The Effect of the Proportions of Roughage to Concentrate on Rumen Fluid Differs Among Various Animal Species
Research Article
The Effect of the Proportions of Roughage to Concentrate on Rumen Fluid Differs Among Various Animal Species
Nuttanun Leamkrajang, Somkiert Prasanpanich, K. Teepalak Rangubhet, Phongthorn Kongmun*
Department of Animal Science, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand.
Abstract | The aim of this study was to evaluate the effect of varying ratios of roughage to concentrate on rumen fluid among different species of ruminants, specifically focusing on rumen fermentation characteristics and the population of rumen microorganisms. The investigation utilized a 3×2 factorial in CRD, incorporating four replications. The inoculum sources included three animal species (swamp buffalo, beef cattle, and goat), while the dietary treatments consisted of two roughages to concentrate ratios: 100:0 and 70:30, using dry leucaena leaves as the source of roughage. The kinetic values of fermentation, gas production, and IVDMD showed significant differences across treatments (p<0.05). The swamp buffalo and the 70:30 ratio exhibited the greatest gas production and IVDMD, while beef cattle followed, and goats produced the least (p=0.004, p<0.001, p=0.001). The swamp buffalo and the 70:30 ratio exhibited the highest concentration of ammonia nitrogen (p<0.001, p=0.002). The ratio of concentrate to roughage influences the overall production of VFAs, the proportion of C2, C3, the C2:C3 ratio, and methane emissions (P<0.05). The 70:30 ratio demonstrated superior performance compared to the 100:0 ratio. Additionally, the interaction effect revealed that a 70:30 feeding ratio in swamp buffalo resulted in a significant increase in the populations of bacteria, fungi, and R. albus (p<0.001). The research indicates that the characteristics of rumen fermentation, digestibility, and the population of rumen microorganisms varying outcomes across different ruminant species. Furthermore, these factors can be enhanced by utilizing a 70:30 ratio of roughage to concentrate.
Keywords | Beef cattle, Goat, Roughage to concentrate ratio, Rumen fermentation, Swamp buffalo
Received | September 07, 2024; Accepted | October 09, 2024; Published | October 29, 2024
*Correspondence | Phongthorn Kongmun, Department of Animal Science, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand; Email: fagrptk@ku.ac.th
Citation | Leamkrajang N, Prasanpanich S, Rangubhet KT, Kongmun P (2024). The effect of the proportions of roughage to concentrate on rumen fluid differs among various animal species. Adv. Anim. Vet. Sci. 12(12): 2499-2511.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.12.2499.2511
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Studying rumen fermentation among ruminant animal species is crucial for enhancing our understanding of ruminant nutrition, improving feed efficiency, and reducing environmental impacts. The rumen, a complex microbial ecosystem, plays a pivotal role in the digestion of plant materials, influencing the overall health and productivity of ruminant animals. The rumen hosts a diverse spectrum of microorganisms, encompassing bacteria, protozoa, and fungi, each of which is crucial for the transformation of complex carbohydrates into simpler compounds that ruminants are capable of absorbing and utilizing. Recent breakthroughs in molecular biology and metagenomics have uncovered a significant variety of uncultured bacteria present in the rumen, which are essential for fiber digestion and have the potential to function as probiotics or sources for industrial enzymes (Firkins et al., 2008; Kobayashi, 2006). Glendinng et al. (2020) indicated that the variations in microbial communities among different ruminant species, such as cows, sheep, reindeer, and red deer, underscore the necessity for species-specific research to fine-tune feed formulations and enhance nutrient absorption. Rumen fermentation plays a crucial role in feed efficiency, which is an important economic characteristic in livestock production. The capacity for efficient feed conversion is correlated with a more diverse and abundant rumen microbiota. Understanding the interplay between rumen microbiota and feed efficiency may lead to the creation of approaches aimed at improving livestock productivity while also decreasing feed costs. The rumen’s ability to convert lignocellulosic plant material into usable compounds mirrors industrial biorefinery processes. Studying rumen fermentation can provide insights into developing efficient bioconversion technologies for industrial applications. The enzymes and microbial processes in the rumen have potential applications in the production of biofuels and other chemicals, highlighting the industrial relevance of rumen studies (Sauer et al., 2012; Zhang et al., 2021).
The investigation into the ratio of concentrate to roughage in ruminant diets is essential for enhancing animal health, productivity, and ecological sustainability. This balance affects various physiological and metabolic processes in ruminants, influencing their digestion, nutrient absorption, and overall well-being. Understanding the implications of different ratios can lead to improved feeding strategies that enhance animal performance and reduce negative environmental impacts. Wanapat et al. (2014) demonstrated that the ratio of roughage to concentrate significantly affects rumen pH, fermentation patterns, and microbial populations. High concentrate diets can lower rumen pH, altering volatile fatty acid (VFA) profiles and reducing the population of cellulolytic bacteria, which are essential for fiber digestion. Adjusting the roughage to concentrate ratio can optimize rumen fermentation, enhancing the production of beneficial VFAs like propionate and improving microbial protein synthesis, which is vital for animal growth and productivity. The digestibility of nutrients varies with different roughage to concentrate ratios. Diets with higher concentrate levels generally improve the digestibility of dry matter, organic matter, and crude protein, but may reduce fiber digestibility. The balance between roughage and concentrate influences the site of nutrient digestion, with higher concentrate diets shifting more protein digestion to the intestines, which can affect the overall efficiency of nutrient utilization (Faría-Mármol et al., 2002). Leng (1993) noted that properly balanced diets can reduce enteric methane emissions, a significant contributor to greenhouse gases, by optimizing the rumen ecosystem and nutrient availability. Feeding strategies that prioritize roughage before concentrates can enhance carbohydrate digestion and bacterial protein synthesis, supporting better health and productivity in dairy cows. Different ruminant species exhibit varying responses to forage and concentrate ratios. For instance, sheep tend to have higher digestibility and intake of forage-rich diets compared to goats and fallow deer, highlighting the need for species-specific feeding strategies (Ramanzin et al., 1997).
Swamp buffaloes, or Bubalus bubalis, are integral to livestock systems in various regions, particularly in Asia. They contribute significantly to agricultural practices, rural economies, and ecological balance. Their importance is multifaceted, encompassing roles in agriculture, nutrition, and environmental management. Research into the rumen of swamp buffaloes provides insights into their unique digestive capabilities, potential dietary enhancements, and the broader implications for sustainable farming practices. The rumen of swamp buffaloes harbors a diverse microbial population, including potent fibrolytic bacteria like Ruminococcus flavefaciens, which enhance fiber digestion. This microbial diversity is crucial for the efficient breakdown of low-quality roughage (Boonsaen et al., 2019). Swamp buffalo rumen fluid contains a higher number of microbes compared to river buffalo and cattle, indicating its potential as a probiotic source for improving livestock health and productivity. A buffalo-derived probiotic strain, SN-6, has demonstrated significant growth-promoting effects in other ruminants by modulating gut microbiota and metabolism, highlighting the potential of swamp buffalo rumen-derived probiotics (Rostini et al., 2018; Yang et al., 2022). Mat Amin et al. (2022) indicated that the morphology of the rumen mucosa in swamp buffaloes varies with production systems, affecting volatile fatty acid concentrations and potentially influencing overall digestive efficiency. The use of swamp buffalo rumen liquid in fodder has been shown to enhance the quality of meat in other livestock, indicating its potential application in improving agricultural outputs (Rostini et al., 2019). Investigating rumen fermentation in beef cattle and goats is essential for maximizing feed efficiency, promoting animal health, and minimizing environmental effects. The rumen, an intricate microbial environment, is fundamental to the digestion and nutrient uptake processes in ruminants. Gaining insights into the mechanisms of rumen fermentation can result in improved productivity and sustainability within livestock agriculture.
