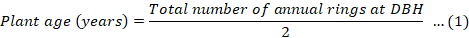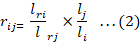The Effects of Plant Age on Phytochemical and Geographical Distribution of Euphorbia helioscopia, (Sun Spurge or Madwoman’s Milk) Euphorbiaceae from Arrang District Bajaur
Research Article
The Effects of Plant Age on Phytochemical and Geographical Distribution of Euphorbia helioscopia, (Sun Spurge or Madwoman’s Milk) Euphorbiaceae from Arrang District Bajaur
Shakir Ullah1* and Lubna Shakir2
1State Key Laboratory of Systematic and Evolutionary Botany (LSEB), Institute of Botany Chines Academy of Science China; 2Department of Botany, Govt Degree College Timergara, Dir Lower, Khyber Pakhtunkhwa, Pakistan.
Abstract | Plant age and geographical location are among the internal and external factors respectively that influence the phytochemical composition of medicinal plants. Euphorbia helioscopia, (sun spurge or madwoman’s milk) (Euphorbiaceae) is a wild medicinal plant that grows nearly in all phytogeographical zones of Bajaur. The plant location, age, and their interactions had a significant influence on the phytochemical composition of E. helioscopia. The plant location had the highest effect on the marginal means of compounds (81.517) while the age influenced a variation by a marginal effect mean of 3.863. Some conjugated and non-conjugated compounds were common in all plant parts and ages whereas others either developed or diminished at certain ages. The conjugated compound (UV+, with blue fluorescence) was present at > 2.0 months in the Arrang and Bajaur regions. while it was present in Ghargu samples at even < 2 months. The phasing out by red fluorescing compounds in stems (SG5) and leaves (SG7) as the plant age increased was attributed to the loss of chlorophyll (green pigments) as a plant part matures. The results indicated that the relationship between phytochemical yield and age of harvesting is not linear. Harvesting at a lower age of < 2 months gives fewer phytochemicals than at 3-4.5 months, which seems to be optimal. It was also found that E. helioscopia, (sun spurge or madwoman’s milk) from Arrang District Bajaur contains the highest phytoconstituents of all studied sites. Additional research is needed to identify the individual phytochemical components fully. Bioassay-guided approaches are recommended, as they provide an analytical method for determining the potency and effect of these phytochemicals on living plants, cells, or tissues.
Received | Juune 18, 2023; Accepted | December 16, 2023; Published | December 26, 2023
*Correspondence | Shakir Ullah, State Key Laboratory of Systematic and Evolutionary Botany (LSEB), Institute of Botany Chines Academy of Science China; Email: Shakirawkum321@gmail.com
Citation | Ullah, S. and L. Shakir. 2023. The Effects of plant age on phytochemical and geographical distribution of Euphorbia helioscopia, (sun spurge or madwoman’s milk) Euphorbiaceae from Arrang District Bajaur Pakistan Journal of Weed Science Research, 29(4): 206-212.
DOI | https://dx.doi.org/10.17582/journal.PJWSR/2023/29.4.206.212
Keywords | Euphorbiaceae, Antioxidant, Phytochemicals, Phytogeography
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Plants have been used as a source of medicine throughout history and continue to serve as the basis for many pharmaceuticals used today (Ginsburg and Deharo, 2011; Javaid et al., 2022; Khan and Javaid, 2023). Euphorbia helioscopia (family Euphorbiaceae), is a wild medicinal plant commonly used in Pakistan. This plant grows in different phytogeographical zones of Bajaur as described by (Nahashon, 2013) including the Arrang region Western-Southern part of the country. These zones are characterized by variations in weather conditions like total rainfall, and temperature. According to the Pakistan Metrological Agency data, temperature ranges for the Arrange region (18-22oC), and Ghargu (22-25°C) while Bajaur was had the highest temperatures, ranging (from 25-33°C). During winter, the temperatures are lower while Ghargu always registers the least, < 10°C. Previous studies on crude extracts and pure compounds of E. helioscopia from Arrang have been traditionally and scientifically confirmed to have bioactive compounds against human and animal infections including anti-Newcastle disease virus, acaricidal activity, anti-infectious, Bursal disease virus and anti-fowl pox disease virus (Nyigo et al., 2016; Credo et al., 2022). The pure compounds; 3β and 3α-friedelanol (Credo et al., 2022), euphol and β-sitosterol (Nyigo et al., 2016), erytrinaccinate