The Influence of Mature Body Weight Variation on Egg Production and Egg Quality Traits of Hy-Line Brown Hens
Research Article
The Influence of Mature Body Weight Variation on Egg Production and Egg Quality Traits of Hy-Line Brown Hens
Elfira Kariane Suawa1*, Juliet R. Roberts2, Greg Parkinson3, Angelina Novita Tethool1
1Faculty of Animal Science, Papua University, Jl. Gunung Salju Amban, Manokwari, West Papua- Indonesia; 2Animal Science, School of Environmental and Rural Science, University of New England, Armidale, New South Wales, Australia; 3Livorno Consulting, 86 Wilson St., Brunswick, Australia
Abstract | The present study was to evaluate the effect of body weight variation of flocks of laying hens on egg production, shell and internal egg quality. Sixty pullets were designed to be both at and significantly above the breeder standard, were allocated into one of 3 groups in accordance with their body weights. Each group had 20 replicates of one bird each. Eggs were processed for measurements of eggshell and egg internal quality variables, scoring of ultrastructural mammillary layer features, completeness of cuticle cover. Body weight at point of lay and mature body weight at 40-45 weeks did not significantly affected egg production, but significantly impacted on egg size eggshell quality and internal egg quality. Cuticle cover (ΔE*ab value), shell breaking strength, deformation, percentage shell and Haugh unit were higher in the light BW group associated with a lower average egg weight. If body weight can be maintained at the level of breeder standards there are significant gains in egg quality characteristics. The correct body weight and high uniformity of the flock at point of lay provide the platform for the maximum expression of genetic potential for production performance and egg shell quality over the laying period. In contrast deviations in body weight are not necessarily associated with lower egg production, but are likely to be associated with significant losses due to compromised egg shell quality.
Keywords | Laying hen, Body weight, Flock uniformity, Egg shell quality
Received | April 16, 2024; Accepted | May 29, 2024; Published | October 29, 2024
*Correspondence | Elfira Kariane Suawa, Faculty of Animal Science, Papua University, Jl. Gunung Salju Amban, Manokwari, West Papua- Indonesia; Email: e.suawa@unipa.ac.id
Citation | Suawa EK, Roberts JR, Parkinson G, Tethool AN (2024). The influence of mature body weight variation on egg production and egg quality traits of hy-line brown hens. Adv. Anim. Vet. Sci. 12(12): 2467-2477.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.12.2467.2477
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The organism’s performance in the field, including chicken, was affected by flock uniformity. Meanwhile, low chicken uniformity affects breeding and reproduction (Sutopo et al., 2021). The low uniformity from the genetic point of view is derived from the poor selection program (Zen et al., 2020). Therefore, the uniformity of chicken needs to be measured to perform an appropriate selection. in commercial practice, flock uniformity is an important performance indicator and economic driver. Uniformity has a significant influence on the growth and final growth performance of the poultry, and the weight of the chicken is generally used as an indicator of the quality of the chick.
Madsen and Pedersen (2010) pointed out that in the USA wholesale purchasers of chicken meat insist on supply of carcasses within a narrow weight range, and that failure to meet these specifications can incur severe economic losses to the processor. From these brief comments it is easy to see that flock uniformity in live weight is a very important matter. The question is just what is a benchmark figure for flock uniformity in commercial practice? Flock uniformity can be expressed as the coefficient of variation in live weight, with increased coefficient variation values synonymous with decreased uniformity, or in other words, a wider spread in live weights above and below the flock average.
Early studies have shown maintaining uniform body weight is a major objective, particularly during the rearing period, as is reinforced in all of the Commercial Breeder manuals (Asensio et al., 2020; Aviagen, 2016; Kosbah et al., 2010). Uniformity provides an estimate of the variability in a given flock at a given age. (Kosba et al., 2010) pointed out that the more uniform the flock, the better the performance of that flock, and the more consistent the responses to nutrition. (Edeh et al., 2020) reported that rearing of pullets according to bodyweight groups is advantageous and could be practiced by poultry farmers in order to obtain improved performance of the flock and offer potential economic savings through more efficient production. Preliminary research undertaken for the AECL in project VAG-1A illustrates highly significant variation in point of lay pullet flock uniformity and in average live weights across 6-7 different farms in Victoria (Parkinson et al., 2007). (England et al., 2022) found that rearing birds as equally mixed-sex results in the lowest coefficient of variatiom percentage for performance parameters and best BW uniformity compared to single-sex birds.
Table 1: Body weight starts at 16 weeks.
|
BW Grouping |
Weight (kg) |
Uniformity Baseline (%) |
|
Light |
1.170 |
100 |
|
Medium |
1.337 |
100 |
|
Heavy |
1.507 |
95 |
Although uniformity is mentioned repeatedly in the Production Manuals for all the major breeds of layer, there has been surprisingly little scientific research conducted into ways of ensuring pullet flock uniformity and then maintaining uniformity throughout lay. A closer examination of these relationships is important in the Australian Egg Industry, because there is good evidence that both pullet weights and subsequent mature weights of commercial layers substantially exceed from the body weight patterns described by the Commercial Breeders. Therefore, the purpose of the present study was to examine the mature body weight variation at point of lay and body weight uniformity on egg production and egg quality traits of Hy-Line brown hens.
MATERIALS AND METHODS
Body Weight and Flock Uniformity
In this research groups of pullets were allocated to three body weight groups that attempted to firstly simulate highly uniform flocks at weights prescribed by the breeder, and two additional body weights groups designed to exceed the breeders recommended body weight by 200 and 300 g (medium and heavy weight, respectively) also with high uniformities. A total of one hundred fifty birds were weighed and sorted according to body weight from the lowest to the highest. Three groups representing light (Breed Standard), medium and heavy weights relative to the mean were selected with mean weights of 1.170, 1.337, and 1.507 kg at 16 weeks (Table 1), respectively.
Each weight grouping was represented by 20 birds with a total of sixty Hy-Line brown birds being housed individually in cages and randomised through of the shed. Body weights (BW) were recorded at the ages of 16, 19, 26, 37, 50, 60, 70 and 80 weeks. Body weight uniformity was calculated as
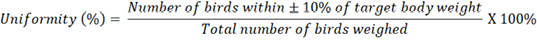
Feed intake was recorded and calculated weekly.
