Kirana D.D. Adiputra1*, Tulus Maulana3 , Ekayanti M. Kaiin3, H. Hasbi2, Herry Sonjaya2
Research Article
The Semen Quality Of Bali And Simmental Bulls Reared In Technical Implementation Unit Of Regional Artificial Insemination Center At Pucak, South Sulawesi
Kirana D.D. Adiputra1*, Tulus Maulana3 , Ekayanti M. Kaiin3, H. Hasbi2, Herry Sonjaya2
1Faculty of Animal Science, Graduate School of Hasanuddin University Jl. Perintis Kemerdekaan Km.10, Makassar, Indonesia 90245; 2Department of Animal Production, Faculty of Animal Science, Hasanuddin University Jl. Perintis Kemerdekaan Km.10, Makassar, Indonesia 90245; 3Research Center for Applied Zoology, National Research and Innovation Agency Jl. Raya Bogor Km.46 Cibinong 16911.
Abstract | This study was conducted to determine the quality fresh and frozen semen of Bali and Simmental bull in the Technical Implementation Unit of Regional Artificial Insemination Center at Pucak, South Sulawesi. Two Bali bulls and two Simmental bulls aged 5-6 were used. The parameters observed in the fresh semen were volume, pH, colour, motility, viability, abnormality, intact plasma membrane, and concentration. Meanwhile, parameters observed in frozen semen were motility, viability, abnormality, intact plasma membrane, acrosome status, DNA integrity, and movement patterns. Viability and abnormality parameters were stained with eosin-nigrosine, intact plasma membrane was stained with Hypo-osmotic (HOS) solution, acrosome status was stained with peanut agglutinin (PNA), and DNA integrity was stained with acridine orange (AO). The results showed that the volume, pH, motility, abnormality, intact plasma membrane, and concentration of fresh semen of Bali and Simmental bull were not significantly different (P>0.05). Likewise, the frozen semen of Bali and Simmental breed did not show any significant differences (P>0.05) in terms of motility, acrosome status, intact plasma membrane, DNA integrity, and movement patterns, but the viability of Bali bull was significantly higher than that of Simmental bull and the abnormality of Simmental bull was significantly higher than that of Bali bull. Based on the results, it can be concluded both Bali and Simmental bull has the same quality of fresh semen, but Bali bull had better viability and abnormality compared to those Simmental breed of frozen semen.
Keywords | Bali Bull, Fresh Semen, Frozen Semen, Semen Quality, Simmental Bull
Received | October 18, 2022; Accepted | November 05, 2022; Published | November 15, 2022
*Correspondence | Kirana D.D. Adiputra, Faculty of Animal Science, Graduate School of Hasanuddin University Jl. Perintis Kemerdekaan Km.10, Makassar, Indonesia 90245; Email: [email protected]
Citation | Adiputra KDD, Maulana T, Kaiin EM, Hasbi H, Sonjaya H (2022). The semen quality of bali and simmental bulls reared in technical implementation unit of regional artificial insemination center at pucak, south sulawesi. Adv. Anim. Vet. Sci. 10(12): 2562-2570.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.12.2562.2570
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
As a livestock production center, South Sulawesi Province has considerable potential as the national cattle barn. This can be seen from the increase in beef cattle population in South Sulawesi, from 1,369,890 in 2019 to 1,431, 533 in 2020 (Statistics Indonesia, 2021). Cattle reproduction is one of the most influential supporting factors in increasing the number of beef cattle populations and the need for meat (Nurjanah et al., 2014). Various government programs have been made to increase the productivity of cattle domestically, including Bali and Simmental breed. The program is stated in the regulation of the Minister of Agriculture no.48/Permentan/PK.210/10/2016 concerning Special Program for the Acceleration of Increasing the Population of Pregnant Cows and Buffaloes signed by the Minister of Agriculture (Upsus Siwab) on October 3, 2016. This program intends to increase the cattle population in a sustainable manner by maximizing the potential of breeding cows in producing calves, improving the quality/genetic of Bali cattle, realizing the government’s commitment to pursue self-sufficiency of beef which is targeted to be achieved by 2026, realizing Indonesia as a country which can independently fulfill foods of animal origin, and at the same time improving the welfare of smallholder farmers. Therefore, the program included the implementation of artificial insemination (AI) activities, the supply and distribution of frozen semen, liquid nitrogen (N2) and containers, and forage fulfilment. In 2020, the there was a follow-up activity known as SIKOMANDAN program or Cow and Buffalo Commodity Mainstay of the Country. Frozen semen production at Artificial Insemination Centers refers to the Regulation of Minister of Agriculture no 10 of 2016 concerning the provision and distribution of frozen semen of ruminants and the Indonesian National Standard (SNI) number 4869.1:2017 on frozen semen. The bulls used as the source of semen to produce frozen semen at the artificial insemination centers are superior bulls (Handayani et al., 2021).