This study is essential for both basic scientific understanding and practical applications in agriculture and industry. Consequently, this research aimed to examine the impact of varying ratios of roughage to concentrate on the fermentation efficiency within the rumen, utilizing rumen fluids from different sources, such as swamp buffalo, beef cattle, and goats.
Table 1: Ingredients and chemical composition of concentrate and dry Leucaena leaf used in the experiment.
|
Ingredients |
Concentrate |
Dry Leucaena leaf |
|
Corn meal |
61 |
|
|
Rice bran |
10 |
|
|
Soybean meal |
23 |
|
|
Molasses |
3 |
|
|
Urea |
2 |
|
|
Mineral premix |
1 |
|
|
Total |
100 |
|
|
Chemical composition |
||
|
Dry matter (DM), (g/kg) |
928 |
875 |
|
DM basis (g/kg) |
||
|
Organic matter (OM) |
914 |
922 |
|
Crude protein (CP) |
146 |
273 |
|
Ether extract (EE) |
37.2 |
39.5 |
|
Neutral detergent fiber (NDF) |
238 |
356 |
|
Acid detergent fiber (ADF) |
121 |
177 |
Materials and Methods
The experiment utilized a 3×2 factorial design in a completely randomized setup with four replications. The inoculum sources encompassed three animal species (swamp buffalo, beef cattle, and goat), while the dietary treatments featured two ratios of roughage to concentrate as follows: 100:0 and 70:30, utilizing dry Leucaena leaves (DLL) as the roughage source. Leucaena contains mimosine and tannins, which can interfere with digestion by binding to proteins and enzymes. However, processing methods such as drying can significantly reduce these antinutritional factors for 32.69%, making Leucaena safer for consumption (Chanchay and Poosaran, 2009). Leucaena leaves (local variety) were collected exclusively from the trees at the Kasetsart University plot, which had an average age of 2-3 years, and subsequently sun-dried. The samples of DLL and concentrate were processed to pass through a 1-mm sieve using a Wiley mill (Thomas Scientific) for the purpose of chemical composition analysis. The samples underwent chemical analysis to determine dry matter (DM), organic matter (OM), crude protein (CP), and ether extract (EE). The analysis of neutral detergent fiber (NDF) and acid detergent fiber (ADF) was carried out following the procedures described by Van Soest (Van Soest, 1994), as detailed in Table 1. The research was carried out in accordance with the guidelines and animal welfare standards of The Institute of Animals for Scientific Purpose Development Thailand, located at Kasetsart University in Bangkok.
Animals and the Process of Preparing Rumen Inoculum
A total of twelve animals were utilized as donors for rumen fluid inoculum: four Thai Swamp Buffaloes (Bubalus bubalis, aged 4 years, weighing 580 ± 20 kg), four Thai beef cattle (Bos taurus indicus, also 4 years old, with a weight of 410 ± 37 kg), and four Anglo-Nubian goats (Capra hircus, 1 year old, weighing 15 ± 2.5 kg). Animals were housed separately and provided with feed twice a day, consisting of a diet made up of 60% rice straw and 40% concentrate that contained 15% crude protein. The animals were maintained on this diet for a duration of 14 days prior to the collection of rumen fluid. An inoculum was created for each donor belonging to the respective animal species. The rumen fluid was filtered through four layers of cheesecloth into pre-warmed thermos flasks. Preparation of artificial saliva was done and rumen fluid from every animal was combined with an artificial saliva solution at a ratio of 1:2 (Menke and Steingass, 1988).
In vitro Gas Production Study
Substrates were carefully prepared and measured to 200 mg of DM into 50 ml bottles. Before introducing 30 ml of the rumen inoculum mixture, the serum bottles containing the substrate treatments were subjected to pre-warming in a water bath maintained at 39 °C for a duration of 1 hour. Gas production was recorded at 0, 2, 4, 6, 8, 12, 24, 36, 48, 60, and 72 hours of incubation. The cumulative gas production data were analyzed using the model proposed by Ørskov and McDonald, (1979) as follows: y = a + b(1-e(-ct)), where a = the gas production from the immediately soluble fraction, b = the gas production from the insoluble fraction, c = the gas production rate constant for the insoluble fraction (b), t = incubation time, (a+b) = the potential extent of gas production, y = gas produced at time “t”.
Rumen fluid inoculum was obtained at 1 and 4 hours of incubation. The rumen fluid samples were divided into two portions; the initial portion was evaluated for pH levels, whereas the subsequent portion was subjected to analysis for ammonia nitrogen (NH3-N) concentration and volatile fatty acids (VFAs) employing high-performance liquid chromatography (Samuel et al., 1997). The remaining portion was preserved at −20 °C for DNA extraction (Yu and Morrison, 2004). At 48 hours after inoculation, a series of samples were analyzed for in vitro true digestibility (IVDMD) in accordance with the methods established by Van Soest and Robertson (1985). In summary, the contents of the bottle were quantitatively transferred into a spoutless beaker by rinsing repeatedly with 100 ml of neutral detergent solution. The mixture was refluxed for one hour and subsequently filtered through pre-weighed Gooch crucibles. The dry matter of the residue was measured, and the IVDMD of the feed was calculated as follows:
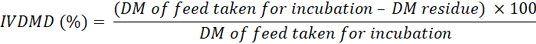
Gas production of methane was calculated by using the equation of Moss et al. (2000). The calculated as follows: Methane production = 0.45 (acetate) – 0.275 (propionate) + 0.4 (butyrate)
Quantitative Analysis of Microbial Populations
Community DNA was obtained from 1.0 ml aliquots of each sample using the RBB+C technique (Yu and Morrison, 2004). A total of 48 samples representing six treatment combinations, with two incubation durations (1 and 4 hours), along with replicates were processed for genomic DNA extraction. The primers utilized in the real-time PCR reactions, which are specific to the target species listed, are presented in Table 2. F. succinogenes primers were selected to allow amplification (446-bp product) of all 10 F. succinogenes strains deposited in GenBank. For R. albus primers allow for a 175-bp product. R. flavefaciens primers were selected to allow species-species amplification of all seven R. flavefaciens strains deposited in GenBank (Koike and Kobayashi, 2001). P. bryantii primers were specific amplication of P. bryantii strain B14T (Tajima et al., 2001). S. bovis primer were selecetd to allow species-species amplication of S. bovis species (Fernando et al., 2010). M. elsdenii primers were specific amplication of M. elsdenii strain YE34 (Ouwerkerk et al., 2002). And S. jonesii primer were selecetd to allow species-species amplication of S. jonesii species (Klieve et al., 2002). For each standard derived from the samples, the copy number concentration was determined. In total, ten real-time PCR standards were created. All PCR reactions were conducted in duplicate.