Egg Production and Quality
Egg production records of laying hens were kept daily throughout the experiment. Group records for production were converted to hen-day egg production percentages (eggs/hen/day as a percentage). Lights stayed for 16 hours.
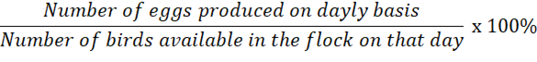
Shell Cuticle Cover
Sixty eggs were collected weekly and used for measurements of cuticle cover estimation and egg shell quality. Shell reflectivity (%) was measured using the TSS shell reflectivity meter. Shell colour was measured using the L*a*b* colour space system, using a Konica Minolta hand-held spectrophotometer (Model CM-2600d; Ramsey, NJ, USA). Eggs were soaked in MST cuticle blue (MS Technologies, Europe Ltd, Kettering, Northamtonshire, UK), made up according to the manufacturer’s recommendation, dye for 1 min. The colour of the eggshell cuticle stained with MST cuticle blue was again measured using the L*, a*, b* colour space system using the same procedures described earlier. For L* has a maximum of 100 (white) and a minimum of 0 (black). For a*, green is towards the negative end of the scale and red towards the positive end. For b*, blue is towards the negative end and yellow towards the positive end of the scale. The difference between the reading before and after staining was measured as a single score.
A single score value (ΔE*ab) was calculated after the method of (Leleu et al., (2011).
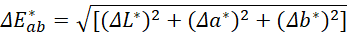
Traditional Eggshell and Egg Internal Quality Measurements
Egg internal and eggshell quality parameters were measured by TSS (Technical Services and Supplies, Dunnington, York, UK) equipment. Shortly, egg weight was measured on a top pan balance. Shell breaking strength and shell deformation were measured by quasistatic compression using TSS QC-SPA (50 N load cell) equipment by placing horizontally individual eggs were in the egg holder of the shell breaking strength machine. Shell weight was measured using a Quintix513-1S balance (Sartorius Lab Instruments GmbH & Co. KG Goettingen, Germany). Shell thickness was measured by a custom-built gauge, based on a Mitutoyo Dial Comparator Gauge Model ID-F150E (Kawasaki, Japan). Percentage shell was calculated as a shell weight to egg weight ratio. For the egg internal quality variables; albumen height, Haugh unit, and yolk colour were measured using the TSS QCEQCM equipment. The same equipment is also used by (Samiullah, et al., 2017).
Egg Shell Ultrastructures
The ultrastructural features of the shell mammillary layer were scored using a JCM-5000 Neoscope benchtop scanning electron microscope (SEM). Small pieces of shell approximately 1 cm square were cut out from around the equator of the eggshell using a Dremel tool (Dremel High Speed rotary tool, 300 series, Robert Bosch Tool Corporation, Gerlingen, Germany), then dried pieces were then plasma etched in a BioRAD RF Plasma Barrel Etcher PT 7150, Hertfordshire, UK, Reid (1983). Each shell pieces was air dusted and mounted on an 9 mm diameter aluminium stub using I005Aqueous conductive silver liquid SEM adhesive (I005-ProSciTech, Townsville, Qld, Australia). The shells were gold sputter coated and viewed under the SEM a Jeol JCM-5000 Neoscope desktop (JEOL, Tokyo, Japan).
A total of thirty eggshell sample was scored for ultrastructural features of the mammillary layer of individual eggshells was scored as described by Bain (2005). Mammillary cap size was scored as 1 (similar), 2 (variable), 3 (highly variable). Mammillary caps were scored according to their quality which was assessed as both the size of the cap in relation to its cone and the degree of membrane attachment from 1 (best) to 5 (worst). Other variables such as confluence, early fusion, late fusion, alignment, Type-A bodies, Type-B bodies, aragonite, cubics, cubic cone formations, changed membrane, cuffing, depression, erosion, holes were each ranked for incidence from 1 (none) to 4 (extensive).
RESULTS AND DISCUSSION
Body Weight, Body Weight Uniformity and Feed Intake
The average body weight of the one hundred and fifty birds from which the body weight groups were selected was 1.3 kg at 16 weeks and average uniformity percentage was 98. The average body weight of birds in the three body weight groups from age 16 weeks to 80 weeks is presented in Table 2. There were significant effects of BW groups and age on the body weight. The body weight increased as the flock aged with the final body weight at 80 weeks of 2.0 kg, 2.2 kg and 2.3 kg for the light (breed standard), medium and heavy BW group respectively. The highest body weight was reached at 60 weeks of age; 2.1 kg, 2.2 kg and 2.4 kg for the light, the medium and the heavy BW groups, respectively.
Body weight uniformity as indicated by percentage for each group is presented in Table 2. Uniformity ranged from 61% to 100%. The highest uniformity 100% was recorded at 16 weeks (the age at which the body weight groups were selected) for the light and the medium BW groups and remained constant until 19 weeks of age in the medium group. As the age of flock increased, the body weight uniformity decreased. Although the uniformity of body weight declined to 67% at 80 weeks in the light BW group, and 70% at 70 weeks in the medium BW group, the body weight uniformity was 84 % and 80% at 80 weeks of age in the heavy and medium groups, respectively.
Table 2: Body weight and uniformity of body weight groups from age 16 weeks to 80 weeks in percentage.
|
Hen Age (weeks) |
Body weight (kg) |
Body weight Uniformity (%) |
||||
|
Light |
Medium |
Heavy |
Light |
Medium |
Heavy |
|
|
16 |
1.2±0.01 |
1.3±0.00 |
1.5±0.01 |
100 |
100 |
95 |
|
19 |
1.6±0.03 |
1.7±0.02 |
1.9±0.03 |
90 |
100 |
89 |
|
26 |
1.7±0.03 |
1.8±0.03 |
2.0±0.03 |
80 |
80 |
89 |
|
37 |
1.9±0.04 |
2.0±0.04 |
2.3±0.04 |
85 |
75 |
89 |
|
50 |
2.0±0.04 |
2.1±0.04 |
2.3±0.04 |
83 |
80 |
89 |
|
60 |
2.1±0.04 |
2.2±0.04 |
2.4±0.04 |
75 |
75 |
84 |
|
70 |
2.0±0.06 |
2.2±0.05 |
2.4±0.04 |
61 |
70 |
84 |
|
80 |
2.0±0.05 |
2.2±0.04 |
2.3±0.05 |
67 |
80 |
84 |
There was significant effect of body weight group and hen age on feed intake. The light BW group consumed less feed than the other BW groups throughout the experiment. Feed intake was related to body size as the heavy BW group consumed more feed, followed by the medium and the light BW group, respectively, as expected. Feed Intake was high above breeder recommendation for all BW group from point of lay 19 weeks to the end of observation period of 80 weeks of age. There was a significant positive correlation between body weight and feed intake, however, a significant negative correlation between body weight and FCR. FCR was calculated as 2.1 for all BW groups (Table 3).