In frozen semen production, superior bulls (both domestic and originated from other countries) are needed because it can be stored in a long period of time and is free from infectious diseases caused by natural mating. The main purpose of frozen semen production is to increase the success rate of pregnancy as much as the natural mating by applying the artificial insemination (AI) biotechnology (Diany et al., 2016). One of the frozen semen providers is the Technical Implementation Unit of Regional Artificial Insemination Center in Pucak, South Sulawesi. One of the efforts to increase the cattle population is by utilizing Artificial Insemination (AI) technology. AI is a reproductive technology which purpose is to increase the efficiency of reproduction and even distribution of superior seeds, as well as to prevent the spread of disease. One of the factors that influence the success rate of AI is the quality of frozen semen. Therefore, its quality must always be maintained in order to maintain good fertility. However, information on semen quality of Bali and Simmental bull in Unit Local of Artificial Insemination and Semen Production in Pucak, South Sulawesi Sulawesi, is still very limited. Thus, this study was conducted to evaluate the quality of semen (fresh and frozen) from bulls breed at Unit Local of Regional Artificial Insemination Center Pucak, South Sulawesi.
MATERIALS AND METHODS
Research Materials
This research was conducted from November 2021 to February 2022 at the Regional Artificial Insemination Center (RAIC) of Pucak-Maros, South Sulawesi and the research center of biotechnology, National Research and Innovation Agency (BRIN). The research material was semen taken from two Bali bulls and two Simmental bulls aged 5-6.
Evaluation of Fresh Semen Quality
Evaluation of the fresh semen quality was conducted at RAIC Pucak, South Sulawesi by observing the semen volume as parameter. Observation was carried out directly in the semen storage tube immediately after storage (in ml). The pH was observed by dripping semen on the pH indicator, and the spermatozoa concentration was calculated by mixing 0.035 ml of semen with 3.5 ml of 0.9% NaCl solution. The solution was then homogenized for 5-7 seconds, transferred into a cuvette to measure its concentration using a photometer SDM 6 (Minitube, Germany) (Prastowo et al., 2018). Normal fresh semen in the storage tube was milky or creamy white and cloudy in color; about 10% of cattle produced normal semen with a yellowish color (Prasetyo et al., 2020). Spermatozoa motility was determined by dripping the sample of semen on a glass slide, covered with a cover glass, and then observed using a microscope (Olympus CX31) (400x magnification). Motility was assessed subjectively by looking at the number of spermatozoa that move straight ahead (progressive). The standard of motility assessment was in the range of 0 - 100%.
Spermatozoa viability is a parameter observed to identify live and dead sperm cells. It was analysed by using eosin-nigrosine staining technique. The principle of this method is that dead spermatozoa will absorb the eosin-nigrosine so that it will turn into red or pink due to the increased cell wall permeability. Ten microns of semen was dropped on a slide, and 20 microns of eosin-nigrosine solution was added. The mixture was homogenized and prepared on a slide glass. The slide was observed under a microscope connected to a monitor at 400x magnification. Dead spermatozoa stained red and live spermatozoa remained colorless (Handayani et al., 2021).

Spermatozoa abnormalities can be classified into three parts, namely head, midpiece, and tail defects. Examination of abnormal spermatozoa can be carried out by using eosin-nigrosine dye of at least 200 cells. The percentage of abnormal sperm is characterized by abnormalities in spermatozoa cells such as in the head, body, and tail of the sperm (Afiati et al., 2015).
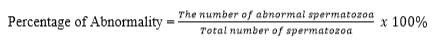
Intact plasma membrane was tested using the Hypoosmotic Swelling method (HOS Test). 10 µl of semen was mixed in 1 ml of HOS solution, homogenized and incubated at 37 °C for 30-45 minutes. The test was performed by dripping a mixture of HOS solution and incubated semen on an object glass, covered with a cover glass and evaluated using a 40 × 10 magnification microscope. Spermatozoa with intact plasma membranes are characterized by coiled or bulging tails, while the damaged ones are characterized by straight tails. The percentage of spermatozoa reacting to the HOS solution was calculated by using the following formula:
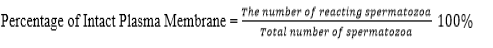
Evaluation of the Frozen Semen Quality
Evaluation of the quality of frozen semen was conducted at BRIN by observing the parameters, motility, viability, abnormality, and intact plasma membrane following the same procedure when evaluating fresh semen. The acrosome status was observed under a Z2 fluorescent imager microscope using peanut agglutinin (PNA) staining. The test was carried out by smearing the sample of semen on a glass object, fixed in 96% ethanol for 10 minutes and dried. Then, droplet of 20-30 micron of PNA was added and incubated at 370 C for 30 minutes. 5 microns of propidium iodine was dropped and incubate for 10 minutes. After that, object glass was washed using aquadest solution, covered using a cover class and observed under a fluorescent microscope at 40 × 10 magnification. Spermatozoa with defected/incomplete acrosome at the tip of the head was green in color while the intact one was reddish orange
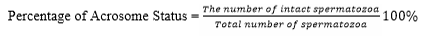
DNA integrity was observed under a Z2 fluorescent imager microscope using acridine orange (AO) staining. The test was performed by smearing the semen sample on a glass object, fixed in Carnoy for 2 hours, and rinsed with distilled water. AO was soaked overnight, rinsed with distilled water and dried. It was then observed under a fluorescent microscope with a magnification of 40 × 10 by observing that the center part of the head. The damaged one was green/dark yellow in color while the intact one was green.