Statistical Analysis
All data were statistically analyzed as a 3×2 factorial arrangement in a CRD using the general linear procedure in PROC ANOVA. The statistical model included terms of animal species, roughage to concentrate ratio, and animal species x roughage to concentrate ratio interactions. Data were analyzed using the model
Yij = µ + τi + βj + γij + εijk
Where;
Yij is the observation, µ is the overall mean, τi is the effect of animal species, βj is the effect of dietary treatment, γij is interaction between animal species and roughage to concentrate ratio, and εijk is residual error. Multiple comparisons
Table 2: PCR primers set used for real-time PCR assay.
|
Target Species |
Primer Sequence (5’-3’) |
Annealing Temp (°C) |
Size (bp) |
Ref. |
|
Total Bacteria |
(F) 5’-CGG CAA CGA GCG CAA CCC-3’ |
60 |
130 |
(Makkar and McSweeney, 2005) |
|
(R) 5’-CCA TTG TAG CAC GTG TGT AGC C-3’ |
||||
|
Total Fungi |
(F) 5’-GAG GAA GTA AAA GTC GTA ACA AGG TTT C-3’ |
60 |
120 |
(Makkar and McSweeney, 2005) |
|
(R) 5’-CAA ATT CAC AAA GGG TAG GAT GAT T-3’ |
||||
|
Total Protozoa |
(F) 5’- GCT TTC GWT GGT AGT GTA TT-3’ |
55 |
223 |
(Denman et al., 2007) |
|
(R) 5’- CTT GCC CTC YAA TCG TWC T-3’ |
||||
|
R. albus |
(F) 5’-CCC TAA AAG CAG TCT TAG TTC G-3’ |
55 |
175 |
(Koike and Kobayashi, 2001) |
|
(R) 5’-CCT CCT TGC GGT TAG AAC A-3’ |
||||
|
R. Flavefaciens |
(F) 5’-CGA ACG GAG ATA ATT TGA GTT TAC TTA GG-3’ |
60 |
132 |
(Koike and Kobayashi, 2001) |
|
(R) 5’-CGG TCT CTG TAT GTT ATG AGG TAT TAC C-3’ |
||||
|
F. succinogenes |
(F) 5’-GTT CGG AAT TAC TGG GCG TAA A-3’ |
60 |
121 |
(Koike and Kobayashi, 2001) |
|
(R) 5’-CGC CTG CCC CTG AAC TAT C-3’ |
||||
|
P. bryantii |
(F) 5’- ACT GCA GCG CGA ACT GTC AGA-3’ |
68 |
540 |
(Tajima et al., 2001) |
|
(R) 5’-ACC TTA CGG TGG CAG TGT CTC-3’ |
||||
|
S. bovis |
(F) 5’-ATT CTT AGA GAT AGG GTT TCT CTT-3’ |
60 |
134 |
(Fernando et al., 2010) |
|
(R) 5’-ACC TTA TGA TGG CAA CTA ACA ATA-3’ |
||||
|
M. elsdenii |
(F) 5’-GAC CGA AAC TGC GAT GCT AGA-3’ |
60 |
128 |
(Fernando et al., 2010) |
|
(R) 5’-TCC AGA AAG CCG CTT TCG CCA CT-3’ |
||||
|
S. jonesii |
(F) 5’-GGA ATA TTG GGC AAT GGG-3’ |
66 |
595 |
(Klieve et al., 2002) |
|
(R) 5’-GTT CTT CGG TTT GCA TCG-3’ |
Table 3: The effect of the proportions of roughage to concentrate on rumen fluid differs among various animal species on fermentation kinetic values, gas production and in vitro true digestibility.
|
Items |
Swamp buffalo |
Beef cattle |
Goat |
SEM |
Effects |
|||||
|
100:0 |
70:30 |
100:0 |
70:30 |
100:0 |
70:30 |
A |
B |
AxB |
||
|
Fermentation kinetic values |
||||||||||
|
a |
5.78 |
6.40 |
5.94 |
6.03 |
4.91 |
5.05 |
1.640 |
0.085 |
0.033 |
0.194 |
|
b |
79.01b |
82.46a |
71.60d |
75.15c |
64.06f |
65.18e |
0.400 |
<0.001 |
<0.001 |
0.047 |
|
c |
0.045d |
0.047c |
0.046b |
0.045d |
0.045d |
0.049a |
0.360 |
<0.001 |
<0.001 |
<0.001 |
|
d |
73.22 |
76.06 |
65.66 |
69.12 |
59.15 |
60.12 |
1.910 |
<0.001 |
0.174 |
0.289 |
|
Gas (72 h, ml) |
72.15b |
75.72a |
64.36d |
68.28c |
58.50f |
59.30e |
0.070 |
<0.001 |
<0.001 |
0.004 |
|
IVDMD,(%) |
81.78 |
85.47 |
73.08 |
78.59 |
68.40 |
74.87 |
1.013 |
<0.001 |
0.001 |
0.246 |
a-f Values on the same row with different superscripts differ (p<0.05). SEM = Standard error of the mean.
A: Animal species (swamp buffalo, beef cattle and goat); B: Roughage to concentrate ratio (100:0 and 70:30); A×B: Interaction between animal species and roughage to concentrate ratio; a: the gas production from the immediately soluble fraction (ml); b: the gas production from the insoluble fraction (ml); c: the gas production rate constant for the insoluble fraction (ml/h); t: incubation time (h); d: the potential extent of gas production (ml); Y: gas produced at time “t” (ml).
among treatment means were performed by Duncan’s New Multiple Range Test (DMRT) (Steel and Torrie, 1980).
RESULTS AND DISCUSSIONS
From Table 3, it was observed that the parameters a, b, c, d, cumulative gas volume at hour 72, and IVDMD exhibited significant differences (p<0.05). The study revealed that the gas production values from the immediately soluble fraction (a) and IVDMD were statistically different due to the impact of the ratio of roughage to concentrate. It was found that the implementation at the 70:30 ratio was greater than at the 100:0 ratio (p=0.033, p=0.001). For the potential extent of gas production (d) and IVDMD values, significant statistical differences were observed because of different animal species. The swamp buffalo group exhibited the highest value, whereas the goat group displayed the lowest value (p<0.001) (74.64, 67.39 and 59.64 ml; 83.63, 75.84 and 71.64 %, respectively). The gas production from the insoluble fraction (b) and cumulative gas volume at hour 72 were found to be highest in the swamp buffalo group that received 70:30 ratio, while the goat group that received 100:0 ratio recorded the lowest value (p= 0.047, p=0.004) (82.46 and 64.06 ml; 75.72 and 58.50 ml, respectively). The gas production rate constant for the insoluble fraction (c) was determined to be the highest in the goat group that received 70:30 ratio (p<0.001).
From Table 4, it is observed that at the 4 hour post-incubation, the concentrations of NH3-N, along with the proportions of C2 and C4, showed significant statistical differences influenced by animal species (p<0.05). The swamp buffalo group exhibited the highest NH3-N concentration and C2 proportion, whereas the goat group recorded the lowest values (p<0.001 and p=0.005) (18.10 and 14.37 mg/dl; 74.07 and 69.94 %). In terms of the C4 proportion, the goat group displayed the highest values, while the swamp buffalo group had the lowest values (p=0.026) (8.41 and 3.30 %). The impact of the ratio of roughage to concentrate at the 4 hour post-incubation was found to affect NH3-N concentration, total VFAs, the proportion of C2, the proportion of C3, the C2:C3 ratio, and methane gas production. It was statistically significant (p<0.05) that the use of the 70:30 ratio can produce a higher NH3-N concentration, total VFAs, and C3 proportion compared to the 100:0 ratio (p=0.002, p=0.004, and p=0.044) (16.94 and 15.48 mg/dl; 80.57 and 65.32 mmol/L; 23.46 and 21.48%, respectively), whereas the 70:30 ratio exhibited a lower proportion of C2 output, C2:C3 ratio, and methane production than the 100:0 ratio (p=0.031, p=0.001, and p=0.034) (70.27 and 72.60 %; 3.40 and 3.00, respectively).