Table 3: Average egg weight, egg mass and fcr at 37 weeks.
|
BW Groups |
Egg Weight (g) |
Egg Mass (g) |
FCR |
|
Low |
59.4 |
392.1 |
2.1 |
|
Mid |
62.1 |
403.6 |
2.1 |
|
High |
64.0 |
438.3 |
2.1 |
Egg production
Egg production records commenced at 20 weeks when the flock reached 5% production, and continued until 80 weeks of age. All BW groups reached production above the breed standard at about 98-100% and maintained high production between 26 and 34 weeks of age. The average body weight during this period of high production was between 1.7 and 1.88 kg; 1.8 and 2.0 kg; and 2.0 and 2.2 kg for light, medium and heavy BW groups, respectively. Although egg production decreased as hen’s aged, it sustained about 90% to 60 weeks. Exception for low BW group from 40 to 45 weeks for the water deprivation incidence. The heavy body weight group produced higher egg mass at 37 weeks (438.3) than other BW groups (Table 4).
Estimation of the Amount of Cuticle
There was a significant main effect of both flock age (Table 4) and BW groups (Table 5) on shell reflectivity (%) and all values of the L*a*b colour space system before and after staining. Shell reflectivity before staining increased to age 70 weeks then decreased slightly. However, after staining, the shell reflectivity increased to age 71-75 weeks then decreased at 76-80 weeks. Both before and after staining, shell reflectivity was highest in the medium and lowest in the light BW group, with the heavy BW group intermediate.
The a* values before staining decreased with increasing flock age, whereas values after staining were varied among the ages being lowest in the age period of 61-75 weeks. The a* values before staining were highest in the light BW group followed by the heavy and medium BW groups, respectively. After staining, a* values were lowest and negative in the light BW group and positive in the heavy and the medium groups which were not significantly different from each other.
Table 4: Main effect of flock age no shell reflectivity and spectrophotometric measurements (l*a*b*) before and after staining.
|
Flock Age (weeks) |
Shell reflectivity % |
L* |
a* |
b* |
|
Before staining |
||||
|
19-25 |
24.1±0.16g |
56.0±0.16i |
18.98±0.11a |
27.6±0.18bc |
|
26-30 |
24.4±0.17f |
56.5±0.17h |
18.7±0.11b |
28.7±0.12a |
|
31-35 |
25.5±0.16e |
57.7±0.15g |
18.2±0.08c |
28.7±0.12a |
|
36-40 |
25.8±0.17de |
58.1±0.17fg |
17.7±0.09d |
27.76±0.13b |
|
41-45 |
26.4±0.21c |
58.9±0.2e |
17.3±0.11e |
27.8±0.11b |
|
46-50 |
25.7±0.21de |
58.2±0.19f |
17.4±0.1de |
27.6±0.13bc |
|
51-55 |
26.5±0.21c |
59.2±0.2de |
16.9±0.11f |
27.4±0.13bcd |
|
56-60 |
27.4±0.23b |
59.7±0.21d |
16.7±0.12f |
27.6±0.14bc |
|
61-65 |
29.2±0.27a |
61.1±0.23c |
16.0±0.13g |
27.3±0.14cd |
|
66-70 |
29.4±0.29a |
61.9±0.24b |
15.6±0.15h |
26.9±0.16ef |
|
71-75 |
28.9±0.31a |
62.7±0.26a |
14.8±0.16i |
26.5±0.18f |
|
76-80 |
26.1±0.27cd |
61.6±0.25b |
15.4±0.15h |
27.1±0.15de |
|
P Value |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
|
After staining |
||||
|
19-25 |
19.4±0.2i |
50.0±0.2i |
1.2±0.33de |
28.6±0.11d |
|
26-30 |
20.4±0.2h |
51.2±0.24h |
1.4±0.29d |
29.7±0.10c |
|
31-35 |
22.0±0.2g |
52.7±0.21g |
2.2±0.29bcd |
30.2±0.09a |
|
36-40 |
22.6±0.2fg |
53.5±0.22f |
1.7±0.32cd |
30.3±0.08a |
|
41-45 |
23.6±0.3cd |
54.5±0.27de |
2.6±0.35ab |
29.8±0.09c |
|
46-50 |
23.3±0.2de |
54.1±0.28ef |
3.4±0.33a |
30.1±0.09ab |
|
51-55 |
23.8±0.3cd |
55.0±0.26d |
2.7±0.33ab |
30.1±0.10ab |
|
56-60 |
24.1±0.3bc |
55.2±0.28d |
1.7±0.35cd |
29.9±0.10bc |
|
61-65 |
24.7±0.3ab |
55.9±0.31c |
-0.3±0.38f |
28.4±0.1d |
|
66-70 |
25.1±0.3a |
56.5±0.32bc |
-0.5±0.39f |
28.3±0.13d |
|
71-75 |
25.3±0.4a |
58.2±0.34a |
0.4±0.38ef |
28.0±0.13e |
|
76-80 |
22.7±0.3ef |
57.1±0.34b |
0.9±0.41de |
28.3±0.11d |
|
P Value |
<0.0001 |
<0.0001 |
<0.0001 |
<0.0001 |
a,b,c,d,e,f,g,h,i Within a column, values with different superscripts are significantly different from each other. Values are Mean ± SE
The b* values, before and after staining, varied with flock age being highest at 26-35 weeks and 31-36 weeks of age, respectively. Before staining, the b* value was highest in the heavy BW group and lowest in the medium BW group with the light BW group intermediate. After staining, the b* value was higher in the heavy and medium BW groups than in the light BW group. The single score values were highest in the light BW group and lowest in the medium BW group with the heavy BW group intermediate. Single score values generally decreased over the course of the experiment.
