Tissue-Specific Response of Peroxidase Activity in Channa striata under Acute Exposure of Pesticides (Deltamethrin + Endosulfan) Mixture
Tissue-Specific Response of Peroxidase Activity in Channa striata under Acute Exposure of Pesticides (Deltamethrin + Endosulfan) Mixture
Aliya Munir1, Sajid Abdullah1*, Huma Naz2*, Khalid Abbas1, Tanveer Ahmed3 and Zahid Farooq2
1Department of Zoology, Wildlife and Fisheries, University of Agriculture Faisalabad, Pakistan
2Department of Zoology, Cholistan University of Veterinary and Animal Sciences Bahawalpur, Pakistan
3Department of Life Sciences, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, Pakistan.
Abstract | The application of pesticides has been increased in agriculture to improve crop production all over the world. This may leads to existence of pesticides and their metabolites in freshwater bodies. Pesticides can trigger stress in organisms by increasing the number of reactive oxygen species. Oxidative stress biomarkers like antioxidants enzymes have been successfully used in ecotoxicology field for evaluating the stress response of organisms exposed to pollutants. Therefore, this work was designed to evaluate the peroxidase (POx) level in kiney, gills and liver of fish Channa striata exposed to LC50 concentration (1.374µgL-1) of deltamethrin+endosulfan (DM+ES) mixture for 4-day. Sampling was performed after a 24-hr period. Fish were sacrificed and organs were collected to calculate the POx activity. It was observed that the exposure of DM+ES significantly accelerated the POx level in gills, liver and kidney of fish in a time dependent manner.
Novelty Statement | The widespread use of insecticide in agriculture sector result in occasional discharge in water bodies and account for significant effects on fish survival. Therefore, this work was designed to assess the toxicity of insecticides on fish.
Article History
Received: June 18, 2019
Revised: July 13, 2020
Accepted: August 02, 2021
Published: August 23, 2021
Authors’ Contributions
AM executed this research work. SA planning the research work. HN performed statistical analysis, KA member of supervisory committee. TA and ZF helped in writing the manuscript.
Keywords
Acute, Organs, Pesticides mixture, Antioxidant enzyme, Fish
Corresponding authors: Sajid Abdullah and Huma Naz
humanaz@cuvas.edu.pk, uaf_sajidabdullah@yahoo.com
To cite this article: Munir, A., Abdullah, S., Naz, H., Abbas, K., Ahmed, T. and Farooq, Z., 2021. Tissue-specific response of peroxidase activity in Channa striata under acute exposure of pesticides (Deltamethrin + Endosulfan) mixture. Punjab Univ. J. Zool., 36(2): 147-151. https://dx.doi.org/10.17582/journal.pujz/2021.36.2.147.151
Introduction
Pesticide and their residues contaminates the aquatic habitats due to widespread use in the agricultural sector (Cerejeira et al., 2003). Pesticides are also highly toxic to non-target animals; among these fish is one of the most prominent (Murthy et al., 2013) because fish has direct contact to theses pescticides (Rao and Pillala, 2001). These chemicals alter the quality of water that is a residence of several aquatic animals (Donohue et al., 2006). Exposure of pesticides at acute concentrations had negative effects on organisms and sometimes causes mass mortalities (Sabae et al., 2014).
Organo-chlorines and pyrethroid pesticides are commonly applied and overlapped in soy-bean crops (Miglioranza et al., 2003). Both pesticides are known as highly toxic to aquatic animals like fish due to harmful impacts (US EPA, 2002; Gu et al., 2007). Deltamethrin (DM) belongs to pyrethroid insecticides (Tomlin, 2006), may badly affect the fish by provoking oxidative stress in various organs of fish (De-Assis et al., 2009). Endosulfan is an off patent organochlorine insecticide and acaricide that is being phased out globally (Guerin, 2001) and extensively applied to control the variety of insect pests (Miglioranza et al., 2003). Pesticides may change the behavior of fish, also alter the haematological and biochemical parameters, histopathological damages and cause genotoxicity (Klumpp et al., 2002).
