The Validation of Different Growth Models of Randall’s Threadfin Bream, Nemipterus randalli (Russell, 1986), in Northeastern Mediterranean Sea
The Validation of Different Growth Models of Randall’s Threadfin Bream, Nemipterus randalli (Russell, 1986), in Northeastern Mediterranean Sea
Sevil Demirci*, Aydın Demirci and Emrah Şimşek
Iskenderun Technical University, Faculty of Marine Sciences and Technology, Department of Marine Technologies, 31200, Iskenderun-Hatay/Turkey
ABSTRACT
The study was conducted to determine the age and the growth of the Lessepsian migrant Randall’s threadfin bream, Nemipterus randalli (Russell, 1986). A total of 587 samples were collected from the eastern Mediterranean coast of Turkey between February 2012 and January 2013. Total length and total weight ranged from 7.7 to 21.0 cm and from 6.90 and 150.99 g, respectively. Length-weight relationship parameters were calculated for all individuals as a= 0.0106 and b= 3.09, and isometric growth was determined. The population constituted five age-classes (0–IV years). Four growth models used to identify the growth characteristics were von Bertalanffy, Logistic, Richards, and Gompertz growth models. Growth type of Randall’s threadfin bream has rapid property; hence the fish population may be continued increasing in the Iskenderun Bay and other of Eastern Mediterranean regions.
Article Information
Received 27 March 2018
Revised 30 May 2019
Accepted 11 September 2019
Available online 11 March 2020
Authors’ Contribution
SD designed the study. SD and ES collected the samples and performed laboratory work. SD and AD analyzed the data and wrote the article.
Key words
Invasive fish, Eastern mediterranean, Growth model
DOI: https://dx.doi.org/10.17582/journal.pjz/20180327130349
* Corresponding author: [email protected]
0030-9923/2020/0003-1113 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Randall’s threadfin bream (Nemipterus randalli) has a common distribution in the Indian Ocean, the Persian Gulf, and the Red Sea (Russell, 1990). Its definite occurrence in the Mediterranean also represents a recent range extension (Lelli et al., 2008). The species is an invasive species, first recorded by Golani and Sonin (2006) in the Mediterranean. Subsequently, Bilecenoglu and Russell (2008) and Gokoglu et al. (2008) recorded this species in the Mediterranean Sea coast of Turkey.
Randall’s threadfin bream has commercially important locations in different areas of the world as following: West Bay of Bengal (Krishnamoorthi, 1971), Andaman Sea (Senta and Tan, 1975), and South China Sea (Devaraj and Gulati, 1988; Kimoto and Johari, 1996). In Iskenderun Bay, the threadfin bream was low in population between 2008 and 2010 (Erguden et al., 2010). Over time, population density in the region increased as a major stocks source for different fishing methods such as trawl, hook, and set net.
Some biological growth parameters of this fish species have been reported from different fishing areas (Samuel, 1990; Kimoto and Johari, 1996; Granada et al., 2004; Wu et al., 2008) and also in the Iskenderun Bay (Erguden et al., 2010). In the Arabian Sea, the growth parameters of the species have been reported by length frequencies data (Kalhoro et al., 2017).
However, there is limited information for this species about growth parameters. Therefore, this study was aimed to estimate growth parameters of this species in terms of length, weight and age for Iskenderun Bay Fishery. Different mathematical growth models have been applied for a more precise result in evaluating age-related growth parameters (Demirci et al., 2016).
MATERIALs AND METHODS
The Randall’s threadfin breams were caught between February 2012 and January 2013 in Iskenderun Bay, in the Eastern Mediterranean. A total of 587 fish were captured via the bottom trawl. Mesh size of trawl net was chosen quite small (24 mm) for this study at depths of 30m-70m. Trawling operations were carried out for 180 minutes at an average speed of 2.7 knots.
