S. frugiperda in corn in Brazil (Muraro et al., 2019), while chlorantraniliprole was found to be effective against S. frugiperda in maize in India (Deshmukh et al., 2020).
In tackling the invasive S. frugiperda population, the use of synthetic insecticides has proven to be an effective emergency-based control method and a crucial component of integrated pest management strategies (Kong et al., 2021). Consequently, evaluating the efficacy of synthetic insecticides against laboratory populations of S. frugiperda is a high-priority task (Ndolo et al., 2019). Hence, there is an urgent need to identify effective insecticides that can manage the infestation while reducing the associated environmental and health risks linked with insecticide overuse. This study aims to evaluate the efficacy of selected insecticides against S. frugiperda and provide insights for developing effective pest management strategies.
Materials and Methods
Insect colony
The collection of egg masses and larvae of S. frugiperda was done from a maize field (32°07’57.3”N 72°41’30.2”E) located at the research farm of the University of Sargodha. The collected eggs and larvae were then reared in the Entomology laboratory. The artificial diet was prepared and offered to neonate larvae. The diet was prepared as suggested by Sorur et al. (2011). After moths emerged, they were paired and confined to oviposition jars, and sugar solution (10%) was provided for feeding. To facilitate oviposition, muslin cloth was hung in plastic jars. The F3 generation of S. frugiperda was selected in further experiments.
Insecticides
In this study, three concentrations of each insecticide; Match (1.0ml/L, 2ml/L and 4ml/L), Voliam flexi (0.4ml/L, 0.8ml/L and 1.6ml/L), Proclaim (1.0ml/L, 2.0ml/L and 4.0ml/L), Coragen (0.25ml/L, 0.5ml/L and 1.0ml/L), Sunitol (2ml/L, 4ml/L and 8ml/L) and Radiant (0.5ml/L, 1.0ml/L and 2.0ml/L) were used. The detail of the selected insecticides, including their different groups and modes of action are presented in Table 1.
Table 1: Detail of selected insecticides tested against Spodoptera frugiperda larvae.
|
Trade name |
Active ingredient |
IRAC group |
Formulation |
Company name |
Mode of action |
Label dose (ml) |
|
Match |
Leufeneuron |
15 Benzoylureas |
50 EC |
Syngenta |
IGR |
200 |
|
Coragen |
Chlorantraniliprole |
1B Organophosphate |
20 SC |
FMC |
Muscle poison |
50 |
|
Proclaim |
Emmamectin benzoate |
6Avermectins |
019 EC |
Syngenta |
Muscle poison |
200 |
|
Voliam flexi |
Thiamethoxam+ chlorantraniliprole |
4A neonicotinoid+28 diamide |
300 EC |
Syngenta |
Nerve & muscle |
80 |
|
Radiant |
Spintoram |
5 Spinosyn |
120 SC |
Arysta |
Neurotoxic |
80 |
|
Sunitol |
Emmamectin+ Betacyfluthrin |
6 Emamectin benzoate + 3A Pyrethroids |
4.3 EC |
Tara group |
Nerve & muscle |
400 |
Table 2: Percent corrected mortality (means±SE) of Spodoptera frugiperda larvae after application of different insecticides at different time interval.