From Table 5 at 1-hour post-incubation, it was observed that the variations in microbial populations within the rumen, including total bacteria, total fungi, total protozoa, and R. albus, were statistically significant (p<0.001) as a result of the animal species. It was noted that swamp buffalo exhibited a higher abundance of most microorganisms, while the total bacteria, total fungi, and R. albus populations were lowest in the beef cattle group, and the total protozoa population was the least in the goat group.
At the 4 hours post-incubation, the differences observed in the populations of total bacteria, total fungi, total protozoa, R. albus, and R. flavefaciens were determined to be statistically significant (p<0.05). The findings from the total bacteria population study revealed significant differences among the treatments (p<0.001). The group that received a 70:30 ratio demonstrated a higher total bacteria population compared to the group that received a 100:0 ratio (p<0.001).
Table 4: The effect of the proportions of roughage to concentrate on rumen fluid differs among various animal species on ruminal pH, ammonia nitrogen, volatile fatty acid production and methane production.
|
Items |
Swamp buffalo |
Beef cattle |
Goat |
SEM |
Effects |
|||||
|
100:0 |
70:30 |
100:0 |
70:30 |
100:0 |
70:30 |
A |
B |
AxB |
||
|
Rumen pH |
||||||||||
|
1 h |
6.69 |
6.73 |
6.66 |
6.70 |
6.74 |
6.71 |
0.205 |
0.587 |
0.540 |
0.849 |
|
4 h |
6.67 |
6.66 |
6.66 |
6.47 |
6.68 |
6.67 |
0.657 |
0.883 |
0.983 |
0.676 |
|
NH3–N, mg/dl |
||||||||||
|
1 h |
12.40 |
14.95 |
12.28 |
12.53 |
12.62 |
13.10 |
2.653 |
0.306 |
0.116 |
0.319 |
|
4 h |
17.35 |
18.84 |
15.03 |
17.32 |
14.05 |
14.68 |
0.387 |
<0.001 |
0.002 |
0.137 |
|
Total VFAs (mmol/L) |
||||||||||
|
1 h |
46.30 |
59.10 |
47.75 |
47.10 |
54.08 |
49.48 |
2.326 |
0.639 |
0.606 |
0.323 |
|
4 h |
69.45 |
83.53 |
68.25 |
78.60 |
58.25 |
79.58 |
2.735 |
0.432 |
0.004 |
0.631 |
|
Acetate (C2), % |
||||||||||
|
1 h |
72.58 |
71.59 |
71.30 |
71.13 |
71.88 |
68.03 |
0.581 |
0.384 |
0.194 |
0.457 |
|
4 h |
76.18 |
71.95 |
71.40 |
69.23 |
70.22 |
69.65 |
0.655 |
0.005 |
0.031 |
0.348 |
|
Propionate (C3), % |
||||||||||
|
1 h |
22.14 |
20.53 |
23.10 |
19.27 |
18.76 |
20.19 |
0.722 |
0.526 |
0.373 |
0.360 |
|
4 h |
21.45 |
23.83 |
22.93 |
23.33 |
20.05 |
23.24 |
0.499 |
0.446 |
0.044 |
0.481 |
|
Butyrate (C4), % |
||||||||||
|
1 h |
5.28 |
7.88 |
5.60 |
9.60 |
9.37 |
11.78 |
0.789 |
0.084 |
0.056 |
0.884 |
|
4 h |
2.38 |
4.22 |
5.68 |
7.45 |
9.71 |
7.11 |
0.797 |
0.026 |
0.814 |
0.361 |
|
Acetate:Propionate (C2:C3) |
||||||||||
|
1 h |
3.3 |
3.5 |
3.1 |
3.7 |
3.8 |
3.4 |
0.142 |
0.515 |
0.591 |
0.483 |
|
4 h |
3.6 |
3.0 |
3.1 |
3.0 |
3.5 |
3.0 |
0.078 |
0.245 |
0.001 |
0.135 |
|
Methane (CH4), mol/100 mol |
||||||||||
|
1 h |
28.68 |
29.73 |
21.97 |
30.55 |
30.94 |
29.77 |
0.498 |
0.591 |
0.432 |
0.344 |
|
4 h |
29.33 |
27.51 |
28.09 |
27.71 |
29.97 |
27.79 |
0.340 |
0.471 |
0.034 |
0.490 |
A: Animal species (swamp buffalo, beef cattle and goat); B: Roughage to concentrate ratio (100:0 and 70:30); A×B: Interaction between animal species and roughage to concentrate ratio.
It was discovered that in the group which received the 70:30 ratio, the total bacterial population in swamp buffalo was higher than that in beef cattle and lower than that in goats (p<0.001) (3.89, 3.52 and 2.91 x 1010 copies/ml of rumen fluid, respectively). The total fungi population was observed to be higher in the group that received a 70:30 ratio compared to the group that received a 100:0 ratio (p<0.001), and it was noted that within the 70:30 ratio group, the total fungi population was greater in swamp buffalo than in beef cattle, with goats having the lowest population (p=0.003) (22.97, 13.85 and 9.85 x 106 copies/ml of rumen fluid, respectively). In the total protozoa population, it was observed that the group receiving a 70:30 ratio exhibited a greater total protozoa count compared to the group that received a 100:0 ratio (p<0.001), and it was also noted that within the group that received the 70:30 ratio, the total protozoa population in beef cattle was more prevalent than in swamp buffalo, with goats showing the least abundance (p<0.001) (9.78, 8.40 and 7.90 x 107 copies/ml of rumen fluid, respectively ). In the case of the R. albus population, it was observed that the group receiving the 70:30 ratio had a larger population compared to the group that received the 100:0 ratio (p<0.001). Additionally, it was noted that within the 70:30 ratio group, the population of R. albus in swamp buffalo was greater than that of goats, while beef cattle exhibited the lowest population (p<0.001) (38.08, 18.03 and 13.48 x 106 copies/ml of rumen fluid, respectively). It was noted that the swamp buffalo group receiving 70:30 ratio exhibited the highest populations of total bacteria, total fungi, and R. albus (p<0.001, p=0.003, and p<0.001, respectively), whereas the beef cattle group administered 70:30 ratio had the greatest population of total protozoa and R. flavefaciens (p<0.001,p<0.001).