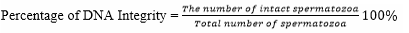
Tests were conducted using Computer-assisted sperm analysis (CASA Sperm Vision Minitube). The characteristic of post thawing motility (PTM) was motility; the distance average path (DAP) was the distance (micron) of the average path of the sperm cell travelled from the beginning to the end of the analysis period; curvilinear distance (DCL): the distance (microns) that the sperm cell travel in one second on the trajectory of the curve from the beginning to the end of the analysis period; distance straight line (DSL): the distance taken by sperm in a straight line from the first frame to the last frame of the analysis period; velocity average path (VAP): the average speed that the sperm cell travelled across from the beginning to the end of the analysis period measured in microns per second; curvilinear velocity (VCL): the average speed that the sperm cell travel across a curved line from the beginning to the end of the analysis period measured in microns per second; velocity straight line (VSL): the speed that sperm cells travelled in a straight line from the beginning to the end of the analysis period measured in microns per second; straightness (STR): VSL/VAP is the relationship between the velocity of a straight line and the velocity of the average path during the measurement period; linearity (LIN): VSL/VCL is the relationship between the velocity of the straight line and the velocity of the curved line during the measurement period; wobble (WOB): VAP/VCL is the relationship between the velocity average path and the velocity curved line during the measurement period; amplitude of lateral head displacement (ALH): the magnitude of the lateral displacement of the sperm head about its average path (microns).
It is stated as the maximum displacement, and beat cross frequency (BCF): the speed measured in Hertz (Hz) that the sperm head moves from side to side during the measurement period (Park, 2013).
Data Analysis
The data obtained was analyzed using a comparative test, namely the T test statistical analysis (T-test Independent sample), to compare the sample of Bali and Simmental bull.
RESULTS AND DISCUSSION
The Quality of Fresh Semen of Bali and Simmental Bull
The semen quality of Bali and Simmental bull is one of the important parameters in semen collection. Evaluation of semen quality includes macroscopic and microscopic tests. Macroscopic quality tests include color, pH, and volume, while microscopic quality tests include motility, viability, abnormality, intact plasma membrane, and concentration. The evaluation results are presented in the Table 1.
Table 1 describes that pH value of semen in both Bali and Simmental bull was 64± 0.0, and this result can be categorized as normal. It was in accordance with Wahyuningsih et al. (2013) who stated that the semen of normal cattle had a pH value ranging from 6.28-7.00. Further, Prastowo et al. (2018) also stated that the factor that affects the pH value of fresh semen is the feedstuffs composition which affects the seminal plasma secretion and accessory glands of the male reproductive organs. The color of the semen of Bali and Simmental cattle in this study was cream. This indicates that the semen color obtained in this study was categorized as normal. These results are in line with Feradis (2010) who argued that normal bull semen was milky white or creamy white and cloudy. The mean volume of Bali bull semen was 6.27±1.19 ml and that of Simmental breed was 6.36±1.42 ml, indicating that there was no significant difference (P > 0.05). The volume of semen produced was still within the normal range. This is in accordance with Hartanti et al. (2012) which stated that the normal volume ranged from 3.2 to 7.3 ml. In fact, this study showed higher results than those reported by Prastowo et al. (2018) which showed a mean value of 5.10±1.26 ml.
Table 1: Evaluation of Fresh Semen Quality of Bali dan Simmental Bull
| Parameters | Mean (± SD) | |
| Bali Bull | Simmental Bull | |
| Macroscopic | ||
| Color | Cream |
Cream |
| Ph | 6.4±0.0 | 6.4±0.0 |
| Volume (ml) | 6.27±1.19 | 6.36±1.42 |
| Microscopic | ||
| Motility (%) | 70 ± 0.0 | 70±0.0 |
| Viability (%) | 96.91±1.59 | 95.62±2.20 |
|
Abnormality (106/ml) |
3.16±1.53 | 4.43±2.09 |
| Intact Plasma Membrane(%) | 93.64±4.69 | 9333±3.83 |
|
Concentration (106/ml) |
1.244±0.29 |
1.247±0.24 |
Note: SD = Standard deviation
The motility found in this study with the average Bali and Simmental bull of 70 ± 0.0% shows no significant difference (P > 0.05). There was no difference between the two breeds of cattle because the bulls used was superior bulls which were selected and had met the standard. Likewise, a research by Kaiin et al. (2013) also reported that the Bali bulls at RAIC Pucak had a motility rate of 70-75%. However, it was lower than that of Fazrien et al. (2020) who found that the average motility of Bali bull spermatozoa obtained was 81.6±8%. Adhyatma et al. (2013) argued that there were several factors affecting the motility of spermatozoa such as age, environment, feedstuffs, semen collection frequency, and body weight of each animal.