Many pesticides include organofluorine, organochlorine, carbamates, organophosphates and pyrethroids, (Di-Giulio and Meyer, 2008) provoke oxidative stress by the creation of reactive oxygen species (ROS) which not only start oxidation of lipids (Koprucu et al., 2008) but also cause variations in antioxidants which scavenge free oxygen radicals in aquatic animals (Livingstone, 2001).
To detoxify ROS fish, possess antioxidant defensive mechanisms contain enzymes such as superoxide dismutase, glutathione peroxidases, catalase, and glutathione S-transferases. Peroxidase is found in the mitochondria and eliminate hydrogen peroxide from the cell and save the cell from oxidative stress (Boeuf et al., 2000). Therefore, in this experiment organ and duration-dependent response of peroxidase in Channa striata due to acute exposure of deltamethrin+endosulfan mixture was examined.
Materials and Methods
Lay-out of experiment
Channa striata were chosen for this experiment. C. striata was captured from natural breeding grounds and brought to Fish Farm at UAF. C. striata were acclimatized to laboratory environment for a couple of weeks. Afterthat, fish (n=10) were shifted to 70-L glass aquarium. C. striata, were exposed to LC50 concentration (1.374µgL-1) of DM+ES for four days (Abdullah et al., 2018).
Physico-chemical parameters
During the trail, pH, temperature and total hardness of water kept stable as 7.00, 30oC and 222 mg L-1, respectively. Other parameters includes sodium, carbon dioxide, magnesium, calcium, potassium, total ammonia, and electrical conductivity were checked according to method of APHA (1998).
Estimation of peroxidase activity
Preparation of homogenate
After each sampling (24-hr interval), fish were sacrificed and organs viz. gills, liver and kidney were removed. Organs homogenate was prepared by adding phosphate buffer (0.2 M) of pH 6.5 in the ratio of 1:4 (w/v) with mortar and pestle. Organ homogenate was centrifuged for 10 mint at 10,000 rpm and 4oC. A clear supernatant was separated for enzyme assay.
Enzyme assay
Civello et al. (1995) protocol was adopted to calculate the peroxidase. The 750µL of guaiacol and 47ml of phosphate buffer added in a test tube and mixed well. After mixing, hydrogen peroxide (0.3 ml) was also added in the test tube. In a cuvette, 3 ml of buffered substrate solution along with 0.06 ml of enzyme extract was taken, and left for 3 min. After 3 min absorbance was noted at 470 nm by using spectrophotometer against control.
Calculation
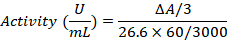
Data analysis
The obtained data from this experiment were analyzed using Statistix Version 8. The organ- and duration specific difference were analyzed by applying One-way ANOVA followed by Tukey’s range test.
Results and Discussion
The inferences of this study demonstrated that the exposure of DM+ES mixture significantly raised the POx level in gills, kidney and liver of C. striata as compared to control. Moreover, level of POx was higher in liver of C. striata than gills and kidney. The POx level gradually enhanced with the passage of time. The POx activity was maximum after 96-hr of exposure to DM+END mixture. Data regarding peroxidase activity is presented in Figure 1-3.
Aquatic animals certainly exposed to a number of pesticides which are different in their structure and toxicity. Effect of single pesticides on fish has been observed by many authors (Joseph and Raj, 2011), but the toxicity associated with pesticides in combined form is comparatively less documented. Therefore, more research work is needed to understand their interactions on the living organisms. The use of biomarkers provides integrative and comprehensive evaluation of biochemical and cellular alterations in living organisms exposed to environmental toxicants (Cazenave et al., 2009; Linde-Arias et al., 2008).
In present work, DM+ES significantly increased POx level in liver, gills and kidney of organs of C. striata in relation to control. The ability of DM and END to cause oxidative stress in various tissues of fish has been extensively studied (Atif et al., 2005). According to Bacchetta et al. (2014) endosulfan exposure activated the peroxidase in fish. Dinu et al. (2010) noted that DM stimulate level of peroxidase in Carassius auratus gibelio liver.