At the 4 hours post-incubation, the impact of the ratio of roughage to concentrate revealed statistically significant differences (p<0.05) in the populations of F. succinogenes, S. bovis, and P. bryantii. The group that received 70:30 ratio
Table 5: The effect of the proportions of roughage to concentrate on rumen fluid differs among various animal species on rumen microorganism population from in vitro incubation with rumen fluid.
|
Items |
Swamp buffalo |
Beef cattle |
Goat |
SEM |
Effects |
|||||
|
100:0 |
70:30 |
100:0 |
70:30 |
100:0 |
70:30 |
A |
B |
AxB |
||
|
Total Bacteria, x 1010 copies/ ml of rumen fluid |
||||||||||
|
1 h |
2.40 |
2.58 |
1.88 |
1.90 |
2.25 |
2.27 |
0.028 |
<0.001 |
0.321 |
0.530 |
|
4 h |
3.73b |
3.89a |
3.23d |
3.52c |
2.11f |
2.91e |
0.124 |
<0.001 |
<0.001 |
<0.001 |
|
Total Fungi, x 106copies/ ml of rumen fluid |
||||||||||
|
1 h |
3.09 |
3.07 |
0.86 |
0.90 |
3.04 |
3.03 |
0.412 |
<0.001 |
0.587 |
0.273 |
|
4 h |
6.71cd |
22.97a |
6.45cd |
13.85b |
5.16d |
9.85c |
5.709 |
<0.001 |
<0.001 |
0.003 |
|
Total Protozoa, x 107 copies/ ml of rumen fluid |
||||||||||
|
1 h |
7.40 |
7.58 |
9.62 |
9.73 |
7.00 |
7.03 |
0.035 |
<0.001 |
0.218 |
0.643 |
|
4 h |
7.20e |
8.40c |
9.31b |
9.78a |
7.02e |
7.90d |
0.015 |
<0.001 |
<0.001 |
<0.001 |
|
Ruminococcus albus, x 106 copies/ ml of rumen fluid |
||||||||||
|
1 h |
6.93 |
6.31 |
0.58 |
0.36 |
1.69 |
1.74 |
0.024 |
<0.001 |
0.421 |
0.220 |
|
4 h |
6.44d |
38.08a |
1.62e |
13.48c |
2.02e |
18.03b |
6.501 |
<0.001 |
<0.001 |
<0.001 |
|
Ruminococcus flavefaciens, x 107 copies/ ml of rumen fluid |
||||||||||
|
1 h |
5.00 |
3.76 |
4.86 |
3.52 |
3.98 |
4.25 |
3.534 |
0.959 |
0.328 |
0.638 |
|
4 h |
3.28de |
4.23bc |
2.48e |
5.30a |
4.60ab |
3.49cd |
0.315 |
0.601 |
0.001 |
<0.001 |
|
Fibrobacter succinogenes, x 108 copies/ ml of rumen fluid |
||||||||||
|
1 h |
7.51 |
6.22 |
8.25 |
8.11 |
1.64 |
5.06 |
1.341 |
0.374 |
0.817 |
0.781 |
|
4 h |
8.57 |
14.08 |
11.16 |
12.09 |
3.20 |
10.40 |
1.135 |
0.113 |
0.034 |
0.428 |
|
Streptococcus bovis, x 106 copies/ ml of rumen fluid |
||||||||||
|
1 h |
12.90 |
6.38 |
0.74 |
4.78 |
3.95 |
5.66 |
9.161 |
0.051 |
0.903 |
0.123 |
|
4 h |
6.54 |
36.58 |
11.01 |
17.55 |
12.27 |
20.53 |
3.489 |
0.359 |
0.002 |
0.059 |
|
Prevotella bryantii, x 104 copies/ ml of rumen fluid |
||||||||||
|
1 h |
12.17 |
12.74 |
12.28 |
8.99 |
6.70 |
10.44 |
5.389 |
0.640 |
0.918 |
0.690 |
|
4 h |
9.14 |
12.36 |
5.73 |
10.41 |
6.00 |
9.12 |
5.434 |
0.245 |
0.034 |
0.906 |
|
Megasphaera elsdenii, x 104 c`opies/ ml of rumen fluid |
||||||||||
|
1 h |
15.46 |
6.51 |
3.00 |
9.29 |
5.61 |
7.89 |
7.674 |
0.691 |
0.979 |
0.446 |
|
4 h |
3.36 |
7.48 |
8.26 |
7.33 |
6.50 |
8.45 |
3.964 |
0.676 |
0.474 |
0.683 |
|
Synergist jonesii, x 105 copies/ ml of rumen fluid |
||||||||||
|
1 h |
5.36 |
6.60 |
2.96 |
1.28 |
0.98 |
2.25 |
1.014 |
0.199 |
0.895 |
0.803 |
|
4 h |
15.40 |
5.76 |
5.36 |
5.27 |
6.74 |
6.26 |
1.111 |
0.078 |
0.083 |
0.086 |
a-f Values on the same row with different superscripts differ (p<0.05); SEM: Standard error of the mean; A: Animal species (swamp buffalo, beef cattle and goat); B: Roughage to concentrate ratio (100:0 and 70:30); A×B: Interaction between animal species and roughage to concentrate ratio.
demonstrated an increase in microbial population compared to the group that received 100:0 ratio. The investigation indicated that the populations of M. elsdenii and S. jonesii did not show significant differences (p>0.05).
The examination of the balance between concentrate and roughage in ruminants is essential for enhancing their nutrition, health, and overall productivity. This R:C ratio significantly influences rumen fermentation, microbial populations, nutrient digestibility, and overall animal performance. By understanding and applying the appropriate concentrate-to-roughage (C: R) ratio, farmers and researchers can enhance feed efficiency, reduce methane emissions, and improve the health and productivity of ruminants. While, studying the fermentation process in the rumen of ruminants offers valuable insights that can be applied to animal feed management, enhancing feed efficiency, optimizing nutrient utilization, and reducing environmental impacts. By understanding the microbial dynamics and fermentation characteristics within the rumen, feed formulations can be tailored to improve animal health and productivity.
Based on the impact of the animal species studied, it was determined that they influence digestibility (IVDMD). The analysis revealed that the swamp buffalo group exhibited the highest level of digestibility, particularly in relation to the insoluble fraction, and it was also noted that the beef cattle group demonstrated a greater ability to digest compared to the goat group, which had the lowest digestibility overall (83.63, 75.84 and 71.64%, respectively). The comparative study of rumen fermentation between swamp buffaloes, beef cattle and goats reveal distinct differences in microbial composition, fermentation efficiency, and nutrient digestibility. These differences are primarily due to the unique adaptations and dietary habits of each species, which influence their rumen ecology and fermentation processes.
Swamp buffaloes have a higher population of cellulolytic bacteria, such as Ruminococcus flavefaciens, it can produces a range of enzymes such as carboxymethyl-cellulase, xylanase, and pectin lyase which enhances their ability to digest low-quality roughages like rice straw. This is attributed to the presence of specific bacterial strains that improve fiber digestion through cross-feeding interactions with non-fibrolytic bacteria. The ability of swamp buffaloes to digest low-quality roughages, which are high fiber content, low nutrient density and poor digestibility, more efficiently than goats is a significant advantage, particularly in regions where such feed resources are abundant. This capability is enhanced by the presence of specific microbial populations that optimize fiber breakdown and nutrient absorption. In goats, the digestibility of organic matter, dry matter, and crude protein is influenced by the bacterial composition, which can be manipulated to improve feed efficiency. However, the overall digestibility may be lower compared to swamp buffaloes, especially when fed on low-quality roughages (Boonsaen et al., 2019; Chanthakhoun et al., 2012; Li et al., 2022).
From this research, variations in the fermentation process within the rumen of beef cattle and goats were identified due to cattle rumen microorganisms are more effective in lignocellulosic biomass degradation, which is crucial for maximizing methane production and biogas yield. This suggests that the cow rumen microorganisms could secrete more extracellular multienzyme complexes to hydrolyze lignocellulosic biomass than the sheep rumen microorganisms in vitro. Goats, on the other hand, show consistent rumen fermentation characteristics across different diets, with stable pH values and VFA concentrations. This indicates a more uniform fermentation process, possibly due to a less diverse microbial community compared to cattle (Ismartoyoa et al., 2024; Xing et al., 2020).