|
Treatment |
Conc. (ml/1000ml) |
Corrected larval mortality (%) |
|||
|
12 HAA |
24 HAA |
48 HAA |
72 HAA |
||
|
Radiant |
0.50 |
36.7±6.273a-d |
53.3±6.494b-e |
75.0±5.637abc |
78.3±5.363abc |
|
1.00 |
50.0±6.509ab |
66.7±6.137abc |
66.7±6.137a-d |
91.7±3.598a |
|
|
2.00 |
63.3±6.273a |
90.0±3.905a |
93.3±3.247a |
95.0±2.837a |
|
|
Voliam flexi |
0.40 |
13.3±4.425d |
33.3±6.137d-g |
41.7±6.418de |
50±6.509d-g |
|
0.80 |
35.0 ±6.209bcd |
58.3±6.418bcd |
66.7±6.137a-d |
70.0±5.966a-d |
|
|
1.60 |
51.7±6.505ab |
76.7±5.506ab |
91.7±3.598a |
91.7±3.598a |
|
|
Coragen |
0.25 |
20.0±5.207cd |
25.0±5.637efg |
35.0±6.209e |
38.3±6.329efg |
|
0.50 |
25.0±5.637bcd |
48.3±6.505b-f |
51.7±6.505cde |
53.3±6.494c-g |
|
|
0.10 |
45.0±6.476abc |
58.3±6.418bcd |
81.7±5.037ab |
81.7±5.037ab |
|
|
Proclaim |
1.00 |
13.3±4.425d |
21.7±5.363fg |
31.7±6.056e |
36.7±6.273efg |
|
2.00 |
21.7±5.363cd |
40.0±6.377c-g |
53.3±6.494b-e |
55.0±6.476b-f |
|
|
4.00 |
38.3±6.329a-d |
53.3±6.494b-e |
73.3±5.757abc |
80.0±5.207abc |
|
|
Match |
1.00 |
11.7±4.179d |
16.7±4.851g |
30.0±5.966e |
36.7±6.273efg |
|
2.00 |
11.7±4.179d |
16.7±4.851g |
35.0±6.209e |
40.0±6.377efg |
|
|
4.00 |
16.7±4.851d |
23.3±5.506fg |
50.0±6.509cde |
58.3±6.418b-e |
|
|
Sunitol |
2.00 |
13.3±4.425d |
23.3±5.506fg |
26.7±5.757e |
26.7±5.757g |
|
4.00 |
13.3±4.425d |
23.3±5.506fg |
28.3±5.866e |
30.0±5.966fg |
|
|
8.00 |
13.3±4.425d |
28.3±5.866efg |
35.0±6.209e |
35.0±6.209efg |
|
|
F10, 1079 |
1.94 |
2.17 |
2.66 |
2.07 |
|
|
P-value |
0.0363 |
0.017 |
0.0032 |
0.0245 |
|
P < 0.05 shows significance, HAA, H after application.
Mortality bioassay
The insecticidal toxicity of each insecticide was evaluated using the direct spray method on third instar larvae obtained from the rearing colony. Prior to application of the insecticide, larvae were starved for 24 h. Each concentration was replicated three times, with each replication consisting of 20 larvae. A volume of 5ml of each concentration was sprayed on Petri plates containing 60 larvae, and the treated larvae were then transferred to new Petri plates. Control larvae were treated with water. After washing, the fresh maize leaves were dried, and provided as food to the larvae. Mortality was recorded at 12, 24, 48, and 72 h after treatment application.
Feeding deterrence bioassay
The most effective insecticides from mortality bioassay were further tested for feeding deterrence activity. For the feeding deterrence bioassay, maize leaf discs were dipped in recommended concentrations of insecticides, and their weight was measured prior to application. The treated leaves were then dried and provided to third-instar larvae. After 48 h of consumption, the remaining leaves were weighed. The feeding deterrence index was calculated by following formula (Hummelbrunner and Isman, 2001).
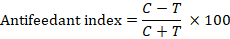
Data analysis
A two-factor factorial analysis of variance (ANOVA) was performed to analyze the effects of insecticides and concentrations on mortality and the means were compared by Tukey’s honest significant difference (HSD) test. The statistical analyses were performed using Minitab 17.0 software.
Results
Laboratory evaluation of synthetic insecticides against S. frugiperda
All insecticides caused significant larval mortality at 12h (F = 1.94; P < 0.05), 24h (F = 2.17; P < 0.05), 48h (F = 2.66; P < 0.05), and 72h (F = 2.07; P < 0.05) after application. The mortality increased over time for all insecticides. Radiant at the recommended concentration (0.50 ml/500ml) caused 91.7% larval mortality after 72 h of application. At the higher concentration (1.00 ml/500ml), the mortality rate was 90.0% at 24 h and increased to 95.0% at 72 h. The higher concentration (0.60 ml/500ml) of Voliam flexi and Coragen (0.25 ml/500ml) caused 91.7% and 81.7% mortality, respectively, after 48 h of application. The mortality rate was 80.0% at 72 h by application of Proclaim (2.00 ml/500ml). The least effective insecticides were Match and Sunitol, with mortality rates of only 58.3% and 35.0% at higher concentrations (2.00 ml/500ml for Match and 4.00 ml/500ml for Sunitol) (Table 2).