The viability of Bali bull in this research was 96.91±1.59% and that of Simmental bull was 95.62±2.20%, indicating no significant difference (P> 0.05). Based on these data, it can be concluded that this fresh semen is suitable for further processing. This is in accordance with Prastika et al. (2018) who stated that the percentage of spermatozoa viability should be at least 60% - 70%, so that it is suitable to be processed into frozen semen. This result is higher than that conducted by Musakkir et al. (2017) which revealed that the average viability of fresh semen of Aceh bull was 86.92 ± 2.87%. The observation result of sperm viability of Bali and Simmental bull can be seen in Figure 5.
This study obtained that the abnormalities of Bali and Simmental bull were 3.16±1.53% and 4.43±2.09% respectively, indicating no significant difference (P> 0.05) between them. It can be concluded that this semen deserves to be processed further. Likewise, Utami et al. (2014) stated that spermatozoa abnormalities were no more than 12% to 23% and the normal percentage was between 77% to 88% in livestock. However, the result of this study was lower than that of Rahmiati et al. (2018) with an average abnormality of 10.34% in Aceh cattle and higher than that of Kaiin and Kaiin and Gunawan (2015) with abnormalities of 1.47% in Simmental bull previously reared at RAIC Pucak. A factor that can influence spermatozoa abnormalities in livestock is ejaculation frequency which can affect the quality and morphology of abnormal spermatozoa in fresh semen that has been collected. Abnormalities in Bali and Simmental bull can be seen in Figure 6.
Incomplete or damaged plasma membrane can affect the success rate of spermatozoa to fertilize an egg (Handayani et al., 2021). Further, Anwar et. al (2014) added that the integrity of the plasma membrane affects the active movement of spermatozoa. The observation result on the intact plasma membranes can be seen in Figure 7.
This study revealed that the intact plasma membrane of Bali and Simmental bull were 93.64 ±4.69% and 93.33 ±3.83% respectively, indicating no significant difference (P > 0.05). This result was higher than that of Muzakkir et al. (2017) with average intact plasma membrane of 88.30 ± 1.61% in fresh semen of Aceh bull and Arvioges et al. (2021) with intact plasma membrane of 59.50±22.96% in Bali cattle.
The concentration of fresh semen obtained in this study was 1,244±0.29 in Bali bull and 1,247±0.24 in Simmental bull, indicating no significant difference (P > 0.05). This result is lower than that previously reported by Kaiin and Gunawan (2015) stating that the sperm concentration of Simmental bull collected at RAIC Pucak was 1,960 x 106 cells/ml, while the concentration in Bali cattle was relatively the same with 1,256.7 x 106 cells/ml (Kaiin et al, 2013). The stress factor is one of the factors that can affect the spermatozoa concentration and any changes in environmental temperature will affect the male reproductive organs (Feradis, 2010). Furthermore, the thermoregulatory function of the scrotum will be disrupted, which then also impact the spermatogenesis process in bull (Garner and Hafez, 2016).
The Quality of Frozen Semen of Bali and Simmental Bull
The main objective of the evaluation of frozen semen is to increase the success rate of pregnancy so that it is similar to natural mating with the application of artificial insemination (AI) biotechnology. Based on the results of the study, the frozen semen quality of Bali and Simmental bull can be seen in Table 2.
The motility of frozen semen of Bali and Simmental Bull showed no significant difference (P>0.05), with 62.41±5.36% vs 61.46±1.52%. The results of this study are regarded as suitable to be used based on the standards set by Indonesian National Standard (SNI) of bull frozen semen serial number 4869.1-2017 which is more than 40% (National Standardization Agency, 2017). The frozen semen motility of Simmental bull obtained in this study was higher than that previously reported by Nalley et al. (2016) with mean motility of 43.60±1.71% in Simmental cattle. According to Gangawar et al. (2016), most of the motility and viability of the spermatozoa decreased at each stage of semen processing due to various treatments which can cause the spermatozoa to die such as a cold shock effect. However, generally around 50% - 60% of the spermatozoa will be able to survive until the thawing stage with the appropriate procedure. Furthermore, Hapsari et al. (2018) reported that during the freezing process, about 50% of spermatozoa will die. Spermatozoa motility can be influenced by many factors including breed, age, management, feedstuff’s nutrition and collection techniques which affect the percentage of individual motility (Rahmawati et al., 2015).