The sampled fish were placed on ice in boxes for transport to the laboratory. Lengths (to nearest mm) and wet weights (nearest 0.01 g) were measured. Sagittal otoliths were analyzed for age estimation (Williams and Bedford, 1974). This analysis was carried out in three stages. Firstly, all right otoliths were used (Jemaa et al., 2015). Secondly, all left otoliths were used. Finally, all right and left otoliths readings were compared for accurate estimation. These readings were carried out twice by the main reader and independently by a second reader. Average percent error (APE) and c.v. were calculated to compare readings between the two readers (Campana, 2001). The length-weight relationship of Randall’s threadfin bream was calculated with W = aLb, where W is the fish weight (g) and L is the total length (cm). Values of the exponent b provide information on fish growth. When b=3, growth is isometric. When the value of b is different from 3, growth is allometric, (positive allometric if b>3, negative allometric if b<3). The hypothesis of the isometric growth (H0: b =3) was tested by t – test, using the statistic: ts = (b-3)/Sb, where Sb is the standard error of the slope, for α=0.05 for testing significant differences among slopes (b) between two regressions for the species (Morey et al., 2003).
In this study, growth parameters that could fit the data were used. Growth curves were fitted using least squares method for all fact using four growth models, the von Bertalanffy growth equation, the logistic growth equation, the Gompertz model and Richards equation (Haddon, 2001). This estimating technique was used for all specimens; all ages and their corresponding lengths were applied to estimate growth parameters, except the classical methods that use age with their mean lengths. The solver routines in Microsoft Office Excel 2016 were used to determine length growth equations:
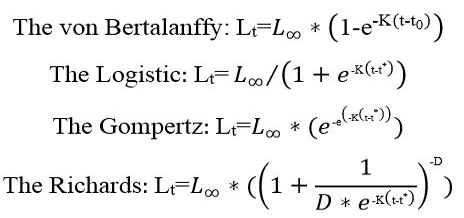
Lt is the total length at age t, L∞ is the maximum theoretical total length, K-1 is the growth coefficient, t0 is the theoretical age at zero length. In logistic equation t* age of the growth inflexion. Finally, D determined the shape of the curve (Tian et al., 1993). For determination age and weight growth equations of Randall’s threadfin bream, in these models, Lt instead of Wt (weight at age t) was made equation change by b has the value of the slope provided by length-weight relationship.

Selection of the best model describing the age–length relationship was performed using both the residual sum of squares (RSS) and inspection of all parameter. The fish growth performance was estimated using Munro’s growth performance (phi-prime) index (Pauly and Munro, 1984) as; φ’ = log(K-1) + 2× log(L∞).
RESULTS
The total sample comprised 587 individuals, with ages ranged from 0 to 4 years. Distributions of length and weight according to age groups were shown in Table I. According to this distribution, population density of I and II age groups was respectively 53.66% and 30.66%. While the smallest individual fish had 7.7 cm length and 6.90 g weight, while the largest fish sample was 21.0 cm long and had 150.99 g weight. The average fish length and weight were 14.24 cm and 43.17 g, respectively.
Table I. Age, weight and length key for Randall’s threadfin bream collected from the Eastern Mediterranean Sea.
|
Age groups |
Total |
||||||
|
0 |
I |
II |
III |
IV |
|||
|
Number of fish |
28 |
315 |
180 |
55 |
9 |
587 |
|
|
|
% |
4.77 |
53.66 |
30.67 |
9.37 |
1.53 |
|
|
Length (cm) |
Mean |
9.65 ±0.18 |
13.02 ±0.08 |
15.70 ±0.07 |
17.93 ±0.09 |
19.71 ±0.23 |
14.24 ±0.09 |
|
Min. |
7.7 |
9.1 |
12.8 |
16.6 |
19.0 |
7.7 |
|
|
Max. |
11.0 |
16.4 |
17.9 |
20.5 |
21.0 |
21.0 |
|
|
Weight (g) |
Mean |
14.18 ±2.20 |
31.31 ±0.65 |
54.64 ±0.75 |
79.04 ±1.04 |
100.03 ±6.93 |
43.17 ±0.71 |
|
Min. |
6.90 |
11.20 |
20.19 |
62.90 |
81.97 |
6.90 |
|
|
Max. |
72.20 |
59.00 |
79.30 |
92.00 |
150.99 |
150.99 |
|
Table II. Comparisons of Randall’s threadfin bream’s length–weight relationship parameters.