Both insecticides were found to exhibit some level of feeding deterrence. Radiant showed a feeding deterrence of -24.57% while Voliam flexi exhibited a feeding deterrence of -22.19%. These results indicate that both insecticides had a suppressing effect on the feeding activity of S. frugiperda when compared to the control group (Figure 1).
Discussion
Spodoptera frugiperda is a destructive insect pest that feeds on a range of crops and results in significant economic losses in agriculture (Mohamed et al., 2022; Ojumoola et al., 2022). The use of insecticides is a common approach to control the population insect pests in the field. In this study, we evaluated the efficacy of selected insecticides against S. frugiperda and found that Radiant and Voliam flexi were the most effective insecticides, causing high mortality rates across all concentrations and time intervals. Our results are consistent with previous studies that have reported the efficacy of insecticides against S. frugiperda (Fernandes et al., 2019; Idrees et al., 2022). Paredes-Sánchez et al. (2021) studied the effectiveness of insecticides in controlling S. frugiperda in maize fields, and they reported that early applications are crucial to prevent crop damage. Belay et al. (2012) examined the impact of various insecticides on controlling S. frugiperda larvae by directly spraying third instar larvae. The mortality rate exceeded 80% in treatments involving chlorantraniliprole, flubendamide, spinosad, indoxacarb, and fenvalerate, 96 h after the application.
Radiant, which contains the active ingredient spinetoram, showed higher efficacy against S. frugiperda in our study. Spinetoram is a member of the spinosyn group of insecticides, which are derived from the natural product spinosad produced by the actinomycete Saccharopolyspora spinosa (Kirst, 2010). Spinosad exerts its insecticidal effect by interacting with the nicotinic acetylcholine receptors in the nervous system of insects, leading to overstimulation and paralysis (Salgado, 1998). Spinosyns have been shown to be highly effective against a wide range of insect pests, including S. frugiperda (Zhao et al., 2020; Bacci et al., 2016). The fast-acting and broad-spectrum properties of spinosyns have made them a popular choice for controlling insect pests in various crops, including corn, cotton, and vegetables (Kodandaram et al., 2010).
Similarly, Voliam flexi, which contains two active ingredients, chlorantraniliprole, and lambda-cyhalothrin, also showed higher efficacy against S. frugiperda in our study. Chlorantraniliprole belongs to the anthranilic diamide group of insecticides (Bentley et al., 2010), while lambda-cyhalothrin is a pyrethroid insecticide (Fetoui et al., 2010). Chlorantraniliprole binds to the ryanodine receptor on the sarcoplasmic reticulum of muscle cells, leading to uncontrolled release of calcium ions and subsequent paralysis of the insect (Lahm et al., 2009). Lambda-cyhalothrin acts on the nervous system by interacting with sodium channels, leading to overstimulation and subsequent paralysis of the insect (Ali, 2012). Together, the two active ingredients in Voliam flexi work synergistically to provide broad-spectrum control of various insect pests, including S. frugiperda. Previous studies have also reported higher efficacy of these insecticides against other pests, such as Helicoverpa armigera and S. litura (Tong et al., 2013; Sharif et al., 2022).
Furthermore, coragen and proclaim also demonstrated good effectiveness against S. frugiperda with both insecticides causing 80.0% mortality rates at higher concentrations and longer exposure times. Previous studies have also reported similar results, demonstrating the efficacy of coragen and proclaim against S. frugiperda (Mian et al., 2022). The lower efficacy of match and sunitol may be due to various factors, including the pest’s resistance to the active ingredient in these insecticides or their mode of action (Dively et al., 2020; Monis et al., 2022).
In addition, radiant and voliam flexi demonstrated more feeding deterrence against S. frugiperda compared to other insecticides. These findings align with previous studies that have also reported the feeding deterrence property of Radiant against S. litura and Spilarctia obliqua (Thakur and Srivastava, 2019). The activation of the ryanodine receptor is responsible for the effects of chlorantraniliprole, including feeding cessation, lethargy, muscle paralysis, and eventual mortality (Cao et al., 2010). The mechanisms underlying this deterrent action are not fully understood but may involve effects on taste receptors or other physiological pathways (Koul, 2008). Our findings suggest that both Radiant and Voliam flexi could be valuable tools for integrated pest management programs targeting S. frugiperda. However, the study was conducted in a controlled laboratory condition, which may not fully replicate the complex and dynamic conditions found in actual field settings. Insects’ behaviors, such as feeding and mortality rate, might differ when subjected to field conditions that include factors like weather fluctuations, plant diversity, and natural predators.