The fluctuation in enzymes activities (enhanced or reduced) under stress situation mainly depends upon different factors such as species, dose, exposure duration and type of the toxicants (John et al., 2001; Cheung et al., 2001). Endosulfan+chlorpyrifos mixture caused the raise in POx activity in all organs of C. catla (Naz et al., 2021). Pandey et al. (2003) documented the higher POx level in kidney, gills and liver of Wallago attu collected from polluted area. DM induced significant increase in kidney and liver GPx level of Channa punctatus as observed by Sayeed et al. (2003). Ullah et al. (2016) recorded the ES induced POx activity in gills and liver of Labeo rohita. Vineela and Reddy (2014) recorded gradual increase in POx activity of Catla catla exposed to Lihocin (Chlorocholinechloride). Isik and Celik (2008) also recorded variations in liver and gills of rainbowtrout (Oncorhynchcus mykiss) after acute exposure to methyl diazinon and parathion. Exposed to malathion significantly increased the glutathione peroxidase in gills of gilthead seabream (Sparus aurata L) (Rosety et al., 2005). Verma and Dubey (2003) also reported the same results. Lemaire et al. (2010) reported that organochlorine compounds significantly augmented the peroxidase activity in Coryphaenoides rupestris. Acute exposure of pesticides accelerated the peroxidase activity in Labeo rohita in relation to control (Dawar et al., 2016). Endoslafn+chlorpyrifos mixture augmented the POx level in kidney, gills and liver of rohu (Naz et al., 2019).
Conclusions and Recommendations
Present study demonstrated that the DM+ES mixture alter the peroxidase level in organs of fish when exposed to lethal dose. It was also concluded that the biochemical parameters like antioxidant enzyme can be successfully used as a bio-marker to examine the pesticides pollution in aquatic ecosystems.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdullah, S., Mateen,A., Abbas,K., Naz,H., Hassan,W. and Anum, S., 2018. Changes in glutathione S-transferase activity in fish Channa striata exposed to different aquatic pollutants (heavy metals and pesticides mixture). Pakistan J. Zool., 13(Suppl. Ser.): 42-47.
APHA, 1998. Standard methods for the examination of water and wastewater. American water works association and water environment federation. 20th Edition. American Public Health Association, Washington DC, USA.
Atif, F., Parvez, S., Pandey, S., Ali, M., Kaur, M., Rehman, H., Khan, H.A. and Raisuddin, S., 2005. Modulatory effect of cadmium exposure on deltemethrin-induced oxidative stress in Channa punctata Bloch. Arch. Environ. Contam. Toxicol., 49: 371-377. https://doi.org/10.1007/s00244-003-9231-4
Bacchetta, C., Rossi, A., Ale, A., Campana, M., Parma, M.J., and Cazenave, J., 2014. Combined toxicological effects of pesticides: A fish multi-biomarker approach. Ecol. Indic., 36: 532-538. https://doi.org/10.1016/j.ecolind.2013.09.016
Boeuf, G., Bauw, G., Legrand, B. and Rambour, S., 2000. Purification and characterization of a basic peroxidase from the medium of cell suspension cultures of chicory. Plant Physiol. Biochem., 38: 217-224. https://doi.org/10.1016/S0981-9428(00)00731-2
Cazenave, J., Bacchetta, C., Parma, M.J., Scarabotti, P.A. and Wunderlin, D.A., 2009. Multiple biomarkers responses in Prochilodus lineatus allowed assessing changes in the water quality of Salado River basin (Santa Fe, Argentina). Environ. Pollut., 157: 3025-3033. https://doi.org/10.1016/j.envpol.2009.05.055
Cerejeira, M.J., Viana, P., Batista, S., Pereira, T., Silva, E., Valerio, M.J., Silva, A., Ferreira, M. and Silva-Fernandes, A.M., 2003. Pesticides in portuguese surface and ground waters. Water Res., 37: 1055-1063. https://doi.org/10.1016/S0043-1354(01)00462-6
Cheung, C.C.C., Zheng, G.J., Li, A.M.Y., Richardson, B.J. and Lam, P.K.S., 2001. Relationships between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels, Pernaviridis. Aquat. Toxicol., 52: 189-203. https://doi.org/10.1016/S0166-445X(00)00145-4
Civello, M.P, Martinez, G.A., Chaves, A.R. and Anon, M.C., 1995. Peroxidase from strawberry fruit (Fragariaananassa Duch.): Partial purification and determination of some properties. J. Agric. Food Chem., 43: 2601. https://doi.org/10.1021/jf00058a008