Table 5: Main effect of body weight groups on shell reflectivity and spectrophotometric l*, a*,b* before and after staining and the single score.
|
BW groups |
Light |
Medium |
Heavy |
P value |
|
Before staining |
||||
|
Shell reflectivity |
25.2±0.11c |
27.9±0.13a |
26.4±0.12b |
<0.0001 |
|
L* |
57.9±0.1c |
60.5±0.12a |
59.1±0.11b |
<0.0001 |
|
a* |
17.8±0.06a |
16.3±0.07c |
17.1±0.07b |
<0.0001 |
|
b* |
27.7±0.07b |
27.2±0.08c |
27.9±0.07a |
<0.0001 |
|
After staining |
||||
|
Shell reflectivity |
20.9±0.13c |
24.8±0.15a |
23.0±0.13b |
<0.0001 |
|
L* |
52.1±0.14c |
56.2±0.15a |
54.5±0.14b |
<0.0001 |
|
a* |
-0.5±0.17b |
2.4±0.17a |
2.4±0.17a |
<0.0001 |
|
b* |
29.1±0.05b |
29.4±0.06a |
29.4±0.06a |
0.0002 |
|
Single score |
19.4±0.18a |
15.0±0.19c |
15.7±0.18b |
<0.0001 |
a,b,c Across a row, values with different superscripts are significantly different from each other. Values are Mean ± SE
Egg Quality Measurements
There were significant effects flock age and of body weight (P<0.0001) for most of the eggshell and internal quality variables (Table 6 and 7). There was a significant interaction between flock age and BW group for both shell reflectivity and egg weight. Flock age significantly affected the translucency score which was highest at 26-30 weeks. Translucency score was also significantly higher in the light BW group (2.6) followed by the heavy BW group (2.5) and the medium BW group (2.4) with all groups being significantly different form one another. Shell reflectivity increased as the flock aged but decreased after 70 weeks of age (Table 7), and was higher in the medium BW group than the other BW groups with all groups being significantly different from one another. Egg weight was correlated with body weight with all groups being significantly different from one another. Egg weight increased from early lay (19 weeks) to the end of the experiment. Shell breaking strength varied with age with the highest breaking strength being at 46-50 weeks before decreasing as the flock aged.Shell breaking strength was higher in the light BW group than the medium and heavy groups which were not significantly different from each other. Shell deformation to breaking point was highest at 19-30 weeks of flock age, after which it decreased to age 80 weeks. Deformation was higher in the light BW group than in the medium and heavy groups which were not significantly different from each other. Shell weight varied among the ages during the experiment, being highest at age 46-50 weeks then decreasing with increasing flock age. Shell weight was significantly different among the body weight groups, being highest in the heavy BW group and lowest in the light body weight group. Percentage shell varied significantly with hen age, generally increasing from 19 to 46-50 weeks of age then decreasing as the flock aged. Percentage shell was significantly different among the BW groups, being highest in the light BW group, lowest in the heavy BW group with the medium BW group intermediate. Shell thickness increased from age 19 weeks to age 46-50 weeks then decreased to 80 weeks. Shell thickness was significantly higher in the light and medium BW groups, than in the heavy BW group.
Egg internal quality, measured by albumen height and Haugh unit, decreased consistently with increasing flock age (Table 7). Albumen height was significantly higher for the heavy BW group than for the light and medium BW groups. Haugh unit also generally declined with increasing hen age.However, there was no statistically significant effect of BW group on Haugh unit.
Table 6: Main effect of BW groups on egg Shell and internal quality parameters.
|
Measurement |
Light |
Medium |
Heavy |
P value |
|
Shell quality |
||||
|
Translucency score (after stain) |
2.6±0.02a |
2.4±0.02c |
2.5±0.02b |
<0.0001 |
|
Shell reflectivity (%) |
25.19±0.11c |
27.91±0.13a |
26.42±0.12b |
<0.0001 |
|
Egg weight (g) |
59.5±0.2c |
61.8±0.2b |
63.7±0.2a |
<0.0001 |
|
Breaking strength (N) |
40.6±0.2a |
38.6±0.2b |
38.8±0.0 b |
<0.0001 |
|
Deformation (µm) |
282.3 ±1.7a |
265.5±1.3b |
267.1±1.2b |
<0.0001 |
|
Shell weight (g) |
5.5±0.02c |
5.6±0.02b |
5.7±0.02a |
<0.0001 |
|
Percentage shell (%) |
9.2±0.03a |
9.1±0.03b |
8.9±0.03c |
<0.0001 |
|
Shell thickness (µm) |
394.6±1.0a |
392.4±0.9a |
389.8±1.0b |
=0.0005 |
|
Internal Quality |
||||
|
Albumen Ht (mm) |
8.8±0.04b |
8.9±0.05b |
9.1±0.04a |
<0.0001 |
|
Haugh Unit |
93.7±0.2a |
93.2±0.2b |
93.7±0.3a |
ns |
|
Yolk Colour Score |
9.7±0.05c |
9.9±0.04b |
10.0±0.04a |
<0.0001 |
a,b,c Across a row, values with different superscripts are significantly different from each other. Values are Mean ± SE
The yolk colour varied over the course of the experiment due to the different batches of feed. Yolk colour was significantly different among the BW groups, being highest in the heavy BW group and lowest in the light BW group with the medium BW group intermediate. There was a statistically significant interaction between flock age and BW group for albumen height, Haugh Unit and yolk colour score.
Table 8: The main effects of BW group on the ultrastructural properties.
|
BW groups |
Alignment |
Changed membrane |
Cap quality |
|
Light |
3.2±0.09ab |
1.2±0.07a |
2.4±0.08b |
|
Medium |
3.2±0.09a |
1.2±0.05a |
2.6±0.07a |
|
Heavy |
3.0±0.09b |
1.0±0.02b |
2.7±0.08a |
|
P Value |
=0.092 |
0.009 |
0.021 |
a,b,c Within a column, values with different superscripts are significantly different from each other. Values are mean ± SE
Ultrastructure Variations of The Eggshell
For shell mammillary layer ultrastructural variations, there was no significant main effect of BW group on mammillary layer ultrastructure except for alignment, changed membrane and cap quality (Table 8). The incidence of alignment was highest for the medium BW group, lowest for the heavy BW group with the light BW group. The incidence of changed membrane was higher for the light and medium BW groups than for the heavy BW group. The cap quality was higher in the medium and heavy BW groups than for the light BW group. There were statistically significant effects of flock age on most of the ultrastructural scores with the exception of the incidence of cubics, cuffing, changed membrane and depression (Table 9). As the flock aged, there was an increase in the incidence of ultrastructural features associated with decreased shell quality such as increased mammillary cap size variability, decreased mammillary cap quality and an increased incidence of late fusion, alignment, type A bodies, type B bodies, aragonite, erosion. There was also a decreased incidence of ultrastructural features associated with good shell quality such as confluence and early fusion.