Conclusions and Recommendations
In conclusion, Radiant and Voliam flexi were the most effective insecticides, followed by Coragen and Proclaim, in controlling S. frugiperda. These insecticides have fast-acting and broad-spectrum properties, making them ideal for controlling a range of insect pests in various crops. However, the long-term effects of their use on the environment and non-target organisms should also be considered. Further studies are needed to evaluate the efficacy of these insecticides in the field and their potential impact on the environment.
Acknowledgment
The authors are thankful to Department of Entomology for providing research facilities.
Conflict of interest
The authors have declared no conflict of interest.
References
Ali, Z.Y., 2012. Neurotoxic effect of lambda-cyhalothrin, a synthetic pyrethroid pesticide: Involvement of oxidative stress and protective role of antioxidant mixture. N. Y. Sci. J., 5(9): 93-100.
Altaf, N., Idrees, A., Ullah, M.I., Arshad, M., Afzal, A., Afzal, M. And Li, J., 2022. Biotic potential induced by different host plants in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects, 13: 921. https://doi.org/10.3390/insects13100921
Bacci, L., Lupi, D., Savoldelli, S. And Rossaro, B., 2016. A review of Spinosyns, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests. J. Ent. Acarol. Res., 48: 40-52. https://doi.org/10.4081/jear.2016.5653
Barros, E.M., Torres, J.B., Ruberson, J.R. And Oliveira, M.D., 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. appl., 137: 237-245. https://doi.org/10.1111/j.1570-7458.2010.01058.x
Belay, D.K., Huckaba, R.M. and Foster, J.E., 2012. Susceptibility of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), at Santa Isabel, Puerto Rico, to different insecticides. Fla. Entomol., 95: 476-478. https://doi.org/10.1653/024.095.0232
Bentley, K.S., Fletcher, J.L. and Woodward, M.D., 2010. Chlorantraniliprole: An insecticide of the anthranilic diamide class. In: Hayes’ handbook of pesticide toxicology. Academic Press. pp. 2231-2242. https://doi.org/10.1016/B978-0-12-374367-1.00102-6
CABI, 2017. New report reveals cost of fall armyworm to farmers in Africa, provides recommendations for control. Retrived from: https://www.cabi.org/news-article/new-report-reveals-cost-of-fall-armyworm-tofarmers-in-africa-provides-recommendations-for-control
Cao, G., Lu, Q., Zhang, L., Guo, F., Liang, G., Wu, K. and Guo, Y., 2010. Toxicity of chlorantraniliprole to Cry1Ac-susceptible and resistant strains of Helicoverpa armigera. Pestic. Biochem. Phys., 98: 99-103. https://doi.org/10.1016/j.pestbp.2010.05.006
De Almeida Sarmento, R., De Souza Aguiar, R.W., Vieira, S.M.J., De Oliveira, H.G. and Holtz, A.M., 2002. Biology review, occurrence and control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in corn in Brazil. J. Biosci., 18: 41-48.