c and Octacosanol (Nyigo et al., 2022) have been reported. Nevertheless, there is strong evidence that plants tend to synthesize secondary metabolites for self-defense against environmental stress (Liu et al., 2016) which in turn serve a medicinal value to relieve or cure various diseases (Ahmad et al., 2015). Plants from Euphorbiaceae (the largest family of flowering plants) have a worldwide distribution thus made to adapt to various sorts of habitats which in turn force them to produce secondary metabolites for survival of defense (Mwine and Van Damme, 2011; Ullah et al., 2023). Since the effectiveness of a plant is a function of its novel active secondary metabolites that they contain (Raya et al., 2015; Soni et al., 2015), any change in chemical composition tends to affect the intended use. There is a proven evidence that plants of the same species tend to display variations in their bioactive phytochemicals depending on how they interact with their environment in development process (Liu et al., 2016, Ullah et al., 2023). are among the notable factors that influence phytochemical distribution in the plants. It is made known that many of these metabolites produced by plants are used by pharmaceutical industries because of their ability to trigger a pharmacological or toxicological effect in humans and animals (Guerriero et al., 2018). It is for this reason it is important to layout a specific and scientifically proven information on phytochemical profiles of E. helioscopia in relation to age and location so as to guide traditional medicine practitioners and the industry of new drug discovery. Previous reports on bioactivity and phytochemical investigations of E. helioscopia, relied on samples from only one location namely, thus miss comparative data. This study aimed at exploring the phytochemical spectrum of E. helioscopia, which grows in three regions (Arrang, Ghargu and Bajaur). This created a need for this study to avail the quality status in roots stems and leaf regions of E. helioscopia.
Materials and Methods
Sampling area and pretreatment
With the aid of a botanist, the fresh plants were collected from three phytogeographical zones in which the plant was readily available. Zone 1, Bajaur (N) within 50 m diameter around an elevation 1656 m S08o47’08.5” E 34o53’22.2”, Zone 2, Arrang region (H), 554 m S 05o34’00.9” E 038o9’57.4” to 567m S 05o34’04.9” E 038o09’54.4” and Zone 3, Ghargu region (C), 1119 m S 06o00’08.5” E 036o58’09.9 to 1132 m S 06o00’05.5” E 036o58’09.9. To suppress the effect of seasonal variation, all samples were collected in November, 2018. The voucher specimen number FM 3672 was deposited at the herbarium of botany department, Govt Post Graduate Collage Timergara Dir Lower Khyber Pakhtunkhwa.
Sampling procedure and measurements
A selective random sampling of at least fifteen E. helioscopia from each site led to a total of 50 samples. For every three sample the diameter at breast height (DBH) was measured and the corresponding number of rings in stems were recorded. A plot of DBH (in centimeters) against rings afforded to obtain the linear regression equation (y = 1.0406x + 0.3533) which was used to calculate the number of rings for uncut stems. Each plant was processed into five separate parts in small pieces of root barks (SG2), root woods (SG3), stem barks (SG5), stem woods (SG6) and leaves (SG7) then parked into reseal able bags. The samples were immediately transferred to liquid nitrogen until drying time. Both samples were dried in cold and dark room (15 oC) to protect the temperature and light sensitive compounds.
Determination of the plant age
The total number of annual rings on stem discs of each plant were used to estimate the plant age (in years) according to the Equation 1 (Verheyden et al., 2004). In this case, time lag of 2 years appeared between the actual age of the tree and the number of growth rings formed at 130 cm height was considered for the diameter at breast height (DBH).
Sample extraction
Dry samples were pulverized separately using a laboratory milling machine (Christy Hunt Engineering Ltd, England) to obtain approximately 1 mm particle size. Each sample was subjected to total maceration with methanol (MeOH) at room temperature in a dark so as to protect volatile and light sensitive compounds. For a maximum extraction, the re-soaking and filtration was done after 48 hours for three consecutive times. Solid residues were obtained by air-drying the samples at room temperature, and stored at 4 oC until further use.