|
Regions |
a |
b |
r2 |
References |
|
Indian Coast |
0.0223 |
2.88 |
- |
Murty (1982) |
|
Arabian Sea |
0.035 |
2.74 |
0.97 |
Kalhoro et al. 2017 |
|
Iskenderun Bay |
0.0130 |
2.69 |
0.98 |
Erguden et al. (2009) |
|
Iskenderun Bay |
0.0011 |
3.06 |
0.98 |
Erguden et al. (2010) |
|
Iskenderun Bay |
0.0106 |
3.09 |
0.97 |
Present study |
Figure 1 shows the length–weight relationship regression calculated for all individuals. The length–weight relationship of Randall’s threadfin bream was W = 0.0106*L3.09 (n = 587), and the exponent of length–weight relationship, b, showed an isometric growth. A comparison concerning the length–weight relationship for the species was also made using results of previous studies (Table II).
Age, length and weight data were used to fit the four growth models parameters (Table III). Acceptable confidence intervals (>70%) length and weight of age growth parameters were observed for each growth models. Figure 2 shows the four-growth model length -age and weight-age growth curves. The results of this current study were compared to previous studies in Table IV.
Table III. The growth parameters and equations of Randall’s threadfin bream by length and weight for von-Bertalanffy, Gompertz, Logistic and Richards fish growth models.
|
Growth models |
Length-age relationships |
Weight-age relationships |
|||||||
|
L∞ |
K-1 |
t0 |
r2 |
φ' |
W∞ |
K-1 |
t0 |
r2 |
|
|
von-Bertalanffy |
27.37 |
0.21 |
-2.09 |
0.74 |
2.19 |
242.87 |
0.04 |
-0.19 |
0.73 |
|
Gompertz |
23.41 |
0.39 |
-0.34 |
0.74 |
2.33 |
153.42 |
0.15 |
2.01 |
0.74 |
|
Logistic |
21.79 |
0.58 |
0.33 |
0.74 |
2.44 |
119.19 |
0.29 |
2.2 |
0.73 |
|
Richards |
23.41 |
0.4 |
-0.34 |
0.74 |
2.34 |
153.41 |
0.14 |
2.06 |
0.74 |
DISCUSSION
The length-weight relationship provides information on growth parameters for fisheries management and fish stock assessment (King, 2013). According to our data, the length-weight relationship of Randall’s threadfin bream in the East Mediterranean coast was calculated to be an isometric growth.
Table IV. Growth parameters comparisons of Randall’s threadfin bream from different areas.
|
Regions |
L∞ |
K-1 |
t0 |
φ' |
References |
|
Eastern Indian |
21.9 |
0.83 |
- |
2.60 |
Murty, 1981 |
|
Iskenderun Bay |
34.96 |
0.21 |
-1.24 |
2.41 |
Erguden et al. 2010 |
|
Arab Sea |
26.25 |
0.32 |
-0.54 |
2.34 |
Kalhora et al. 2017 |
|
Iskenderun Bay |
27.37 |
0.21 |
-2.09 |
2.19 |
Present study |
While the finding “a” and “b” for Randall’s threadfin bream in the East Mediterranean coast in this current study was found to be similar to that of the same fish species in Ergüden’s (2010) study from the same area but different location in Mediterranean Sea. It was found to be different from other regions e.g., Indian Coast (Murty, 1981), Arabian Sea (Kalhoro et al., 2017), Iskenderun Bay (Erguden et al., 2009). These differences could be the results of ecological and environmental factors.
The increase in the population of this fish species can be expressed as a successful invader lessepsian fish species in the Eastern Mediterranean. Iskenderun Bay in Eastern Mediterranean coastal waters are intense species diversity and overfishing areas. In addition to overfishing, very different of fishing methods are used in these fishing areas such as otter trawl, purse seine, set net long line. As it can be predicted that the overfishing of the economically important target fish species can result in its decline in a short period (Demirci et al., 2018).