Deshmukh, S., Pavithra, H.B., Kalleshwaraswamy, C.M., Shivanna, B.K., Maruthi, M.S. and Mota-Sanchez, D., 2020. Field efficacy of insecticides for management of invasive fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on maize in India. Fla. Entomol., 103: 221-227. https://doi.org/10.1653/024.103.0211
Divekar, P.A., Narayana, S., Divekar, B.A., Kumar, R., Gadratagi, B.G., Ray, A. and Behera, T.K., 2022. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci., 23: 2690. https://doi.org/10.3390/ijms23052690
Dively, G.P., Patton, T., Barranco, L. and Kulhanek, K., 2020. Comparative efficacy of common active ingredients in organic insecticides against difficult to control insect pests. Insects, 11: 614. https://doi.org/10.3390/insects11090614
Fernandes, F.O., Abreu, J.A., Christ, L.M. and Rosa, A.P.S.A., 2019. Efficacy of insecticides against Spodoptera frugiperda (Smith, 1797). J. Agric. Sci., 11: 494-503. https://doi.org/10.5539/jas.v11n1p494
Fetoui, H., Makni, M., Garoui, E.M. and Zeghal, N., 2010. Toxic effects of lambda-cyhalothrin, a synthetic pyrethroid pesticide, on the rat kidney: Involvement of oxidative stress and protective role of ascorbic acid. Exp. Toxicol. Pathol., 62: 593-599. https://doi.org/10.1016/j.etp.2009.08.004
Ganiger, P.C., Yeshwanth, H.M., Muralimohan, K., Vinay, N., Kumar, A.R.V. and Chandrashekara, K., 2018. Occurrence of the new invasive pest, fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), in the maize fields of Karnataka, India. Curr. Sci., 115: 621-623. https://doi.org/10.18520/cs/v115/i4/621-623
Goergen, G., Kumar, P.L., Sankung, S.B., Togola, A. and Tamò, M., 2016. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS One, 11: e0165632. https://doi.org/10.1371/journal.pone.0165632
He, L.M., Zhao, S.Y., Gao, X.W. and Wu, K.M., 2021. Ovipositional responses of Spodoptera frugiperda on host plants provide a basis for using Bt-transgenic maize as trap crop in China. J. Integr. Agric., 20: 804-814. https://doi.org/10.1016/S2095-3119(20)63334-2
Hummelbrunner, L.A. and Isman, M.B., 2001. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Fd. Chem., 49: 715-720. https://doi.org/10.1021/jf000749t
Idrees, A., Qadir, Z.A., Afzal, A., Ranran, Q. and Li, J., 2022. Laboratory efficacy of selected synthetic insecticides against second instar invasive fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae. PLoS One, 17: e0265265. https://doi.org/10.1371/journal.pone.0265265
Kirst, H.A., 2010. The spinosyn family of insecticides: Realizing the potential of natural products research. J. Antibiot., 63: 101-111. https://doi.org/10.1038/ja.2010.5
Kodandaram, M.H., Rai, A.B. and Halder, J., 2010. Novel insecticides for management of insect pests in vegetable crops: A review. Veg. Sci., 37: 109-123.
Kong, F., Song, Y., Zhang, Q., Wang, Z. and Liu, Y., 2021. Sublethal effects of chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) Moth: Implication for Attract and Kill Strategy. Toxics, 9: 1-9. https://doi.org/10.3390/toxics9020020
Koul, O., 2008. Phytochemicals and insect control: An antifeedant approach. Crit. Rev. Pl. Sci., 27: 1-24. https://doi.org/10.1080/07352680802053908
Lahm, G.P., Cordova, D. and Barry, J.D., 2009. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem., 17: 4127-4133. https://doi.org/10.1016/j.bmc.2009.01.018
Makgoba, M.C., Tshikhudo, P.P., Nnzeru, L.R. and Makhado, R.A., 2021. Impact of fall armyworm (Spodoptera frugiperda) (JE Smith) on small-scale maize farmers and its control strategies in the Limpopo province, South Africa. Jamba: J. Disaster Risk Stud., 13: 1-9. https://doi.org/10.4102/jamba.v13i1.1016
Mian, F.M., Khan, I., Ullah, N., Gondal, A.H., Ajmal, M.S., Qureshi, M.S. and Jabbar, A., 2022. Efficacy of Insecticides against Fall Armyworm, Spodoptera frugiperda (Lepidoptera, Noctuidae) in Maize. J. Bioresour. Manage., 9: 133-139.
Mohamed, H.O., El-Heneidy, A.H., Dahi, H.F. and Awad, A.A., 2022. First record of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on Sorghum Plants, A new invasive pest in Upper Egypt. Egypt. Acad. J. Biol. Sci., 15: 15-23. https://doi.org/10.21608/eajbsa.2022.214719
Monis, C., Malan, A.P., De Waal, J.Y. and Johnson, S.A., 2022. Insecticide efficacy against trimen’s false tiger moth, Agoma trimenii (Lepidoptera: Agaristidae). South Afr. J. Enol. Vitic., 43: 1-9.