The impact of different animal species on the rumen fermentation characteristics observed in the 4 hours post-incubation, such as the levels of NH3-N and the ratio of C2, was noted to be highest in the swamp buffalo group and lowest in the goat. Ruminants rely on rumen microorganisms to degrade nitrogenous compounds, leading to NH3 production. This process is essential for microbial protein synthesis, which is a significant source of amino acids for the host animal (Zurak et al., 2023). The rate of NH3 production varies with the substrate and microbial population. Swamp buffaloes exhibit higher NH3-N concentrations in the rumen compared to beef cattle. This is attributed to their ability to efficiently recycle nitrogen, which is reflected in higher ruminal NH3-N levels (Thanh, 2014). Chanthakhoun et al. (2012) indicated that swamp buffaloes have a higher microbial population, particularly cellulolytic bacteria, which may contribute to their enhanced ability to digest low-quality roughages and maintain higher NH3-N levels. While, Li et al. (2022) reported that cattle and goat exhibit a different microbial community structure compared to buffaloes, which affects their ruminal fermentation and NH3-N production. Goats require a minimum NH3-N level of 3.43 mg/dl to maintain microbial activity, with optimal levels around 14.5 mg/dl for efficient microbial community function (Lopes et al., 2020).
The greatest percentage of C2 was observed in swamp buffalo due to they have a more efficient rumen fermentation process compared to cattle and goats, which leads to higher acetate production. The rumen environment, including pH and microbial population, plays a crucial role in determining the types and amounts of VFAs produced. The microbial population in the rumen of buffaloes is adapted to break down fibrous plant materials more effectively, leading to increased acetate production. This is supported by the presence of specific microbial communities that are more efficient in converting carbohydrates to acetate (Xu et al., 2024). Physiological differences between swamp buffaloes, cattle, and goats also contribute to variations in acetate production. Buffaloes have a larger rumen volume and longer retention time for feed, allowing for more extensive fermentation and acetate production. The acetate turnover rate, which reflects the rate at which acetate is produced and utilized, is higher in buffaloes compared to cattle. This indicates a more dynamic acetate metabolism in buffaloes, supporting their higher production levels (Davis, 1967). The activity of enzymes involved in acetate metabolism, such as acetyl-CoA synthetase, is higher in buffaloes, facilitating the conversion of acetate into energy and other metabolic products. This enzymatic activity supports the continuous production and utilization of acetate in buffaloes (Smith et al., 2018).
From the impact of various animal species on distinct microbial communities in the rumen, it was discovered that swamp buffalo exhibited the largest populations of total bacteria, total fungi, and R. albus, while the total protozoa population was highest in beef cattle. This suggests that swamp buffalo rumen fluid could serve as a potential probiotic source for other livestock (Rostini et al., 2014). The rumen of swamp buffaloes contains a diverse fungal community, with aerobic fungi such as Ascomycota and Basidiomycota being dominant under various feeding conditions. This diversity is crucial for the breakdown of fibrous plant materials, enhancing the digestive capabilities of swamp buffaloes (Wang et al., 2023). In beef cattle, the abundance of rumen fungi is influenced by diet and breed. While specific studies on rumen fungi in goats are limited, the bacterial composition suggests a symbiotic relationship with fungi to optimize fermentation and nutrient absorption. The presence of certain bacterial genera correlates with improved fermentation parameters, indicating a potential interaction with fungal populations (Li et al., 2022; Zhang et al., 2022).
Clemmons et al. (2021) indicated that protozoa are essential to the rumen ecosystem by managing bacterial populations and engaging in nitrogen cycling. They play a substantial role in the microbial biomass and contribute to the breakdown of starch and fiber, thereby improving feed efficiency. The metabolic activities of protozoa, such as the production of volatile fatty acids and involvement in methanogenesis, are essential for the overall energy metabolism in cattle. These activities can influence the efficiency with which cattle convert feed into body mass, impacting production traits like marbling and methane emission (Conteville et al., 2023). The presence of specific bacterial families within the protozoa-associated communities, such as Lachnospiraceae, further supports the metabolic functions of protozoa, aiding in the digestion process and nutrient absorption (Levy and Jami, 2018).
High-concentration diets (e.g., 100:0 C:R) lead to an increase in gas production along with enhanced digestibility of both dry matter and organic matter. This enhancement is attributed to the greater availability of fermentable carbohydrates, which boost microbial activity and fermentation efficiency (Al-Galbi and Majeed, 2022). On the other hand, a higher roughage ratio (e.g., 80:20 R:C) generally results in decreased gas production but may improve fiber digestibility due to the heightened activity of cellulolytic bacteria. The most effective R:C ratio for achieving equilibrium between gas production and digestibility is likely to be in the range of 40:60 or 50:50, as this combination encourages a balanced interaction between fiber and fermentable carbohydrates, enhancing both microbial activity and fermentation efficiency (Nagadi, 2019; Suriyapha et al., 2021).
Wang et al. (2023) reported that raising the proportion of concentrates in the diet usually causes a drop in rumen pH, potentially stimulating propionic acid production but increasing the risk of acidosis if not carefully monitored. An increased roughage content aids in stabilizing rumen pH and boosts the level of acetic acid, which is advantageous for fiber digestion and the overall health of the rumen. The concentration of total volatile fatty acids (TVFA) generally rises with elevated concentrate levels, improving energy availability for the host animal (Chen et al., 2021).
The R:C ratio plays a key role in shaping the rumen microbiota’s composition. Diets high in concentrates boost the population of amylolytic bacteria, known for their effectiveness in starch fermentation, whereas diets rich in roughage promote cellulolytic bacteria, which are crucial for fiber degradation (Wang et al., 2023). The production of methane tends to be reduced with greater concentrate diets because fermentation shifts toward propionate production, which utilizes hydrogen that would typically contribute to methane formation. The consequences of an unbalanced roughage-to-concentrate ratio on rumen health and animal productivity are complex, impacting microbial diversity, nutrient absorption, fermentation characteristics, and overall well-being of the animals. Increasing the concentrate ratio usually boosts nutrient digestibility and growth results, but it could endanger rumen health by modifying microbial communities and fermentation dynamics. In contrast, a higher roughage ratio enhances rumen stability but might limit improvements in productivity. Thus, achieving an ideal equilibrium between roughage and concentrate is crucial for optimizing both animal welfare and productivity levels. This balance varies according to the specific needs of the animal and particular production goals (Nagadi, 2019; Phesatcha et al., 2021).
The roughage to concentrate (R:C) ratio in ruminant diets significantly influences the rumen microbial ecosystem, including S. bovis, F. succinogenes, and P. bryantii. These microbial populations are crucial for rumen fermentation, fiber digestion, and overall animal health. S. bovis, an amylolytic bacterium, thrives in high-starch environments typical of high-concentrate diets. As the concentrate proportion increases, the population of amylolytic bacteria like S. bovis also increases, which can lead to rapid fermentation and potential acidosis. Polyorach et al. (2014) indicated that the increase in S. bovis is associated with a decrease in rumen pH, as observed in diets with higher concentrate levels, which can further promote the growth of this bacterium. While, F. succinogenes, a cellulolytic bacterium, is more prevalent in high-roughage diets. Its population decreases as the concentrate level increases, which can negatively impact fiber digestion. A study found that F. succinogenes numbers were higher in diets with a higher roughage proportion, such as an 80:20 R:C ratio, supporting its role in fiber degradation (Wanapat et al., 2014). The findings of this study contrast with previous results, revealing that the population of F. succinogenes rises when concentrates are incorporated into the diet. Moreover, P. bryantii, involved in protein and carbohydrate metabolism, is influenced by the R:C ratio. It is generally known that this bacterium adapts to varying dietary conditions, often increasing with higher concentrate levels due to its role in starch and protein breakdown (Polyorach et al., 2014). The overall bacterial population, including P. bryantii, tends to increase with higher concentrate diets, enhancing the fermentation of non-fiber carbohydrates (Phesatcha et al., 2020).