Table 2: Evaluation of Frozen Semen Quality of Bali dan Simmental Bull
| Parameters | Mean (± SD) | |
| Bali Bull | Simmental Bull | |
| Motility (%) | 62.41± 5.36 | 61.46±1.52 |
| Viability (%) |
94.50±2.05a |
92.71±3.21b |
| Abnormality (%) |
3.69±0.81a |
5.71±2.06b |
| Acrosome Status (%) | 93. 34±3.31 | 93.97±3.35 |
| Intact Plasma Membrane (%) | 92.24±3.44 | 92.76±2.73 |
| DNA Integrity (%) | 96.41±3.02 |
95.67±2.43 |
Note: Different superscripts in the same line shows significant difference (P<0.05)
The viability of frozen semen of Bali bull was significantly higher than that of Simmental bull (P<0.05) with 94.50±2.05% vs 92.71±3.21%. This shows that Bali cattle semen has better durability than that of Simmental breed in the freezing process. The result of this study was higher than what previously reported by Handayani et al. (2021) with spermatozoa viability percentage value of 82.65±4.7%. The percentage of sperm viability of Bali and Simmental bull was classified as very good with an average percentage value of above 90%. Furthermore, Garner and Hafez (2016) added that the spermatozoa viability for producing thawed or frozen semen need to have at least 60% to 75% of live spermatozoa. Spermatozoa abnormalities in Bali bull showed a significant difference (P < 0.05) which was lower than Simmental cattle, with 3.69 ± 0.81% vs 5.71 ± 2.06. It indicates that the spermatozoa of Bali cattle were more resistant to changes in temperature during the processing of frozen semen production than that of Simental semen. It may also be due to breed differences. Likewise, Chandolia et al. (1999) also argued that post-thawing heat shock can influence the resistance of spermatozoa, one of the causing factors is genetic factor. Based on the result of the study, the spermatozoa abnormality of Simmental breed was higher than that found by Kusumawati et al. (2016) with a mean score of 4.88 ± 0.08 in the same breed.
There was no difference between the acrosome status of Bali and Simental bull (P>0.05) in this study. The result implies that the percentage of spermatozoa with damaged acrosome status was very low when compared to the previous study reported by Fatah et. al. (2018) with 40% found in Bali bull and 50% in Simmental bull during the observation of the hood acrosome intact. Sitepu and Marisa (2019) claimed that the acrosome plays an important role in the fertilization process for successful artificial insemination. The spermatozoa binding initiation to the zona pellucida will trigger the acrosome reaction which lead to the release and activation of acrosome enzymes, so that the spermatozoa are able to penetrate the zona pellucida. The observation result of the acrosome status of Bali and Simmental bull can be seen in Figure 8.
The acrosome membrane of spermatozoa which consists of an Inner Acrosome Membrane (IAM) and an Outer Acrosome Membrane (OAM) that serve as a protective acrosome for the spermatozoa will be stained. Specific proteins that make up the spermatozoa membrane will be stained so that they can be distinguished. If part of the acrosome is damaged or incomplete, the spermatozoa cells are unable to properly absorb the color and the staining result will look faded. Irregularity in the head can also be used as an indication of damaged membrane which has the potential to damage the acrosome (Nofa et al., 2017). The intact plasma membranes of spermatozoa taken from Bali and Simmental bull were 92.24±3.44 and 92.76±2.73 respectively, indicating no significant difference (P > 0.05). The decreasing value of the intact plasma membrane corelates closely with the success in decreasing the spermatozoa ability to fertilize oocytes (Handayani et al., 2021).
DNA integrity of spermatozoa of Bali and Simmental bull were 96.42 ±3.01 vs 95.67±2.43, indicating no significant difference (P > 0.05). The result of this study showed less damaged spermatozoa DNA than that of previous research by Priyanto et al. (2018) which revealed that each nation has a different level of DNA damage. Brahman bull showed the lowest DNA damage (93.17±1.25%) and the highest was Simental bull (90.66±3.76%). The observation result of the DNA integrity of Bali and Simmental bull can be seen in Figure 9.
Prabowo et al. (2016) added that damage to chromatin deoxyribose nucleic acid (DNA) of spermatozoa was an important factor which cause infertility. In the head area of spermatozoa, there is a nucleus containing DNA which is an important component in the fertilization process. All genetic information that will be passed down from one generation to the next is contained in the DNA strands in the nucleus of spermatozoa (Saili et al., 2006). DNA damage of spermatozoa can occur during spermatogenesis, the freezing process and after thawing. It may occur due to several factors, including age, infection in the testes, lack of protamine elements, hormones, contaminated with toxic chemicals, and drugs (Priyanto et al., 2015).
Spermatozoa Movement Pattern of Bali and Simmental Bull
The movement pattern of spermatozoa is an important factor to support the fertilization process. Spermatozoa movement patterns were analyzed using CASA. The pattern of movement of spermatozoa in Bali and Simmental cattle can be seen in Table 3.
Table 3 shows that the DAP, DCL, DSL, VAP, VCL, VSL, STR, LIN, WOB, ALH, and BCF value of frozen semen of Bali and Simmental bull do not show significant differences (P>0.05). The results of DAP, DCL, and DSL analysis on Bali and Simmental bull have relatively the same value as the research of Diansyah et al. (2022) and Sarastina et al. (2007).
Table 3 shows that Bali and Simmental bull have good VCL and VSL values this is the same as the research of Rounge (2003) and Suzuki et al (2003) which reported that VCL values in Bali bull have optimally moving spermatozoa with VCL values > 100 μm/s as suggested by that the progressive motility of spermatozoa is characterized by a VAP of > 25.0 μm/s. Donnelly et al. (1998) reported that the high value of VCL, VSL and VAP possess high probability of pregnancy rate, because VCL and VSL are parallel to fertilization ability, while VAP value indicates a high correlation with pregnancy rate.