Body weight at point of lay is important as it can influence egg production and egg quality over the entire laying period. The results of the present study show that average body weight at 19 weeks of age was 1.595 kg, 1.741 kg, and 1.870 kg for the light, medium and heavy BW groups, respectively, and this is similar to what is seen in many commercial flocks. The light BW group achieved the minimum weight at that age as recommended by the breeder manual (1.53kg - Hy-Line Brown, Performance standards manual), and continued to have body weights similar to that of the breeder recommendation although body weight was lower at 26 weeks and higher at 60 weeks than the recommended standard. At 26 weeks, the medium BW group was lower than the breeder standard by 60 g whereas the light BW group was below the breeder standard from 26 to 50 weeks of age. The body weight in both medium and heavy BW groups continued to exceed the breeder standard by an average of 170-370 g during the experiment. Although the body weight exceeded the recommended weight at point of lay, the medium group
Table 9: The main effect of flock age on the mammillary ultrastructure scores of the eggshell.
|
Ultrastructure properties |
Flock age (weeks) |
P Value |
||||||
|
20 |
30 |
40 |
50 |
60 |
70 |
80 |
||
|
Mammillary cap size |
2.4±0.1bc |
2.5±0.1b |
2.7±0.1ab |
2.7±0.1ab |
2.9±0.1a |
2.8±0.1a |
2.8±0.1a |
0.0008 |
|
Confluence |
3.3±0.1a |
2.4±0.1b |
2.3±0.1bc |
2±0.2bcd |
2.1 ±0.2bcd |
1.7 ±0.1d |
2±0.2cd |
<0.0001 |
|
Caps quality |
2.2±0.1 |
2.7±0.1 |
2.7±0.1 |
2.6±0.1 |
2.4±0.1 |
2.5±0.1 |
2.8±0.1 |
0.0042 |
|
Early fusion |
3.4±0.1a |
3.3±0.2 ab |
3.1±0.2abc |
3.1±0.2abc |
3±0.2bc |
2.8±0.2c |
2.7±0.2c |
0.03 |
|
Late fusion |
2.8±0.1c |
3±0.2bc |
3±0.1bc |
3.2±0.1abc |
3.3±0.1ab |
3.4±0.1ab |
3.5±0.1a |
0.018 |
|
Alignment |
2.5±0.1d |
2.8±0.1cd |
3.1±0.1bc |
3.3±0.1ab |
3.2±0.1ab |
3.5±0.1a |
3.3±0.1ab |
<0.0001 |
|
Type A |
1.5±0.1d |
1.6±0.1cd |
1.8±0.1bc |
1.9±0.1ab |
1.9±0.1ab |
2.2±0.1a |
2.2±0.1a |
<0.0001 |
|
Type B |
2.2±0.1c |
2.5±0.1bc |
2.5±0.1bc |
2.6±0.1ab |
2.5±0.1bc |
2.9±0.1a |
3.1±0.1a |
0.0001 |
|
Aragonite |
1.6±0.2cd |
1.8±0.1bcd |
1.4±0.1d |
1.5±0.2cd |
1.9±0.1abc |
2.2±0.2ab |
2.3±0.2a |
0.0001 |
|
Cubics |
1.2±0.1 |
1.4±0.1 |
1.4±0.1 |
1.4±0.1 |
1.4±0.1 |
1.3±0.1 |
1.4±0.1 |
NS |
|
Cubic cone formation |
2.2±0.1a |
2±0.1ab |
1.8±0.1bc |
1.9±0.1bcd |
1.6±0.1cd |
1.6±0.1d |
1.4±0.1d |
<0.0001 |
|
Cuffing |
1±0.0 |
1±0.0 |
1±0.0 |
1±0.0 |
1.1±0.05 |
1.0±0.03 |
1.0±0.0 |
NS |
|
Changed membrane |
1.1±0.1 |
1.2±0.1 |
1.1±0.1 |
1.1±0.1 |
1.3±0.1 |
1.3±0.1 |
1.1±0.1 |
NS |
|
Depression |
1.1±0.1 |
1.1±0.1 |
1.0±0.0 |
1.0±0.0 |
1.0±0.0 |
1.0±0 |
1.1±0.1 |
NS |
|
Erosion |
1.1±0.1cd |
1.4±0.1b |
1.1±0.1cd |
1.0±0.03d |
1.3±0.1bc |
1.5±0.1b |
1.9±0.1a |
<0.0001 |
a,b,c,d Across a row, values with different superscripts are significantly different from each other. Values are Mean ± SE
reached 5% production at age 20 weeks whereas both the heavy and the light groups reached 5% production at 21 weeks. All groups attained significantly higher egg production than the breeder recommendation with the exception of the light BW group at 41-45 weeks where the birds inadvertently experienced water restriction. However, the light BW group recovered rapidly after the incident, reaching their highest production at 46-50 weeks. Dunnington and Siegel (1984) studied the age at onset of sexual maturity and egg production in relation to body weight from high (HA) and light (LA) lines of antibody production to sheep erythrocyte antigen in single Comb White Leghorn chickens. These authors concluded that egg-type chickens must attain a minimum age and body weight before commencing egg production.
In this experiment, birds were allocated to the body weight groups at 16 weeks of age so that all groups had extremely high body weight uniformity (90 – 100 %) at point of lay, in order to assess the maximum productive potential of different body weight categories at the onset of sexual maturity. Body weight uniformity remained high during the trial, generally decreasing with increasing flock age. Commercial egg producers and pullet growers aim for a particular average body weight and uniformity of the flock at the age of sexual maturity. Producers aim to have at least 80% of the pullets within a range of ±10% of the average weight of the flock (Akanbi and Goodman, 1982) in order to reach optimum egg production. Furthermore, (Akanbi and Goodman, 1982) found that body weight uniformity at 19 weeks of age was 80.7, 84.0, and 81.2% for pullets from the lowest, middle and heaviest groups, respectively, with restricted-feeding for the middle and heavy groups to attain the recommended weight at 19 weeks of age. They assumed that separation of pullets based on weight at 9 weeks might be used as an indicator of their genetic potential for growth. Others, including (Leeson et al., 2005) found that body weight uniformity improved with step-down lighting. (Frikha et al., 2009) found that body weight uniformity of pullets was not affected by dietary treatment when corn was substituted by wheat. However, (Sweeney et al., 2022) found that feeding a high fiber diet on an every day basis during rearing, improved body weight uniformity in rearing, encouraged early lay, improved eggshell quality and increased egg weight.