Standardization of samples and chromatographic constants
The pulverized samples (about 1 mm particle size) were subjected to total maceration using methanol and extracted three times consecutively after every 72 hours. All crude extract solutions were air dried to solid residues and each was standardized to a concentration of 5 mg/mL. Three pure compounds were used as the chromatographic constants; trimethoprim (TMP), Sulphathiazole (SMX) and Sulphadoxine SDX) that were selected according to their availability and polarity strength to suit the polarity range of the crude extracts under study. A concentration of 10 parts per million (ppm) for each compound was sufficient for visualization on thin-layer chromatography paper.
In each case a 4.0 µL of the sample were spotted on the TLC paper and eluted at different solvent systems. The selected combination of chromatographic constant was simultaneously spotted on the same TLC and eluted along with study samples. At each elution, the relative retention factor (Rf) values for observed compounds were recorded and compared. A number of compound elution trials on the TLC papers were made using different solvent systems including dichloromethane, ethyl acetate and methanol. Suitable solvent systems of 2, 10 and 18 % Methanol (MeOH)/ dichloromethane (DCM) were selected for a maximum elution.
Calculation of the relative retention factor (rij)
The formula for calculating the retention factor of the component phytochemicals relative to the selected standards was adopted from (Berezkin 2006) as indicated in Equation 2.
Where: lri= net retention distance for compound i, li= virtual distance for I; lrj= net retention distance for standard j, and lj= virtual distance for standard compound j.
Data analysis
A descriptive analysis was carried out to describe the characteristics of the data. An analysis of variation of the plant location, age and plant parts with their interaction was performed through a two-way analysis of Variance (ANOVA) with assistance of IBM SPSS version 25. The study analyzed the number of compounds present in each plant part as the dependent variable.
Results and Discussion
General trend in phytoconstituents of euphorbia helioscopia
A comparison of the minimum number of compounds observed in each part showed that the stem bark had the highest number while the stem wood had the least (Figure 1). A large number of compounds in the stem and root barks of E. helioscopia can be related to how greatly these parts of are forced to interact more with the environment for survival.
A descriptive analysis results (Table 1 and 2) indicated the strong correlation between the chemical composition and plant age, geographical location and the plant part with (p <0.05). Among the test conditions, the plant part with the highest marginal mean of total compounds was identified. This indicates that a change in plant part would result in a change in chemical composition by 81.52.A simultaneous change in location and age, caused the lowest variation in the number of chemical compounds by 0.628.
Table 1: Descriptive analysis of the association in the number of compounds against plant age, location and plant part factors in Euphorbia helioscopia.
|
Source |
Type III sum of squares |
df |
Mean square |
F |
Sig. |
|
Corrected model |
1026.073a |
109 |
9.414 |
75.308 |
0.000 |
|
Intercept |
13702.936 |
1 |
13702.93 |
109623.48 |
0.000 |
|
Location |
135.064 |
2 |
67.532 |
540.256 |
0.000 |
|
Age |
42.497 |
11 |
3.863 |
30.907 |
0.000 |
|
Part |
326.069 |
4 |
81.517 |
652.137 |
0.000 |
|
Location * Age |
17.483 |
9 |
1.943 |
15.541 |
0.000 |
|
Location * Part |
75.126 |
8 |
9.391 |
75.126 |
0.000 |
|
Age * Part |
28.662 |
44 |
0.651 |
5.211 |
0.000 |
|
Location * Age * Part |
19.466 |
31 |
0.628 |
5.023 |
0.000 |
|
Error |
5.000 |
40 |
0.125 |
||
|
Total |
19557.00 |
150 |
|||
|
Corrected total |
1031.073 |
149 |
Effect of age on chemical distribution
The analyzed plant samples aged between 1-5 months. The majority plants in sampled areas were between 1-2 months of which 72.22 % Arrang, 66.11% Ghargu and 66.67 % Bajaur. The highest age made up to only 5.56 % in all areas. The absence of mature plants in the sample areas may be due to over harvesting for medicinal purposes and other human activities, such as clearing land for farming. Despite a small significance on the influence of age on phytochemicals profiles of E. helioscopia (9.39), the change was not linear (Figure 2). At lower ages of <1 months, there is a minimal number of compared to 3 or 4.5 months. There was noticed a linear increase in means of number of phytochemicals at the age >5.5 months. The analysis showed that there was no significant alteration in the overall number of compounds present in the stem regions. This implied that the stem is not exposed more to the environmental stress thus a few compounds synthesized.