Table 3: The Movement Pattern of Frozen Spermatozoa in Bali and Simmental Bull
| Parameters | Mean (± SD) | |
| Bali Bull | Simmental Bull | |
| DAP (µm) | 31. 24±1.52 | 28.88±7.11 |
| DCL (µm) |
44.14±6.93 |
40.90±7.81 |
| DSL (µm) |
24.07±6.83 |
22.07± 7.38 |
| VAP (µm/s) | 68.97±17.37 | 65.25±17.39 |
| VCL (µm/s) | 107.83±11.1 | 99.27±16.39 |
| VSL (µm/s) | 52.45±7.01 | 47.72±7.37 |
| STR | 0.71±0.06 | 0.68±0.06 |
| LIN | 0.48±0.06 | 0.47±0.07 |
| WOB | 0.68±0.19 | 0.68±0.05 |
|
ALH (μm) |
5.19±0.97 | 5.10±1.63 |
| BCF (Hz) | 25.89±2.92 |
25.89±3.66 |
Note: SD = Standard Deviation
LIN values in Bali bull 0.48±0.06 (48%) and Simmental bull 0.47±0.07 (47%) this shows spermatozoa swimming linearly according to research reported by Setiyono et al. (2020) that spermatozoa swim linearly when LIN values > 35%. The LIN value in spermatozoa may indicate the characteristics of the movement direction or the straightness of spermatozoa movement. (El-Bahrawy, 2017).
ALH values in Bali and Simmental bull were 5.19±0.97 μm and 5.10±1.63 μm, respectively, while the BCF values of Bali and Simmental bull were 25.89±2.92 Hz and 25.89±3.66 Hz. ALH and BCF values were relatively higher with the ALH and BCF values reported by Ratnawati et al. (2018). The ALH parameter indicates the average amplitude of spermatozoa head ascillations (vibration) during swimming (Kathiravan et al., 2011) while BCF is the number of time the path crosses an average spermatozoa per second grove where is useful parameter to identify change in the pattern of flagellar beat.
CONCLUSION
Based on the results, it can be concluded both Bali and Simmental bull has the same quality of fresh semen, but Bali bull had better viability and abnormality compared to those Simmental breed of frozen semen.
ACKNOWLEDGEMENT
The authors are thank to Regional Artificial Insemination Center South Sulawesi and the research center of biotechnology, National Research and Innovation Agency (BRIN) for their assistance in facilitated this research.
conflict of interest
The authors declared that they had no conflicts of interests.
novelty statement
Information on semen quality of Bali and Simmental bull in Unit Local of Artificial Insemination and Semen Production in Pucak, South Sulawesi Sulawesi, is still very limited. Thus, this study was conducted to evaluate the quality of semen (fresh and frozen) from bulls breed at the Local Unit of Regional Artificial Insemination Center Pucak, South Sulawesi.
authors contribution
All authors contributed equally in conducting and writing the manuscript.
REFERENCES
Adhyatma M., Nurul I., Nuryadi N. (2013). The effect of weight on simmental cattle semen quality and quantity. Ternak Trop. J. Trop. Anim. Prod., 14(2): 53-62.
Afiati F., Yulnawati Y., Riyadi M., ArifiantinI R. I. (2015). Spermatozoa abnormality with different semen collection frequency in ram. In Prosiding Seminar Nasional Masyarakat Biodiversitas Indonesia. 1(3): 930-934.
Anwar P., Ondho Y.S., Samsudewa D. (2014). The effect of filteret sugarcane juice in egg yolk citrate on quality of Bali catle semen. J. Peterna.11(2): 48-54.
Arvioges A., Anwar P., Jiyanto J. (2021). Effect of thawing temperature on intact plasma membrane (mpu) and intact acrosome cap (tau) spermatozoa of Bali cattle . Green Swarnadwipa: J. Pengembangan Ilmu Pertanian. 10(2): 342-350.
Bernecic NC, Gadella BM, Leahy T, de Graaf SP. (2019). Novel methods to detect capacitation-related changes in spermatozoa. T Heriogenol. 137: 56-66. https://doi.org/10.1016/j.theriogenology.2019.05.038
Chandolia R. K., Reinertsen E. M, Hansen P. J. (1999). Lack of breed differences in responses of bovine spermatozoa to heat shock. Short Comunication. J. Dairy Sci. 82 : 2617 – 2619.
Diansyah A. M, M. Yusuf A. L. Toleng2, M. I. A. Dagong. (2022). Characteristic and Kinematics of Bali-Polled Bull Sperms. Adv. Anim. Vet. Sci. 10(8): 1787-1796.