Additionally, the success of an invasive species can negatively affect the population of local fish species. After this lessepsian fish species was seen in the eastern Mediterranean, the catching of red pandora (Pagellus erythrinus), declined in the region since 2008. (unpublished material in Fisheries Department of the Government). In addition, this species has been evaluated as “Least Concern” according to the IUCN Red List of Threatened Species since 2014 (IUCN, 2014; Şimşek and Demirci, 2018). As a result, the decreasing in red pandora and increase in Randall’s threadfin bream was an evident from the change in the local fish market’s items. Additionally, the visual similarity of these two species (red color) plays an important role in this replacement. These fish have different taste and red pandora was the number one chosen fish at first. However, declining of the red pandora in amount and increasing of the Randall’s threadfin bream made people get used to this fish species in a very short period. As a result Randall’s threadfin bream became a preferred product and has got a new place in the tables in this region.
Successful invaders like Randall’s threadfin bream often exhibit in a wide variation in life-history traits, with differences evident between native and non-native populations or with dynamic shifts occurring over the course of an invasion (Fox and Copp, 2014). This new dynamic structure should be investigated to bring out the accurate estimation of the biological parameters of the Randall’s threadfin bream. This useful knowledge has importance in terms of sustainable fisheries in the future.
Biological traits that would be useful during the early phase of an invasion may be different from those that would be useful at a later phase. Thus, ‘r-selected’ life history traits may be favored during the initial stages of a species’ establishment and spread in a novel environment, because this species has low density and, in many cases, high food availability per individual ‘K-selected’ life-history traits. These traits can be expressed as lower reproductive investment and greater investment in individual offspring, may be favored where density levels are high enough to produce a more competitive environment (Bøhn et al., 2004; Villeneuve et al., 2005; García-Berthou, 2007; Fox and Copp, 2014; McKnight et al., 2017).
The morphometric properties are presents important parameters to biological studies (Qamar and Panhwar, 2019). In this study, length-age and weight-age growth parameters of Randall’s threadfin bream were estimated with four different growth models. It would not be right to compare the L∞, K-1, and to values in these four different growth models. The comparison of data of this study can be made with statistical confidence intervals in these four mathematical growth models. According the data of our study’s findings, these statistical comparison values are found to be the same (0.74, Table III) for mathematical growth models. However, this confidence interval r2 value in this current study was just above 0.70, which can be accepted lower limit levels in this kind of field studies. The closeness of these values (r2) is generally considered not only primarily due to a mathematical model, but also due to also age-related growth data. When we considered the results of the four different growth parameter’s models in this current study, Gompertz and Richards’s model could be evaluated the best one than all other models regarding the length distribution for the fish species.
Figure 2A shows L∞ and to values are found to be closer to each other. The similar positive result for Gompertz and Richards’ model age weight and growth equations are also shown in the same figure. Residual values in Logistic and Richards fish growth models differentiated from each other.
Comparing with previously reported studies, only the von Bertalanffy growth model can be used for estimation of length age parameters (Ergüden et al., 2010; Demirci et al., 2016). In the same fishing area, the maximum theoretical lengths in the Iskenderun Bay were slightly smaller than study reported by Ergüden et al. (2010) for Randall’s threadfin bream (Table IV). At the same time length distribution of this species was similar for Iskenderun Bay. The cause of this similarity could be defined as effects of overfishing pressure in the area and same environmental conditions. Although the length ranges of the fish in this study and findings of Ergüden et al. (2010)’s study were very similar to each other, there are some statical differences between them.