The physiological differences in rumen structure between swamp buffalo, beef cattle, and goats significantly influence their response to changes in roughage to concentrate (R:C) ratios. Swamp buffaloes have a larger omasum compared to cattle and goats, which influences their ability to process fibrous diets. This structural advantage allows for a higher microbial count in the rumen, particularly cellulolytic bacteria, which are essential for breaking down fibrous plant material (Wanapat et al., 2003). Cattle have a more complex enteric nervous system in their forestomach, which supports diverse motility patterns necessary for processing various diets. Goats have a smaller omasum relative to their body size compared to buffaloes and cattle, which may limit their ability to process large amounts of fibrous material This anatomical feature influences their feeding behavior and nutrient absorption efficiency, making them more suited to diets with higher concentrate ratios. (Mcsweeney, 1988).
The study’s findings revealed that swamp buffalo paired with a feeding ratio of 70:30 (R:C) proved to be the most effective. Additionally, the interaction effect indicates that a 70:30 feeding ratio in swamp buffalo significantly impacts the fermentation kinetic values and gas production, as well as the optimal populations of bacteria, fungi, and R. albus. In contrast, the 100:0 dietary approach for goats manifested the least influence, while the population of protozoa was notably elevated in cattle on the 70:30 diet. Swamp buffaloes, known for their ability to digest low-quality roughages, have a unique rumen fermentation process that can be influenced by various dietary supplements. R. albus, a key cellulolytic bacterium, plays a significant role in this process. The interaction between swamp buffalo rumen fermentation and R. albus can be enhanced through specific dietary interventions, which can improve fermentation efficiency, nutrient digestibility, and reduce methane emissions.
Swamp buffaloes have a higher population of cellulolytic bacteria, including R. albus, compared to cattle, which enhances their ability to digest fibrous feeds like rice straw (Chanthakhoun et al., 2012). This is crucial for efficient rumen fermentation, as these bacteria break down cellulose and hemicellulose into volatile fatty acids (VFAs), which are the primary energy source for ruminants (Emilio M. Ungerfeld et al., 2023). R. albus plays a significant role in rumen fermentation, particularly in the breakdown of fibrous plant materials and hydrogen production. This bacterium is a key member of the rumen microbiome, contributing to the conversion of complex carbohydrates into simpler compounds that can be utilized by the host animal. The fermentation of cellulose by R. albus results in the production of acetate, a volatile fatty acid that serves as a major energy source for ruminants. This process is facilitated by the bacterium’s ability to ferment cellulose to acetate and ethanol at mesophilic temperatures (Christopherson et al., 2014).
The population of protozoa in cattle is notably influenced by the dietary composition, particularly the level of concentrate in the diet. High-concentrate diets tend to elevate the protozoal population in the rumen, which plays a significant role in ruminal fermentation and nutrient metabolism. This response is due to the increased availability of dietary starch and changes in ruminal conditions that favor protozoal growth. In cattle fed diets with varying concentrate levels, the total number of rumen ciliate protozoa is higher in those receiving a higher concentrate diet. For instance, cattle on a 60% concentrate diet exhibited a higher protozoal count compared to those on a 30% concentrate diet (Santra and Pathak, 2001). Similarly, in a study involving barley-based diets, a medium-concentrate diet supported a higher protozoal population than a high-concentrate diet, although Entodinium spp. remained predominant in both cases (Hristov et al., 2001).
This research indicates that the efficiency of rumen microorganisms in swamp buffalo exceeds that of beef cattle and goats in terms of population and the production of rumen fermentation end products, suggesting a subject worthy of deeper exploration in the future. For example, the study of cellulolytic bacteria in swamp buffaloes for enhancing the efficiency of roughage utilization in ruminants, identifying key cellulolytic organisms, and exploring methods to optimize their activity for improved fiber digestion.
CONCLUSIONS AND RECOMMENDATIONS
Variations in the ratios of concentrate to roughage produce distinct effects on the fermentation process and its end product from the rumen fermentation in swamp buffalo, beef cattle, and goat. Research on animal species revealed that swamp buffalo exhibited the highest efficiency in fermentation kinetic value, gas production and IVDMD, with beef cattle following, while goats demonstrated the least efficiency, all with statistical significance. Moreover, regarding the impact of the R:C ratio, it was determined that the 70:30 ratio enhanced rumen fermentation efficiency more effectively than the 100:0 ratio, which was also statistically significant. Furthermore, the interaction effect revealed that a 70:30 feeding ratio in swamp buffalo has a significant influence on the efficiency of the fermentation process, as well as on the optimal populations of bacteria, fungi, and R. albus. This study concludes that the fermentation process in the rumen varies among different ruminant species, and that the efficiency of this process can be enhanced by utilizing concentrate and roughage in suitable proportions. This research can be utilized in the management of animal feed and in the formulation of feed ration for various ruminant species that exhibit distinct reactions to the rumen fermentation process.
ACKNOWLEDGEMENTS
This research is supported by the Graduate Program Scholarship from the Graduate School of Kasetsart University.
Novelty Statement
From this research, it was revealed that the capability to utilize feed among swamp buffalo, beef cattle, and goats varies, with swamp buffalo demonstrating the highest efficiency in feed utilization. This can be attributed to the distinct functioning of cellulolytic bacteria present in the rumen, which differs from that of other ruminant species.
AUTHOR’S CONTRIBUTIONs
Nuttanun Leamkrajang: Conceptualization, methodology, data curation and formal analysis, writing original draft.
K. Teepalak Rangubhet and Somkiert Prasanpanich: Writing review and editing.
Phongthorn Kongmun: Project administration and resources, writing-review and editing, supervision.
All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors have declared no conflict of interest.
REFERENCES
Al-Galbi HAJ, Majeed MS (2022). Effect of Concentrate: Roughage Ratio and the Addition of Kefir on the Production Characteristics of Ruminant in vitro. Arch. Razi Inst., 77(1): 323-331.
Boonsaen P, Poonko S, Kanjanapruetipong J, Phiriyangkul P, Sawanon S (2019). Isolation and partial characterization of Ruminococcus flavefaciens from the rumen of swamp buffalo. Buffalo Bull., 38(2): 311-325.
Chanchay N, Poosaran N (2009). The reduction of mimosine and tannin contents in leaves of Leucaena leucocephala. Asian J. Food Agro-Ind., 2.