Dawara, F.U., Zuberi, A., Azizullah, A. and Khattaka, M.N.K., 2016. Effects of cypermethrin on survival, morphological and biochemical aspects of rohu (Labeo rohita) during early development. Chemosphere, 144: 697-705. https://doi.org/10.1016/j.chemosphere.2015.09.007
De-Assis, H.C.S., Nicareta, L., Salvo, L.M., Klemz, C., Truppel, J.H. and Calegari, R., 2009. Biochemical biomarkers of exposure to deltamethrin in freshwater fish Ancistrus multispinis. Braz. Arch. Biol. Technol., 52: 1401-1407. https://doi.org/10.1590/S1516-89132009000600012
Di-Giulio, R.T. and Meyer, J.N., 2008. Reactive oxygen species and oxidative stress. In: Di-Giulio RT, Hinton DE, editors. The Toxicology of Fishes. Boca Raton: CRC Press, Taylor and Francis Group. pp. 273-324. https://doi.org/10.1201/9780203647295.ch6
Dinu, D., Marinescu, D., Munteanu, M.C., Staicu, A.C., Costache, M. and Dinischiotu, A., 2010. Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus gibelio liver and intestine. Arch. Environ. Contam. Toxicol., 58: 757-764. https://doi.org/10.1007/s00244-009-9401-0
Donohue, I., Style, D., Coxon, C. and Irvine, K., 2006. Importance of spatial and temporal patterns for assessment of risk of diffuse nutrient emissions to surface waters. J. Hydrol., 304: 183-192. https://doi.org/10.1016/j.jhydrol.2004.10.003
Gu, B.G., Wang, H.M., Chen, W.L., Cai, D.J. and Shan, Z.J., 2007. Risk assessment of λ-cyhalothrin on aquatic organisms in paddy field in China. Regul. Toxicol. Pharmacol., 48: 69-74. https://doi.org/10.1016/j.yrtph.2007.01.005
Guerin, T.F., 2001. Abiological loss of endosulfan and related chlorinated organic compounds from aqueous system in the presence and absence of oxygen. Environ. Poll., 15: 219-230. https://doi.org/10.1016/S0269-7491(01)00112-9
Isik, I. and Celik, I., 2008. Acute effects of methyl parathion and diazinon as inducers for oxidative stress on certain biomarkers in various tissues of rainbowtrout (Oncorhynchus mykiss). Pestic. Biochem. Physiol., 92: 38–42. https://doi.org/10.1016/j.pestbp.2008.06.001
John, S., Kale, M., Rathore, N. and Bhatnagar, D., 2001. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem., 12: 500-504. https://doi.org/10.1016/S0955-2863(01)00160-7
Joseph, B. and Raj, S.J., 2011. Impact of pesticide toxicity on selected biomarkers in fishes. Int. J. Zool. Res., 7: 212-222. https://doi.org/10.3923/ijzr.2011.212.222
Klumpp, D.W., Huasheng, H., Humphrey, C., Xinhong, W. and Codi, S., 2002. Toxic contaminants and their biological effects in coastal waters of Xiamen, China. I. Organic pollutants in mussel and fish tissues. Mar. Poll. Bull., 44: 752-760. https://doi.org/10.1016/S0025-326X(02)00053-X
Koprucu, S.S., Yonar, E. and Seker, E., 2008. Effects of deltamethrin on antioxidant status and oxidative stress biomarkers in freshwater mussel Unio elongatulus eucirrus. Bull. Environ. Contam. Toxicol., 81: 253-257. https://doi.org/10.1007/s00128-008-9474-x
Lemaire, B., Priede, I.G., Collins, M.A., Bailey, D.M., Schtickzelle, N., Thome, J.P. and Rees, J.F., 2010. Effects of organochlorines on cytochrome P450 activity and antioxidant enzymes in liver of round nose grenadier Coryphaenoides rupestris. Aquat. Biol., 8: 161-168. https://doi.org/10.3354/ab00227
Linde-Arias, A.R., Inacio, A.F., Novo, L.A., De-Alburquerque, C. and Moreira, J.C., 2008. Multibiomarker approach in fish to assess the impact of pollution in a large Brazilian river, Paraiba do Sul. Environ. Pollut., 156: 974-979. https://doi.org/10.1016/j.envpol.2008.05.006
Livingstone, D.R., 2001. Contaminant stimulated reactive oxy- gen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull., 42: 656-666. https://doi.org/10.1016/S0025-326X(01)00060-1
Miglioranza, K., Moreno, J. and Moreno, V., 2003. Trends in soil science. Organochlorine pesticides in Argentinean soils. J. Soils. Sediments, 3: 264-265. https://doi.org/10.1007/BF02988686
Murthy, K.S., Kiran, B.R. and Venkateshwarlu, M., 2013. A review on toxicity of pesticides in fish. Int. J. Open Sci. Res., 1: 15-36.