L∞ infinite value of the fish in this current study were calculated to be 27.37 which is in accordance with Murty (1981) and Kalhora et al. (2017) while it was lower than given in Ergüden et al. (2010) L∞ infinite value (34.90 cm) which is considered too high in the size distribution of the species according to King (2013). Additionally, it will not be correct to compare only L∞ in another region results. Instead, the K coefficient should be taken into account. Estimate of the growth coefficient K in this study was in parallel those of other studies (Ergüden et al., 2010; Murty, 1981; Kalhoro et al., 2017). Our results on this issue were found to be lower than value recorded by Erguden et al. (2010) in North East Mediterranean Coast and Murty (1981) and Kalhoro et al. (2017) in the Indian coast and Pakistan. The best way to be used in these comparisons is growth performance index (φ’). This study indicates a lower growth parameter according the index to the previous studies. The difference in these comparisons is natural, depending on the different variables in the regional ecological and fishery dynamics also the environment. It is clear that the difference in the study done in the same region is due to L∞ values as previously mentioned. In the Von Bertalanffy growth model used, the calculation of the confidence interval as a statistic will make the predicted values more realistic. The confidence interval was not given in the previous study of Ergüden et al. (2010).
Inspite of geographic differences the size structure of local fish populations depends on factors such as water temperature, food availability and assimilative capabilities. The fishing method might also cause different results for growth parameters (Campana, 2001).
The values of growth parameters L∞ and K-1 variables can effect each other positively. However, results of the study can be more useful for the sustainable use of fisheries management. Because the maximum length was observed to be 21.0 cm and the estimated value of L∞, was calculated to be 27.37 cm, which can be considered as a very high value by von Bertalanffy growth model (Sparre, 1987; Pauly and Munro, 1984). This species has showed fast growth characteristic with coefficients compared to most of the fish populations in the region. We believe that this species less affected by fishing pressure in region compared to other fish species which has same growth property. Considering this issue, Randall’s threadfin bream can be defined as a kind of opportunistic fish species in the Regional Fisheries management and life-history traits of this species is found to be K-selected. Therefore, it can be expected this species populations will be sustainable in the region. Litarature have supported this idea by indicating distribution of the species other parts of the Mediterranean (Gulsahin and Kara, 2013; Stern et al., 2014; Aydın and Akyol, 2016).
CONCLUSION
Similar studies in this field should be made for all fish compositions in the region to analyse population dynamics . Because dynamic structure of marine ecosystem may change continuously because of different fishing pressure and global climate change etc. In addition to, these differences also may affect the fish growth parameters from Iskenderun Bay and North East Mediterranean.
ACKNOWLEDGEMENTS
Authors are grateful to BAP foundation of Mustafa Kemal University (Hatay, Turkey) for their financial support (project no: 301).
Statement of conflict of interset
The authors declare there is no conflict of interset.
REFERENCES
Aydın, I. and Akyol, O., 2016. Occurrence of Nemipterus randalli Russell, 1986 (Nemipteridae) of Izmir Bay, Turkey. Egyptian J. aquat. Res., 39: 267-274.
Bilecenoglu, M. and Russell B.C., 2008. Record of Nemipterus randalli Russell, 1986 (Nemipteridae) from Iskenderun Bay, Turkey. Cybium, 32: 279-280.
Bøhn, T., Sandlund, O., Amundsen, P. and Primicerio, R., 2004. Rapidly changing life-history during invasion. Oikos, 106: 138-150. https://doi.org/10.1111/j.0030-1299.2004.13022.x
Campana, S.E., 2001. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol., 59: 197-242. https://doi.org/10.1006/jfbi.2001.1668
Demirci, S., Demirci, A. and Şimşek, E., 2018. Spawning season and size at maturity of a migrated fish, Randall’s Threadfin Bream (Nemipterus randalli) in Iskenderun Bay, Northeastern Mediterranean, Turkey. Fresen. Environ. Bull., 27: 503-507.
Demirci, S., Şimşek, E. and Demirci, A., 2016. Assessment of age and growth parameters of the nakedband gaper Champsodon nudivittis (Ogilby, 1895) with different models in the Eastern Mediterranean. Pakistan J. Zool., 48: 891-894.
Devaraj, M. and Gulati, D., 1988. Assessment of the stock of threadfin bream (Nemipterus japonicus) in the northwest continental shelf of India. In: The first Indian fisheries forum (ed. M.J. Mohan). Proc. Asian Fisheries Society. Indian Branch, Mangalore, pp. 159-164.