Muraro, D.S., Garlet, C.G., Godoy, D.N., Cossa, G.E., Rodrigues Junior, G.L.D.S., Stacke, R.F. and Bernardi, O., 2019. Laboratory and field survival of Spodoptera frugiperda (Lepidoptera: Noctuidae) on Bt and non-Bt maize and its susceptibility to insecticides. Pest Manag. Sci., 75: 2202-2210. https://doi.org/10.1002/ps.5347
Ndolo, D., Njuguna, E., Adetunji, C.O., Harbor, C., Rowe, A., Den Breeyen, A. and Hospet, R., 2019. Research and development of biopesticides: challenges and prospects. Outlooks Pest Manag., 30: 267-276. https://doi.org/10.1564/v30_dec_08
Ojumoola, O.A., Omoloye, A.A. and Umeh, V.C., 2022. Seasonal difference in fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) abundance and plant injury on selected maize varieties in Ibadan, Southwest Nigeria. Int. J. Pest Manage., 2022: 1-11. https://doi.org/10.1080/09670874.2022.2055809
Overton, K., Maino, J.L., Day, R., Umina, P.A., Bett, B., Carnovale, D. and Reynolds, O.L., 2021. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot., 145: 105641. https://doi.org/10.1016/j.cropro.2021.105641
Paredes-Sánchez, F.A., Rivera, G., Bocanegra-García, V., Martínez-Padrón, H.Y., Berrones-Morales, M., Niño-García, N. and Herrera-Mayorga, V., 2021. Advances in control strategies against Spodoptera frugiperda. A review. Molecules, 26: 1-19. https://doi.org/10.3390/molecules26185587
Salgado, V.L., 1998. Studies on the mode of action of spinosad: Insect symptoms and physiological correlates. Pestic. Biochem. Phys., 60: 91-102. https://doi.org/10.1006/pest.1998.2332
Sharif, M.N., Iqbal, M.S., Alam, R., Awan, M.F., Tariq, M., Ali, Q. and Nasir, I.A., 2022. Silencing of multiple target genes via ingestion of dsRNA and PMRi affects development and survival in Helicoverpa armigera. Sci. Rep., 12: 10405. https://doi.org/10.1038/s41598-022-14667-z
Singh, G.M., Xu, J., Schaefer, D., Day, R., Wang, Z. and Zhang, F., 2022. Maize diversity for fall armyworm resistance in a warming world. Crop Sci., 62: 1-19. https://doi.org/10.1002/csc2.20649
Sorur, M.A., Khannss, O., Abd El–Wahab, A.S., El–Sheikh, M.A.K. and Abul–Ela, S., 2011. An ecomomically modified semi – synthetic diet for mass rearing the Egyptian cotton leaf worm Spodoptera littoralis. Acad. J. Entomol., 4: 118–123.
Thakur, H. and Srivastava, R.P., 2019. Sub-lethal and antifeedant effect of spinosyn and diamide insecticides against Spodoptera litura (Fab.) and Spilarctia obliqua (Wlk.). J. Entomol. Res., 43: 431-438. https://doi.org/10.5958/0974-4576.2019.00076.8
Tong, H., Su, Q., Zhou, X. and Bai, L., 2013. Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. J. Pest Sci., 86: 599-609. https://doi.org/10.1007/s10340-013-0505-y
Veres, A., Wyckhuys, K.A., Kiss, J., Tóth, F., Burgio, G., Pons, X. and Furlan, L., 2020. An update of the Worldwide Integrated Assessment (WIA) on systemic pesticides. Part 4: Alternatives in major cropping systems. Environ. Sci. Pollut. Res., 27: 29867-29899. https://doi.org/10.1007/s11356-020-09279-x
Womack, E.D., Williams, W.P., Smith, J.S., Warburton, M.L., Bhattramakki, D. and Hesler, L., 2020. Mapping quantitative trait loci for resistance to fall armyworm (Lepidoptera: Noctuidae) leaf-feeding damage in maize inbred Mp705. J. Econ. Entomol., 113: 956-963. https://doi.org/10.1093/jee/toz357
Zhao, Y.X., Huang, J.M., Ni, H., Guo, D., Yang, F.X., Wang, X. and Gao, C.F., 2020. Susceptibility of fall armyworm, Spodoptera frugiperda (JE Smith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem. Physiol., 168: 1-5. https://doi.org/10.1016/j.pestbp.2020.104623
To share on other social networks, click on any share button. What are these?