Diany E., Muhandri T. (2016). Marketing strategy of beef cattle frozen semen in aia lembang. Manajemen IKM: J. Manajemen Pengembangan Industri Kecil Menengah, 11(1): 61-71. https://doi.org/10.29244/mikm.11.1.61-71
Donnelly E. T., Lewis S. E., McNally J. A., Thompson W. (1998). In vitro fertilization and pregnancy rates: the influence of sperm motility and morphology on IVF outcome. Fertil. Sterilit., 70(2): 305-314. https://doi.org/10.1016/s0015-0282(98)00146-0
El-Bahrawy KA (2017). The influence of caffeine supplementation and concerted utilization of enzymatic and mechanical semen liquefaction on freezability of dromedary camel spermatozoa. Int. J. Vet. Med. 5: 121- 127. https://doi.org/10.1016/j.ijvsm.2017.09.005.
Fatah. K., D. Dasrul, M. A. N. Abdullah. (2018). Comparison of frozen semen quality of Aceh cattle, Bali cattle, Brahman cattle and Simmental cattle and the relationship with the success level of artificial insemination in female aceh cattle. Agripet. 18(1). https://doi.org/10.17969/agripet.v18i1.8709
Fazrien W. A., Herwijanti E., Isnaini N. (2020). Effect of individual variation on fresh and frozen bali bulls semen. Sains Peternakan: Ju. Penelitian Ilmu Peternakan, 18(1), 60-65. https://doi.org/10.20961/sainspet.v18i1.37986
Feradis. (2010). Bioteknologi Reproduksi pada Ternak. Alfabeta. Bandung.
Fuerst-Waltl B., H. Schwarzenbacher, C. Perner, J.S. Olkner. (2006). Effects of age and environmental factors on semen production and semen quality of Austrian Simmental bulls. Anim. Reprod. Sci. 95(1): 27–37. https://doi.org/10.1016/j.anireprosci.2005.09.002.
Gangawar C., S.D. Kharche, S. Kumar, S.K. Jindal. (2016). Cryopreservation of goat semen : status and prospects. Indian J. Small Rumin. 22(1): 1–10. https://doi.org/10.5958/0973-9718.2016.00005.2.
Garner D L, E. S. E. Hafez. (2016). Spermatozoa and Seminal Plasma. In: Reproduction in Farm Animals. Baltimore, Maryland, USA: Lippincott Williams & Wilkins. pp. 96–109. https://doi.org/10.1002/9781119265306.ch7.
Handayani E., Supriatna I., Tumbelaka L. I., Kaiin, E. M. (2021). Comparative analyzis of quality sni certified and non sni certified frozen semen. J. Vet. 22: 207-15. https://doi.org/10.19087/jveteriner.2021.22.2.207.
Hapsari R D., Y. Khalifah, N. Widyas, A. Pramono, S. Prastowo. (2018). Age effect on post freezing sperm viability of Bali cattle (Bos javanicus). IOP Conference Series: Earth Environ. Sci. 142: 012007. https://doi.org/10.1088/1755-1315/142/1/012007.
Hartanti D., Setiati E. T., Sutopo (2012). comparison of yolk egg citrate acid and yolk egg tris as semen diluter on percentage of sperm viability in brebes javanese cattle. Anim. Agric. J., 1(1): 33–42.
Kaiin E.M, M. Gunawan, F. Afiati, S. Said dan, B. Tappa. (2013). Production of frozen sexing sperm separated with BSA column method with standardizedon Artificial Inseminartion Center. Proceedinds Int. Conference Biotechnol. 2012. Pp 67-72.
Kaiin E.M., M. Gunawan. (2015). Uji coba produksi sperma sexing sapi simmental di UPTD-IB Pucak, Sulawesi Selatan. Prosiding Seminar Nasional “Bioresources Untuk Pembangunan Ekonomi Hijau” pp. 234-242.
Kathiravan P., Kalatharan J., Karthikeya G., Rengarajan K., Kadirvel G. (2011). Objective sperm motion analysis to assess dairy bull fertility using computer-aided system - A Review. Reprod. Dom. Anim. 46: 165-172. https://doi.org/10.1111/j.1439-0531.2010.01603.x
Kusumawati E. D., Krisnaningsih A. T. N., Romadlon R. R. (2016). The quality of Simental frozen semen based on the temperatures and duration of thawing. Jurnal Ilmu-Ilmu Peternakan. Indonesian J. Anim. Sci., 26(3): 38-41. https://doi.org/10.21776/ub.jiip.2016.026.03.06.
Muzakkir, Dasruh, S. Wahyuni, M. Akmal. M. Sabri. (2017). His study was done to investigate effects of equilibration time on the quality of Aceh cattle semen
frozen using Andromed® diluents. J. Ilmiah Peter. 5 (2): 115-128.
Nalley W. M., Pratama A., Arifiantini R. I. (2016). Evaluate sperm motility, velocity and to calculate the sperm concentration in one straw produce by the regional artificial insemination center in Indonesia.
National Standardization Agency (2017). SNI Frozen Semen- Part 1: Bulls. BSN.
Nofa Y., Karja N. W. K., Arifiantini R. I. (2017). Acrosome Status and Quality of Post - Thawed Sperm from Several Cattle Breed of Two Artificial Insemination Centre. Acta Vet. Indonesiana., 5(2): 81-88. https://doi.org/10.29244/avi.5.2.81-88.