The most interesting finding, however, was the gradation of uniformities at 70 weeks for the different body weight groups, with the light and medium groups having low uniformities, the egg production was 80% above. However, there was no significant correlation between flock uniformity and egg production. The medium body weight group produced 350 eggs to 72 weeks of age, with a body weight range of 1.86 to 2.37 kg, a uniformity at 70 weeks of 70% and a pullet weight of 1.7 kg at 19 weeks of age, compared to the light BW (322 eggs) and heavy BW (348 eggs) at that age.
For the light body weight group, the egg production to 40-42 weeks was extremely high (93-94%) with a body weight range of 1.53 to 2.22 kg at 37 weeks of age, and a uniformity of 85%. The light BW group had the poorest correlation between BW at point of lay and the 50 weeks weight, and this seems likely to reflect the greater variation in age at sexual maturity and potential for growth in individual birds. In the heavy body weight group, the lower correlation (R2=0.2519) between 19 and 50 weeks body weights probably reflects the differences in the potential of the heavier birds to deposit body fat, compared with the medium sized birds that have the strongest correlation between 19 and 50 week body weights. The significant correlation between BW at point at lay and egg production shows that maintaining BW at point of lay is very important for reaching the peak and sustaining egg production.
Feed intake was directly associated with body weight in the present study. Birds in the medium and heavy BW groups consumed about 104 and 107 g feed per bird, respectively, above the recommendation feed intake. Heavy birds consuming more feed have been reported by other studies (Leeson et al., 2005; Parkinson and Stanhope, 2011). The poultry house used in the present study was not environmentally controlled and it is assumed that this caused the birds to consume more feed, resulting in body weight exceeding the recommended levels.
However, egg production for all groups was higher than recommended guidelines, with some variation among the groups. By controlling feed intake, producers will maintain the average target body weight and flock uniformity and, as a result, will reach maximum production.
There was a significant effect on the FCR of hen age (P<0.0001) and BW group (P=0.0112). The regression analysis showed that there was a negative correlation between body weight and FCR (P=0.0492) showed in. The high values for FCR are due to the very low egg production at 19 weeks in all BW groups. However, there was a positive correlation between body weight and egg mass (R2= 0.141) as shown in. The low values in this are due to the low egg production at 19 weeks in all BW groups. Management is the key point to controlling flock uniformity
The SCI L* component of the L*a*b system measures the grading between white and black and gives results that are similar to shell reflectivity, which simply indicates the colour lightness of the shell. In the current study, the increasing L* value with increasing flock age indicated that the amount of colour deposited decreased with flock age and this was observed despite the staining with cuticle blue dye. There was a significant correlation between shell reflectivity and L* value with R2 = 0.8962. This finding indicated that shell colour became lighter as the flock aged except for the decreased reflectivity and slightly decreased L* value at 76-80 weeks of age. A possible explanation is that, as the flocks get older, birds produced larger eggs which had rougher surfaces and some had speckled shells. The speckled shell surfaces were darker in colour which affected the results recorded by the reflectivity meter and the spectrophotometer.
The a* value measures the grading between red and green with green towards the negative end of the scale. A more negative value following staining with cuticle blue dye indicates the presence of more eggshell cuticle. Eggs with good quality intact cuticle stained well; eggs with patchy cuticle acquired patchy stain whereas the eggs did not stain at all in the absence of cuticle. Our results indicate that the cuticle cover was greater in the light BW group which produced smaller eggs than the other groups. Cuticle cover was higher at the early stage of lay (20 weeks) and higher again in late lay (age 51-70 weeks). A possible explanation for our findings of greater cuticle cover in the light BW group is more protein distributed in the cuticle, because eggs are smaller than those of the other. (Mikšík et al., 2007) reported that 70% of protoporphyrin, the main pigment of the eggshell, was found in the cuticle layer. This finding was contradicted by (Samiullah and Roberts, 2013) who found only 13-20% of protoporphyrin was located in the cuticle. The finding of better cuticle cover in late lay contradicts the findings of (Rodríguez-Navarro et al., 2013) who found that thinner and more irregular cuticle was found on eggs laid by old flock s (70 weeks) than on eggs of young flocks which had thicker and more regular cuticle. (Roberts et al., 2013) noted that, although the cuticle cover on eggshells was not significantly different based on age, the thickest cuticle was found at mid lay (40-55 weeks) and late lay (55-65 weeks). In the current experiment, we analysed eggs from individual birds from the beginning of lay to the age of 80 weeks, whereas the study of (Roberts et al., 2013) collected eggs randomly from the flock. The cause of thicker cuticle found in older flocks is uncertain.
Flock body weight at point of lay affected the internal and eggshell quality of the eggs. Shell reflectivity was significantly different among the BW groups. The light BW group had darker shell colour and lower egg weight than the other groups. It has been suggested that, regardless of egg size, the same amount of colour is distributed over the shell surface (Odabaşi et al., 2007). This may explain why small eggs have darker shell colour than larger eggs. The heavy BW group had significantly higher in egg weight and shell weight than the other BW groups. A strong correlation between body weight and egg weight and other studies report that differences in egg weight generally reflected differences in body weight, with heavy birds producing larger eggs than smaller birds (Kırıkçı et al., 2004; Lacin et al., 2008).
Shell breaking strength (BS) measures the shell resistance to breakage when force is applied to the surface of the eggshell. In the current study, BS and shell deformation were higher in the light BW group indicating stronger shells. This might be related to egg weight as this BW group produced the smallest eggs (59.5 g). However, (Lacin et al., 2008) did not find a significant effect of body weight on shell strength and shell thickness.
For egg internal quality, albumen height and HU were higher in the heavy BW group, which produced larger eggs, than the other BW groups. These results are contradictory to the findings of (Lacin et al., 2008) who reported a higher albumen index and HU in light weight birds. Yolk colour was higher in the heavy BW group. The primary determinant of yolk colour is plant pigment (xanthophyll) content in the diet and is influenced by feed intake. This result most likely reflects the level of feed consumed by heavy BW groups. Fluctuations in yolk colour score with the age of the flock will reflect feed intake and the amount of yolk colour pigment in the different batches of feed.