Ergüden, D., Turan, C. and Gürlek, M., 2009. Weight-length relationships for 20 Lessepsian fish species caught by bottom trawl on the coast of Iskenderun Bay (NE Mediterranean Sea, Turkey). J. appl. Ichthyol., 25: 133-135. https://doi.org/10.1111/j.1439-0426.2008.01198.x
Ergüden, D., Turan, C., Gürlek, M., Yaglıoğlu, D. and Güngör, M., 2010. Age and growth of the Randall’s threadfin bream Nemipterus randalli (Russell, 1986), a recent Lessepsian migrant in Iskenderun Bay, northeastern Mediterranean. J. appl. Ichthyol., 26: 441-444. https://doi.org/10.1111/j.1439-0426.2009.01387.x
Fox, M.G. and Copp, G.H., 2014. Old world versus new world: life-history alterations in a successful invader introduced across Europe. Oecologia, 174: 435-446.
García-Berthou, E., 2007. The characteristics of invasive fishes: what has been learned so far? J. Fish Biol., 71: 33-55. https://doi.org/10.1111/j.1095-8649.2007.01668.x
Gokoglu, M., Guven, O., Balci, B. A., Colak, H. and Golani, D., 2008. First records of Nemichthys scolopaceus and Nemipterus randalli and second record of Apterichthus caecus from Antalya Bay, Southern Turkey. Mar. Biodiv. Rec., 2: e29. https://doi.org/10.1017/S175526720800033X
Golani, D. and Sonin, O., 2006. The Japanese threadfin bream Nemipterus japonicus, a new Indo-Pacific fish in the Mediterranean Sea. J. Fish Biol., 68: 940-943. https://doi.org/10.1111/j.0022-1112.2006.00961.x
Granada, V.P., Masuda, Y. and Matsuoka, T., 2004. Age and growth of the yellowbelly threadfin bream Nemipterus bathybius in Kagoshima Bay southern Japan. Fish. Sci., 70: 497-506. https://doi.org/10.1111/j.1444-2906.2004.00831.x
Gulsahin, A. and Kara, A., 2013. Record of Nemipterus randalli Russell, 1986 from the southern Aegean Sea (Gökova Bay, Turkey). J. appl. Ichthyol., 29: 933-934. https://doi.org/10.1111/jai.12187
Haddon, M., 2001. Modeling and quantitative methods in fisheries. Chapman and Hall ⁄ Crc. Boca Raton, London, New York, Washington D.C, pp. 406.
IUCN, 2014. The IUCN red list of threatened species-Pagellus erythrinus (Becker, Common Pandora, King of the Breams, Pandora, Spanish Sea Bream) http://www.iucnredlist.org/details/170224/0.
Jemaa, S., Bacha, M., Khalaf, G., Dessailly, D., Rabhi, K. and Amara, R., 2015. What can otolith shape analysis tell us about population structure of the European sardine, Sardina pilchardus, from Atlantic and Mediterranean waters? J. Sea Res., 96: 11-17. https://doi.org/10.1016/j.seares.2014.11.002
Kalhoro, M.A., Tang, D., Ye, H., Morozov, E., Liu, Q., Memon K.H. and Kalhoro, M.A., 2017. Population’s dynamic of the Randall’s threadfin bream Nemipterus randalli from Pakistani waters, Northern Arabian Sea. Indian J. Geo-Mar. Sci., 46: 551-561.
Kimoto, H. and Johari, I., 1996. The stock status and management scheme of kerisi (Nemipterus peronii) on the east coast of peninsular Malaysia. SEAFDEC MFRDMD ⁄ RES ⁄ 1. Marine Fishery Resources Development and Management Department of Southeast Asian Fisheries Development Center, Kuala Terengganu, Malaysia.
King, M., 2013. Fisheries biology, assessment and management. John Wiley and Sons. https://doi.org/10.1002/9781118688038.ch5
Krishnamoorthi, B., 1971. Biology of the threadfin bream Nemipterus japonicus (Bloch). Indian J. Fish., 18: 1-21.