The TLC profiles of methanolic extracts (Figures 3 and 5) contained a variety of highly polar and nonpolar compounds. Some compounds were less and others more polar than the selected chromatographic constants. A less polar and UV- compound with rij of 4.99 was present in all five categories (SG1-7) although it was in small quantities (faint spot) in stem woods (SG6). Leaves were among the samples that did not show major difference in compounds’ profiles. This can be attributed to the fact that leaves develop seasonal wise thus tend to have closely similar age before they are shed off. The tree sheds its leaves during summer, and regenerates them in the wet season. For this reason, stem part samples (SG5) contained complex mixtures including the chlorophyll, the red fluorescing compounds (Figure 3A).
An Illumination of stem bark extracts with UV light at 365 nm indicated presence of many conjugated compounds (black or fluorescing spots) in (Figure 5a) than the non-conjugated (purple spots after chemical treatment.
Table 2: Representative relative retention factors for compounds from Arrang samples as observed at both ultra violet light (254 and 365 nm) and chemical (vanillin reagent) treatment.
|
S. No |
Relative retention values (rij) |
||||
|
NSG2 |
NSG3 |
NSG5 |
SG6 |
NSG7 |
|
|
1 |
139.46 |
0.96 |
26 |
16 |
|
|
2 |
6.64 |
0.98 |
4.94 |
4.1 |
4.04 |
|
3 |
4.27 |
0.17 |
3.43 |
0.72 |
2.58 |
|
4 |
2.42 |
0.72 |
1.27 |
0.48 |
1.25 |
|
5 |
1.34 |
0.35 |
0.34 |
0.17 |
0.82 |
|
6 |
1.11 |
0.17 |
9.75 |
0 |
0.45 |
|
7 |
1.38 |
0.11 |
2.53 |
0.05 |
0.12 |
|
8 |
0.72 |
0.09 |
0.06 |
0.03 |
0.06 |
|
9 |
0.34 |
0.03 |
0.02 |
0.02 |
0.02 |
|
10 |
0.52 |
0.19 |
0.01 |
0.01 |
0 |
|
11 |
0.52 |
0.05 |
0.005 |
NE |
|
|
12 |
0.52 |
0.03 |
0.004 |
|
|
|
13 |
0.52 |
0.02 |
0.004 |
|
|
|
14 |
NE |
0 |
0.001 |
|
|
|
15 |
|
|
NE |
|
|
NE, no elution at 18 % MeOH/DCM
In Figure 4a and b, the concentration of some compounds increased with plant age. E. helioscopia, roots samples indicated several compounds were commonly distributed in all sampled plants although differed in their concentrations. For example, a less polar UV- compound (rij= 4.99), that reacted purple with vanillin reagent (terpene characteristic), was found in high concentration as the plant age increased. This compound could not be detected in < 1 month age. In SG5 and SG7 from all study sites, the red fluorescing (fR) compounds decreased in concentration as the plant age increased. This was linked with a decrease in chlorophyll (green pigment) as the plant matures. According to (Nobossé et al., 2018), the increase in leaf age had a remarkable effect on the phenolic contents, flavonoids and the chlorophyll which in turn influenced a variation in antioxidant activity of the extracts. In this view.
Effects of geographical location on chemical profiles
The results as indicated in Figure 6, E. helioscopia, from the high attitude area (Najombe) had the highest number of total compounds while Morogoro region had the lowest. This could be due to the variation in geographical climate and high altitude in Najombe, making it the coldest region. It could be ascertained that the high altitude favored the phytoconstituent accumulation (Nayeem, 2017; Gololo et al., 2019). In a study by (Muraina et al., 2008) it showed similar trend for the extract yields of E. helioscopia from colder and hot. The low number of compounds in Morogoro samples (Figure 6) may be attributed to various factors, such as the soil type in the specific area. A Similar phytochemical variation with location was also recorded in Morus nigra (Khattak et al., 2015). Some compounds had the highest concentration in Tanga, followed by Najombe, while the concentration was least for Morogoro samples (Figure 5a-c). In a study conducted by (Gololo et al., 2019) variations in antioxidant capacity were observed among E. helioscopia leaves from different locations.