Overall, BW groups and flock age had significant effects on all measures of eggshell and egg internal quality. As flock age increased, shell reflectivity, egg weight, shell weight and shell thickness increased, and translucency, BS, and shell deformation decreased. These results are comparable to the findings of several authors (Guesdon and Faure, 2004; Lacin et al., 2008; Rodriguez-Navarro et al., 2002; Gu et al., 2021). Then (Rodriguez-Navarro et al., 2002) correlated BS with the orientation of calcite crystals in the eggshell and, found in their studies that eggs from older flocks had lower BS values compared to younger flock eggs. Change in components of the organic matrix with flock age might be a contributing factor to poor shell strength in older hens (Fraser et al., 1999; Gautron and Nys, 2006; Panheleux et al., 1999).
There was a tendency to higher incidence of confluence in the light BW group as compared to the other groups. The reason for this result could probably be the smaller egg size produced in this group. As hen age increased, the incidence of ultrastructural features that have been shown to be associated with good shell quality, such as early fusion and confluence, decreased. At the same time, the incidence of ultrastructural features known to be associated with poorer shell quality increased: alignment of mammillae, type-A bodies, type-B bodies, aragonite, late fusion and pitting.
The significant effects of flock age on early fusion, late fusion, alignment, aragonite, erosion are evidence of deterioration in shell quality. (Bain, 2005) noted that late fusion and alignment in the mammillae predispose an egg to initiation and propagation of cracks. However, cuffing improved shell strength (Bain, 2005). The incidence of ultrastructural features that have been shown to be associated with good shell quality, such as early fusion and confluence have been reported by other workers to decrease as the flock increased in age (Samiullah et al., 2017; Bain, 2005). As the flock age increased, a number of structural variations altered in the mammillary layer increased and reduced the shell membranes bonding (Samiullah et al., 2017; Marzec et al., 2019) thus weakening the shell strength (Bain, 2005). The tendency of higher incidence of type-A bodies, type-B bodies and aragonite in the heavy BW group showed that the eggshells were poorer in quality. The scoring of ultrastructural features under the SEM and the photographs taken revealed that weak eggshells can possess major ultrastructural abnormalities.
CONCLUSIONS AND ECOMMENDATIONS
Body weight exceeded the breed standard by an average of 170-370 g for the medium and heavy BW groups, and body weight uniformity decreased as hen age increased. The uniformity of the heavy BW group was steady at 89% from 19 to 50 weeks which may be responsible for maintaining the peak production higher than the breed standard. Body weight at point of lay does affect the overall egg quality.
ACKNOWLEDGEMENTS
The authors want to thank to Australian Egg Corporation Limited, Australia.
NOVELTY STATEMENT
The body weight uniformity at point of lay is a critical fac-tor in egg industry.
AUTHORS CONTRIBUTIONS
The authors of the current experiment contributed equally.
Conflict of Interest
The authors have declared no conflict of interest.
REFERENCES
Asensio X, Piedrafita J, Puente A, Barroeta AC (2020). Effect of body weight on uniformity, livability, and skeletal development and strength of broiler breeder females. J. Appl. Anim. Res., 48(1): 320-325. https://doi.org/10.1080/09712119.2020.1789647
Aviagen. 2016. Uniformity of female broiler breeders. Huntsville, AL: Aviagen, Inc.
Akanbi O, Goodman BL (1982). The influence of increased uniformity of body weight in pullets at 19 weeks of age on subsequent production. Poult. Sci. J., 6:855-860. https://doi.org/10.3382/ps.0610855
Bain MM (2005). Recent advances in the assessment of eggshell quality and their future application. World’s Poult. Sci. J., 6: 268-277. https://doi.org/10.1079/WPS200459
Bell D (2011). The challenges of management for today’s high performing laying hens. Part 1: egg production and egg size. Lohmann Info, 46: 8-15.
Dunnington EA, Siegel PB (1984). Age and body weight at sexual maturity in female White Leghorn chickens. Poult. Sci., 63: 828-830. https://doi.org/10.3382/ps.0630828
Edeh HO, Osita CO, Nwoga CC, Ani AO, Okwor J (2020). The effect of bodyweight variation on laying performances of Shaver brown hen in humid tropical environment. Niger. J. Anim. Sci., 22(1): 83-90.
England AD, Gharib-Naseri K, Kheravii SK, Wu SB (2022). Rearing broilers as mixed or single-sex: relevance to performance, coefficient of variation, and flock uniformity. Poult. Sci., 101(12): 102176. https://doi.org/10.1016/j.psj.2022.102176
Fraser AC, Bain MM, Solomon SE (1999). Transmission electron microscopy of the vertical crystal layer and cuticle of the eggshell of the domestic fowl. Br. Poult. Sci., 40: 626-631. https://doi.org/10.1080/00071669987016
Frikha M, Safaa, HM, Serrano MP, Arbe X, Mateos GG (2009). Influence of the main cereal and feed form of the diet on performance and digestive tract traits of brown-egg laying pullets. Poult. Sci., 88: 994-1002. https://doi.org/10.3382/ps.2008-00480
Gautron J, Nys Y (2006). Eggshell matrix proteins and natural defences of the egg. Paper presented at the Symposium COA/INRA Scientific Cooperation in Agric. Tainan,(Taiwan, R.O.C). https://doi.org/10.1007/978-3-540-37885-3_16
Gu YF, Chen YP, Jin R, Wang C, Wen C, Zhou YM (2021). A comparison of intestinal integrity, digestive function, and egg quality in laying hens with different ages. Poult. Sci., 100(3): 100949. https://doi.org/10.1016/j.psj.2020.12.046
Guesdon V, Faure JM (2004). Laying performance and egg quality in hens kept in standard or furnished cages. Anim. Res., 53: 45-57. https://doi.org/10.1051/animres:2003045
Hudson BP, Lien RJ, Hess JB (2001). Effects of body weight uniformity and pre-peak feeding prog on broiler breeder hen performance. J. Appl. Poult. Res., 10: 24-32. https://doi.org/10.1093/japr/10.1.24
Hughes RJ, Heberle N, Barekatain R, Edwards NM, Hynd PI (2017). Flock Uniformity-is it important, and how is it assessed? In: Proceedings of the Australian Poultry Science Symposium at Sydney, Australia, pp. 93-95.