Lelli, S., Colloca, F., Carpentieri, P. and Russell, B. C., 2008. The threadfin bream Nemipterus randalli (Perciformes: Nemipteridae) in the eastern Mediterranean Sea. J. Fish Biol., 73: 740-745. https://doi.org/10.1111/j.1095-8649.2008.01962.x
McKnight, E., García-Berthou, E., Srean, P. and Rius, M., 2017. Global meta-analysis of native and nonindigenous trophic traits in aquatic ecosystems. Glob. Change Biol., 23: 1861-1870. https://doi.org/10.1111/gcb.13524
Morey, G., Moranta, J., Massuti, E., Grau, A., Linde, M. Riera, F. and Morales-Nin, B., 2003. Weight-length relationships of littoral to lower slope fishes from the Western Mediterranean. Fish. Res., 62: 89-96. https://doi.org/10.1016/S0165-7836(02)00250-3
Murty, V.S., 1981. Observations on some aspects of biology of threadfin bream Nemipterus mesoprion (Bleeker) from Kakinada. Indian J. Fish., 28: 199-207.
Pauly, D. and Munro, J.L., 1984. Once more on the comparison of growth in fish and invertebrates. Fishbyte (ICLARM), 2: 21.
Russell, B.C., 1990. FAO species catalogue. Family Nemipteridae. An annotated and illustrated catalogue of Nemipterid species known to date. FAO Fish. Synop., 125: 12.
Samuel, M., 1990. Biology, age, growth and population dynamics of threadfin bream Nemipterus japonicus. J. mar. biol. Assoc. India, 32: 66-76.
Senta, T. and Tan, K.S., 1975. Species and size-composition of threadfin snappers in the South China Sea and Andaman Sea. Singapore J. Primatol. India, 3: 1-11.
Sparre, P., 1987. A method for the estimation of growth, mortality and gear selection/recruitment parameters from length frequency samples weighted by catch per effort. (eds. D. Pauly and G.R. Morgan), Length-based methods in fisheries research ICLARM Conf. Proc., 13: 75-102.
Stern, N., Levit, Y., Galil, B.S., Diamant, A., Yokeş M.B. and Goren, M., 2014. Distribution and population structure of the alien Indo-Pacific Randall’s threadfin bream Nemipterus randalli in the eastern Mediterranean Sea. J. Fish Biol., 85: 394-406. https://doi.org/10.1111/jfb.12421
Şimşek, E. and Demirci, A., 2018. Barotrauma treatment effects on survival rates for some discarded fish by trawl fishery. Fresen. environ. Bull., 27: 4867-4873.
Qamar, N. and Panhwar, S.K., 2019. Otolith dimensions versus fish lengths estimated for five carangids (Pisces) in Pakistan. Pakistan J. Zool., 51: 1963-1965.
Tian, X., Leung, P. and Hochman, E., 1993. Shrimp growth functions and their economic implications. Aquacult. Eng., 12: 81-96. https://doi.org/10.1016/0144-8609(93)90018-7
Williams, T. and Bedford, B.C., 1974. The use of otoliths for age determination. In: The aging of fish. (ed. T.B. Bagenal). Unwin Brothers, Old Woking, Surrey, UK. pp. 114–123.
Villeneuve, F., Copp, G.H., Fox, M.G. and Stake˙nas, S., 2005. Interpopulation variation in the growth and life-history traits of the introduced sunfish, pumpkinseed Lepomis gibbosus, in Southern England. J. appl. Ichthyol., 21: 275-281. https://doi.org/10.1111/j.1439-0426.2005.00679.x
Wu, C.C., Weng, J.S., Liu, K.M. and Su, W.C., 2008. Reproductive biology of the notchedfin threadfin bream, Nemipterus peronii (Nemipteridae), in waters of southwestern Taiwan. Zool. Stud., 47: 103-113.
To share on other social networks, click on any share button. What are these?