The observed variation in the number and concentration of compounds could be attributed to how much the plant struggles to survive the harsh environment. Figure 5d and Figure 7 shows that despite its decrease at higher ages, a blue fluorescing compound (fb) had the highest concentration in Arrang samples (Figure 5c, d).
Therefore, the wide spectrum of compounds in Euphorbia helioscopia, from different location of Tanzania reasons for the biotic and abiotic environment District Bajaur in which they grow. In turn they are expected to form a large pool of medicinal plant derived drugs.
Conclusions and Recommendations
The findings indicated that both tested factors of location and age influenced the phytochemicals compounds distribution in all of its parts of this plant. However, the location factor had the highest influence on the phytochemical profiles. Of all plant parts assessed, the stem barks indicated to contain the highest number of chemical compounds followed by the root barks. The Ghargu and Arrang sample have highest concentration of phytochemicals. From this study we can concluded that the plants phytochemicals are affected by climatic changes, biotic and abiotic factors. It is however recommended to conduct the bioassay guided experiments that provide an analytical method for determining the potency and effect of these phytochemicals on living plants, cells, or tissues so as to determine key plant ages from each study location that contain most pharmacologically active compounds.
Acknowledgement
The state key laboratory plants evaluation and systematics play a crucial role in conducting this research work, and we greatly appreciate their contribution.
Novelty Statement
The phytochemical composition of E. helioscopia is significantly influenced by both plant location and age. Plant location demonstrated the highest effect on the marginal means of compounds, emphasizing the importance of geographical variation.
Author’s Contribution
Shakir Ullah Supervision and Wrote the Manuscript
Lubna Shakir Performed Main Research Work
Conflicts of interest
The authors have declared no conflict of interest.
References
Ahmad, Z., M. Bhardwaj and A. Kumar. 2015. Phytochemical analysis and antimicrobial activity of Commiphora wightii plant (guggul) extract. Res. J. Pharma. Biol. Chem. Sci. Phytochem., 6(3): 1759–1766.
Berezkin, AV., Mathematical modeling of interfacial polycondensation. 2006. Journal of Polymer Science Part B: Polymer Physics 44, 18: 2698-2724.
Credo, D., F.P. Mabik, F. Machumi, M. Chacha, C. Cornett and B. Styrishave. 2022. Anti-newcastle disease virus activity of 3β and 3α Friedelanol triterpenoids from the leaves of Synadenium glaucescens Pax. Trop. Biomed., 39(2): 1–8. https://doi.org/10.47665/tb.39.2.016
Ginsburg, H. and E. Deharo. 2011. A call for using natural compounds in the development of new antimalarial treatments. An introduction. Malaria J., 10: l-1. https://doi.org/10.1186/1475-2875-10-S1-S1
Gololo, S.S., D. Nthai and M.A. Mogale. 2019. Phytochemical contents and antioxidant capacity of the leaves of Aloe greatheadii var. davyana from Four South African provinces. Asian J. Chem., 31(8): 1830-1834. https://doi.org/10.14233/ajchem.2019.22019
Guerriero, G., R. Berni, J.A. Muñoz-Sanchez, F. Apone, E.M. Abdel-Salam, A.A. Qahtan, A.A. Alatar, C. Cantini, G. Cai, J.F. Hausman, K.S. Siddiqui, S.M.T. Hernández- Sotomayor and M. Faisal. 2018. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes, 9: 309. https://doi.org/10.3390/genes9060309
Javaid, A., F.A. Chaudhury, I.H. Khan and M.F.H. Ferdosi. 2022. Potential health-related phytoconstituents in leaves of Chenopodium quinoa. Adv. Life Sci., 9(4): 574-578.
Khan, I.H. and A. Javaid. 2023. Identification of pharmaceutically important constituents of quinoa root. Jordan J. Pharma. Sci., 15(1): 96-102. https://doi.org/10.35516/jjps.v16i1.1071
Khattak, K.F. and T.R. Rahman. 2015. Effect of geographical distributions on the nutrient composition, phytochemical profile, and antioxidant activity of Morus nigra. Pak. J. Pharma. Sci., 28(5):1671-1678.