Naz, H., Abdullah, S., Abbas, K., Hassan, W., Batool, M., Perveen, S., Maalik, S. and Mushtaq, S., 2019. Toxic Effect of Insecticides Mixtures on Antioxidant Enzymes in Different Organs of Fish, Labeo rohita. Pak. J. Zool., 51: 1355-1361. https://doi.org/10.17582/journal.pjz/2019.51.4.1355.1361
Naz, H., Abdullah, S., Ahmed, T., Abbas, K. and Ijaz, M.U., 2021. Regression analysis for predicting the duration dependent response of oxidative stress dynamics and nuclear anomalies in Catla catla exposed to chlorpyrifos and endosulfan. J. Anim. Plant Sci., 31: 1167-1173. https://doi.org/10.36899/JAPS.2021.4.0314
Pandey, S., Parvez, S., Sayeed, I., Haque, R., Bin-Hafeez, B. and Raisuddin, S., 2003. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total. Environ., 309: 105-115. https://doi.org/10.1016/S0048-9697(03)00006-8
Rao, A.S. and Pillala, R.R., 2001. The concentration of pesticides in sediments from Kollerulake in India. Pest Manage. Sci., 57: 620-624. https://doi.org/10.1002/ps.336
Rosety, M., Rosety-Rodriguez, M., Ordonez, F.J. and Rosety, I., 2005. Time course variations of antioxidant enzyme activities and histopathology of gilthead sea bream gills exposed to malathion. Histol. Histopathol., 20: 1017-1020.
Sabae, S.Z., El-Sheekh, M.M., Khalil, M.A., Elshouny, W.A.E. and Badr, H.M., 2014. Seasonal and regional variation of physicochemical and bacteriological parameters of surface water in El-Bahr El- Pherony, Menoufia, Egypt. World J. Fish Mar. Sci., 6: 328-335.
Sayeed, I., Parvez, S., Pandey, S., Bin-Hafeez, B., Haque, R. and Raisuddin, S., 2003. Oxidative stress biomarkers of exposure to deltamethrin in freshwater fish Channa punctatus Bloch. Ecotox. Environ. Saf., 56: 295-302. https://doi.org/10.1016/S0147-6513(03)00009-5
Tomlin, C.D.S., 2006. The pesticide manual: A world compendium, 14th Ed.; British Crop. Protection Council. Alton, Hampshire, pp. 186-187.
Ullah, S., Hasan, Z. and Dhama, K., 2016. Toxic effect of endosulfan on behavior, protein contents and antioxidant enzyme system in gills, brain, liver and muscle tissues of rohu, Labeorohita. Int. J. Pharmcol., 12: 1-10. https://doi.org/10.3923/ijp.2016.1.10
US E.P.A., 2002. Reregisteration eligibility decision (RED) document for endosulfan. N◦738-F-02-012. Office of the Pesticide Programs (OPP), US EPA, Washington, DC.
Verma, S. and Dubey, R.S., 2003. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci., 164: 645-655. https://doi.org/10.1016/S0168-9452(03)00022-0
Vineela, D. and Reddy, S.J., 2014. Impact of lihocin on immuno haematological and antioxidant enzyme indices of carp fish. Int. J. Pharm. Life Sci., 5: 3517-3525.
To share on other social networks, click on any share button. What are these?