Nurjanah T., Hartono M., Suharyati S. (2014). The influential factors of conception rate on cattle after estrous synchronization in Pringsewu regency. J. Ilmiah Peternakan Terpadu., 2(1).
Park S. (2013). Effect of sow, boar, and semen traitson sow reproduction. Thesis. University of Nebraska. Lincoin.
Prabowo T.A., R.I. Arifiantini, D. Sajuthi, dan U. Saefullah. (2016). Development method of livestock sperm dna damage identification. J. Sain Vet. 32(2): 166-171. https://doi.org/10.22146/jsv.27538.
Prastika Z., Susilowati S., Agustono B., Safitri E., Fikri F., Prastiya R. A. (2018). Motility and Viability of Rambon Cattle Spermatozoa in Kemiren Village Banyuwangi. J. Medik Vet., 1(2): 38-42. https://doi.org/10.20473/jmv.vol1.iss2.2018.38-42.
Prastowo S., Dharmawan P., Nugroho T., Bachtiar A., Pramono A. (2018). Age Effect on Bali Cattle (Bos Javanicus) Fresh Semen Quality. J. Ilmu Ternak Universitas Padjadjaran, 18(1): 1-7. https://doi.org/10.24198/jit.v18i1.17684.
Prasetyo H., Y. S. Ondho, D.Samsudewa. (2020). Kualitas makroskopis semen segar pejantan sapi Peranakan Ongole Kebumen pada umur yang berbeda. J. Anim. Res App. Sci., 2(1):1-5.
Priyanto L., Arifiantini R. I., Yusuf T. L. (2015). Detection of sperm dna damage infresh and frozen semen using toluidine blue staining. J. Vet., 16(1): 48-55. https://doi.org/10.33230/JPS.7.1.2015.7081.
Priyanto L., Budiyanto A., Kusumawati A., Arifiantini I. (2018). Comparison of post thawing spermatozoa dna damage examination between sperm-bos-halomax® and toluidine blue. J. Peternakan Sriwijaya., 7(1). https://doi.org/10.33230/JPS.7.1.2018.7081.
Rahmawati M.A., T. Susilawati, dan M.N. Ihsan. (2015). Semen quality and frozen semen
production of beef cattle at different month collection. J. Ilmu-ilmu Peternakan. 25 (3) : 25 - 36.
Rahmiati R., Eriani K., Dasrul D. (2018). Kualitas dan morfologi abnormal spermatozoa sapi aceh pada berbagai frekuensi ejakulasi. Prosiding Biotik, 3(1).
Ratnawati D., N. Isnaini., dan T. Susilawati. (2017). Pemanfaatan casa dalam observasi motilitas spermatozoa semen cair sapi madura dalam pengencer berbeda. J. Ilmu-Ilmu Peternakan. 27 (1): 80-95. https://doi.org/10.21776/ub.jiip.2017.027.01.07.
Rounge M. (2003). Sperm Motility. Sperm Motility.
Saili T., W.E. Prasetyaningtyas, M.A. Setiadi, S. Agungpriyono, dan A. Boediono. (2006). DNA integrity of sheep spermatozoa after the freeze drying process. J. Ilmu Ternak dan Vet. 11(3): 215-221.
Sarastina T., Susilawati, G Ciptadi (2007). Analisa beberapa parameter motilitas sperma pada berbagai ternak menggunakan Computer Assisted Semen Analysis (CASA). J.Ternak Troika., 6(2):1-12.
Setiyono A., Setidyadi M.A., Kaiin E.M, Karja N.W.K. (2020). Pola gerakan spermatozoa sapi setelah diinkubasi dalam media fertilisasi dengan imbuhan heparin dan/atau kafein. J. Vet., 21(3): 458-469. http://doi.org/10.19087/jveteriner.2020.21.3.458
Sitepu S. A., Marisa J. (2019). Percentage value of membrane integrity and acrosome integrity spermatozoa in simmental liquid semen with addition penicillin and sweet orange essential oil. In IOP Conference Series: Earth Environ. Sci., 327 (1): 012027. https://doi.org/10.1088/1755-1315/327/1/012027.
Suzuki K., M. Geshi., N. Yamaguchi., T. Nagai, (2003). Functional Changes and Motility Characteristic of Japanese Black Bull Spermatozoa Separated by Percoll. Anim. Reprod. Sci. 77: 157-172. https://doi.org/10.1016/S0378-4320(03)00035-6.
Utami T., Tophianon T. C. (2014). The Effect of Thawing Temperature on Sperm Quality of Friesian Holstein Bulls. J. sain Vet., 32(1): 0126-0421.
Wahyuningsih A., D.M. Saleh dan Sugiyatno. (2013). Effect of male age and storage frequency on volume and motility of fresh semen of Simmental cattle at the Lembang Artificial Insemination Center. J. Ilmiah Peternakan.1(3):947-953.
To share on other social networks, click on any share button. What are these?