Kirikci K, Çetin O, Günlü A, Garip M (2004). Effect of hen weight on egg production and some egg quality characteristics in pheasants (Phasianus colchicus). Asian-Aust. J. Anim. Sci., 17: 684-687. https://doi.org/10.5713/ajas.2004.684
Kosba MA, Zeweil HS, Ahmed MH, Shabara SM, Debes AA (2010). Selection for uniformity in Alexandria local chickens: 2-correlated response for productive and reproductive traits. Egyptian Poult. Sci. J., 30: 114-136.
Lacin E, Yildiz A, Esenbuga N, Macit M (2008). Effects of differences in the initial body weight of groups on laying performance and egg quality parameters of Lohmann laying hens. Czech. J. Anim. Sci., 53:466-471. https://doi.org/10.17221/341-CJAS
Leeson S, Caston L, Lewis PD (2005). Rearing and laying performance following various step-down lighting regimens in the rearing period. Poult. Sci., 84: 626-632. https://doi.org/10.1093/ps/84.4.626
Leeson S, Summers JD (1987). Effect of immature body weight on laying performance. Poult. Sci., 66: 1924-1928. https://doi.org/10.3382/ps.0661924
Leleu S. Messens W, De Reu K, De Preter S, Herman L, Heyndrickx M, De Baerdemaeker J, Michiels CW, Bain M (2011). Effect of egg washing on the cuticle quality of brown and white table eggs. J. Food Prot., 74: 1649–1654. https://doi.org/10.4315/0362-028X.JFP-11-013
Madsen TG, Pedersen JR (2010). Broiler flock uniformity. Feedstuffs, 82: 12-13.
Marzec A, Damaziak K, Kowalska H, Riedel J, Michalczuk M, Koczywąs E, Cisneros F, Andrzej L, Niemiec J (2019). Effect of hens age and storage time on functional and physiochemical properties of eggs. J Appl. Poult. Res., 28(2): 290-300. https://doi.org/10.3382/japr/pfy069
Mikšík I, Eckhardt A, Sedláková P, Mikulikova K (2007). Proteins of insoluble matrix of avian (Gallus gallus) eggshell. Con. Tis. Res., 48: 1-8. https://doi.org/10.1080/03008200601003116
Odabaşi AZ, Miles RD, Balaban MO, Portier KM (2007). Changes in brown eggshell colour as the hen ages. Poult. Sci., 86: 356-363. https://doi.org/10.1093/ps/86.2.356
Panheleux M, Bain, M Fernandez MS, Morales I, Gautron J, Arias JL, Solomon SE, Hincke M, Nys Y (1999). Organic matrix composition and ultrastructure of eggshell: a comparative study. Br. Poult. Sci., 40: 240-252. https://doi.org/10.1080/00071669987665
Parkinson G, Cransberg BPH, Fadavi-Firooz R (2007). Influence of flock uniformity on productivity, eggshell quality and bird welfare. Sydney, NSW: An EPRID report for the Australian Egg Corporation Limited.
Parkinson GB, Stanhope W (2011). Alternative genetics to improve egg layer efficiency. Proc. Aust. Poult. Sci. Symp., 22: 104-111.
Reid, J (1983). The use of the plasma chemistry unit as an aid to the scanning electron microscope study of avian egg-shell structure. Br. Poult. Sci., 24: 233-235. https://doi.org/10.1080/00071668308416734
Roberts JR, Brackpool C (1995). Egg shell ultrastructure and the assessment of egg shell quality. Armidale: University of New England.
Roberts JR, Choulsalkar K, Samiullah S (2013). Egg quality and age of laying hens: implications for product safety. Anim. Prod. Sci., 53: 1291-1297. https://doi.org/10.1071/AN12345
Rodriguez-Navarro AB, Kalin O, Nys Y, Garcia-Ruiz JM (2002). Influence of the microstructure on the shell strength of eggs laid by hens of different ages. Br. Poult. Sci., 43: 395-403. https://doi.org/10.1080/00071660120103675
Rodríguez-Navarro AB, Domínguez-Gasca N, Muñoz A, Ortega-Huertas M (2013). Change in the chicken eggshell cuticle with hen age and egg freshness. Poult. Sci., 92: 3026-3035. https://doi.org/10.3382/ps.2013-03230
Samiullah S, Roberts JR (2013). The location of protoporphyrin in the eggshell of brown-shelled eggs. Poult. Sci., 92: 2783-2788. https://doi.org/10.3382/ps.2013-03051
Samiullah S, Omar AS, Roberts JR, Chousalkar K (2017). Effect of production system and flock age on eggshell and egg internal quality measurements. Poult. Sci., 96(1): 24-258. https://doi.org/10.3382/ps/pew289
Saraswati YV, Mustofa F, Sari APZNL, Sasongko H, Ariyadi B, Maharani D (2023). Live Weight Uniformity on The Day-Old Chick of Indonesian Local Chickens. Malays. Appl. Biol., 52(2): 153-156. https://doi.org/10.55230/mabjournal.v52i2.2599
Solomon SE (1991). Egg and Eggshell Quality London:Wolfe Publishing Limited.
Sutopo, Kurnianto E, Setiatin ET, Lestari DA, Setiaji A, Suryawijaya A, Samsudewa D( 2021). Growth evaluation of native chicken on different type of hen body weight. J. Sain Peternakan Indonesia, 16(4): 292-296. https://doi.org/10.31186/jspi.id.16.4.292-296
Sweeney KM, Aranibar CD, Kim WK, Williams SM, Avila LP, Starkey JD, Starkey CW, Wilson JL. (2022). Impact of every-day versus skip-a-day feeding of broiler breeder pullets during rearing on body weight uniformity and reproductive perfor-mance. Poultry Science, 101(8), 101959. https://www.sci-encedirect.com/science/article/pii/S0032579122002516
Thiele HH (2012). Management tools to influence egg weight in commercial layers. Lohman Info, 47: 21.
Zen AA, Ondho YS, Sutiyono S (2020). Seleksi pejantan ayam kampung berdasarkan breeding value terhadap gerak massa, abnormalitas dan motilitas spermatozoa. J. Sain Peternakan Indonesia, 15(3): 339-347. https://doi.org/10.31186/jspi.id.15.3.339-347
To share on other social networks, click on any share button. What are these?