Liu, W., D. Yin, N. Li, X. Hou, D. Wang, D. Li and J. Liu. 2016. Influence of environmental factors on the active substance production and antioxidant activity in Potentilla fruticosa L. and its quality assessment. Sci. Rep., 6: 1–18. https://doi.org/10.1038/srep28591
Muraina, I., A. Adaudi, M. Mamman, H. Kazeem and J. Eloff. 2008. Effects of geographical location on the yield and bioactivity of Anoigeissus leiocarpus. J. Pharm. Bioresour., 5(2). https://doi.org/10.4314/jpb.v5i2.52995
Mwine, T.J. and P.V. Damme. 2011. Why do Euphorbiaceae tick as medicinal plants. A review of the Euphorbiaceae family and its medicinal features. J. Med. Plants Res., 5(5): 652–662.
Nahashon, M., 2013. Conservation of Wild-harvested Medicinal Plant Species in Tanzania; Chain and consequence of commercial trade on medicinal plant species. Examensarbete Hållbar Utveckling, pp. 124.
Nayeem, N., 2017. Influence of altitude on the phytoconstituents and anti-oxidant activity of the leaves of Tectona granditis. Indo-Am. J. Pharma. Sci., 4(10): 3919–3922.
Nobossé, P., E.N. Fombang and C.M.F. Mbofung. 2018. Effects of age and extraction solvent on phytochemical content and antioxidant activity of fresh Moringa oleifera L. leaves. Food Sci. Nutr., 6(8): 2188–2198. https://doi.org/10.1002/fsn3.783
Nyigo, V.A., X. Peter, F. Mabiki, H.M. Malebo, R.H. Mdegela, G. Fouche, F.M. Abiki, H.M. Malebo, R.h. Mdegela and G. Fouche. 2016. Isolation and identification of euphol and β-sitosterol from the dichloromethane extracts of Synadenium glaucescens. J. Phytopharmacol., 5(3): 100–104. https://doi.org/10.31254/phyto.2016.5302
Nyigo, Vitus A., Hamisi M. Malebo, Faith M, and Robinson Mdegela. (2022). Isolation and identification of long-chain aliphatic compounds from Synadenium glaucescens.” The Journal of Phytopharmacology 11, 3: 151-154.
Raya, K.B., S.H. Ahmad, S.F. Farhana, M. Mohammad, N.E. Tajidin and A. Parvez. 2015. Changes in phytochemical contents in different parts of Clinacanthus nutans (Burm. F.) Lindau due to storage duration. Bragantia, 74(4): 445–452. https://doi.org/10.1590/1678-4499.0469
Shakir, L., U. Shakir, S. Mohammad, U. Rihan and S. Muhammad. 2023. Phytochemical analysis, antipyretic and antifungal activities of Solanum nigrum L. Natl. J. Pharma. Sci., 3(2): 06-12.
Soni, U., S. Brar and V.K. Gauttam. 2015. Effect of seasonal variation on secondary metabolites of medicinal plants. Int. J. Pharm. Sci. Res., 6(9): 3654–3662.
Ullah, S., A. Said, H. Ihtisham, U. Ikram, B. Rizwana, A. Asghar, K. Yaseen and Muhammad. 2023. Ethnobotanical study of village Darangal Kambat Tehsil Samarbagh, District Dir Lower, Khyber Pakhtunkhwa, Pakistan. J. Agric. For. Res., 2: 5.
Ullah, S., L. Shakir and R. Ullah. 2023. Morphological and phytochemical study of cirsium arvense from District Mardan Pakistan. J. Bioinf. Biotechnol. Res., 1(1): 1-7. https://doi.org/10.61440/JBBR.2023.v1.01
Verheyden, A., J.G. Kairo, H. Beeckman and N. Koedam. 2004. Growth rings, growth ring formation and age determination in the mangrove Rhizophora mucronata. Ann. Bot., 94(1): 59–66. https://doi.org/10.1093/aob/mch115
To share on other social networks, click on any share button. What are these?