Chanthakhoun V, Wanapat M, Kongmun P, Cherdthong A (2012). Comparison of ruminal fermentation characteristics and microbial population in swamp buffalo and cattle. Livestock Sci.,143(2): 172-176. https://doi.org/10.1016/j.livsci.2011.09.009
Chen H, Wang C, Huasai S, Chen A (2021). Effects of dietary forage to concentrate ratio on nutrient digestibility, ruminal fermentation and rumen bacterial composition in Angus cows. Sci. Rep.,11(1): 17023. https://doi.org/10.1038/s41598-021-96580-5
Christopherson MR, Dawson JA, Stevenson DM, Cunningham AC, Bramhacharya S, Weimer PJ, Kendziorski C, Suen G (2014). Unique aspects of fiber degradation by the ruminal ethanologen Ruminococcus albus 7 revealed by physiological and transcriptomic analysis. BMC Genomics,; 15(1): 1066. https://doi.org/10.1186/1471-2164-15-1066
Clemmons BA, Shin SB, Smith TPL, Embree MM, Voy BH, Schneider LG, Donohoe DR, McLean KJ, Myer PR (2021). Ruminal Protozoal Populations of Angus Steers Differing in Feed Efficiency. Animals (Basel), 11(6). https://doi.org/10.3390/ani11061561
Conteville LC, da Silva JV, Andrade BGN, Cardoso TF, Bruscadin JJ, de Oliveira PSN, Mourão GB, Coutinho LL, Palhares JCP, Berndt A, de Medeiros SR, Regitano LCA (2023). Rumen and fecal microbiomes are related to diet and production traits in Bos indicus beef cattle. Frontiers in Microbiology, 14:1282851. https://doi.org/10.3389/fmicb.2023.1282851
Davis CL (1967). Acetate Production in the Rumen of Cows Fed Either Control or Low-Fiber, High-Grain Diets. J. Dairy Sci.,50(10): 1621-1625. https://doi.org/10.3168/jds.S0022-0302(67)87683-5
Denman SE, Tomkins NW, McSweeney CS (2007). Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol., 62(3): 313-322. https://doi.org/10.1111/j.1574-6941.2007.00394.x
Emilio M, Ungerfeld, Nathaly Cancino-Padilla, Vera-Aguilera, N (2023). Fermentation in the rumen. https://doi.org/10.1002/9781119850007.ch4
Faría-Mármol J, González J, Rodríguez CA, Alvir MR (2002).Effect of diet forage to concentrate ratio on rumen degradability and post-ruminal availability of protein from fresh and dried lucerne. Anim. Sci., 74: 337-345. https://doi.org/10.1017/S1357729800052498
Fernando SC, Purvis HT, Najar FZ, Sukharnikov LO, Krehbiel CR, Nagaraja TG, Roe BA, DeSilva U (2010). Rumen Microbial Population Dynamics during Adaptation to a High-Grain Diet. Appl. Environ. Microbiol.,76(22): 7482-7490. https://doi.org/10.1128/AEM.00388-10
Firkins JL, Karnati SKR, Yu Z (2008). Linking rumen function to animal response by application of metagenomics techniques. Aust. J. Exp. Agric., 48(7): 711-721. https://doi.org/10.1071/EA08028
Glendinning L, Genç B, Wallace RJ, Watson M (2020). Metagenomic analysis of the cow, sheep, reindeer and red deer rumen. Sci. Rep., 11. https://doi.org/10.1101/2020.02.12.945139
Hristov AN, Ivan M, Rode LM, McAllister TA (2001). Fermentation characteristics and ruminal ciliate protozoal populations in cattle fed medium- or high-concentrate barley-based diets2. J. Anim. Sci.,79(2): 515-524. https://doi.org/10.2527/2001.792515x
Ismartoyoa I, Islamiyatia R, Rusdya M (2024). Rumen Fermentation of Local Grasses Fed to Native Goat. Hasanuddin J. Anim. Sci., 5:28-35. https://doi.org/10.20956/hajas.v5i1.24777
Klieve AV, Ouwerkerk D, Turner AF, Roberton RF (2002). The production and storage of a fermentor-grown bacterial culture containing Synergistes jonesii, for protecting cattle against mimosine and 3-hydroxy-4(1H)-pyridone toxicity from feeding on Leucaena leucocephala. Crop and Pasture Sci., 53: 1-5. https://doi.org/10.1071/AR00121
Kobayashi Y (2006). Inclusion of novel bacteria in rumen microbiology: Need for basic and applied science. Anim. Sci. J., 77: 375-385. https://doi.org/10.1111/j.1740-0929.2006.00362.x
Koike S, Kobayashi Y (2001). Development and use of competitive PCR assays for the rumen cellulolytic bacteria: Fibrobacter succinogenes, Ruminococcus albus and Ruminococcus flavefaciens. FEMS Microbiol. Lett., 204(2):361-366. https://doi.org/10.1016/S0378-1097(01)00428-1
Leng RA (1993). Quantitative ruminant nutrition a green science. Crop and Pasture Science,; 44:363-380. https://doi.org/10.1071/AR9930363
Levy B, Jami E (2018). Exploring the Prokaryotic Community Associated With the Rumen Ciliate Protozoa Population. Front. Microbiol., 9: 2526. https://doi.org/10.3389/fmicb.2018.02526
Li J, Yan H, Chen J, Duan C, Guo Y, Liu Y, Zhang Y, Ji S (2022). Correlation of Ruminal Fermentation Parameters and Rumen Bacterial Community by Comparing Those of the Goat, Sheep, and Cow In Vitro. Fermentation, 8(9): 427. https://doi.org/10.3390/fermentation8090427
Lopes ASM, de Oliveira JS, Santos EM, Medeiros AN, Givisiez PEN, Lemos MLP, Santos FNS, Silva NMV, Azevedo PS, Sousa LS, Pereira DM, Oliveira CJB (2020). Goats fed with non-protein nitrogen: ruminal bacterial community and ruminal fermentation, intake, digestibility and nitrogen balance. The J. Agric. Sci.,158(8-9): 781-790. https://doi.org/10.1017/S0021859621000162
Makkar PSH, McSweeney CS (2005). Methods in Guts Microbial Ecology for Ruminants, Vii. IAEA. https://doi.org/10.1007/1-4020-3791-0
Mat Amin F, Mohd Azmi AF, Hakim Idris L, Abu Hassim H, Saad MZ, Abu Bakar MZ (2022). Rumen Volatile Fatty Acids and Morphology of the Rumen Mucosa of Swamp Buffalo Raised under Semi-Intensive and Extensive System in Tropical Environment. Pertanika J. Trop. Agric. Sci., https://doi.org/10.47836/pjtas.45.1.01
Mcsweeney C (1988). A comparative study of the anatomy of the omasum in domesticated ruminants. Aus. Vet. J., 65(7): 205-207. https://doi.org/10.1111/j.1751-0813.1988.tb14458.x
Menke HH, Steingass H (1988). Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev., 28: 7-55.
Moss AR, Jouany JP, Newbold J (2000). Methane production by ruminants: its contribution to global warming. Ann. Zootechnie, 49(3): 231-253. https://doi.org/10.1051/animres:2000119
Nagadi SA (2019). In vitro Gas Production, Methane Emission and Rumen Fermentation Characteristics with Increasing Roughage to Concentrate Ratios. J. King Abdulaziz Univ. Meteorol. Environ. Arid Land Agric. Sci.,
Orskov ER, McDonal I (1979). The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. The J. Agric. Sci., 92: 499-503. https://doi.org/10.1017/S0021859600063048
Ouwerkerk D, Klieve AV, Forster RJ (2002). Enumeration of Megasphaera elsdenii in rumen contents by real‐time Taq nuclease assay. J. Appl. Microbiol., 92(4): 753-758. https://doi.org/10.1046/j.1365-2672.2002.01580.x
Phesatcha K, Chunwijitra K, Phesatcha B, Wanapat M, Cherdthong A (2021). Addition of Active Dry Yeast Could Enhance Feed Intake and Rumen Bacterial Population While Reducing Protozoa and Methanogen Population in Beef Cattle. Fermentation, 7(3): 172. https://doi.org/10.3390/fermentation7030172
Phesatcha K, Phesatcha B, Wanapat M, Cherdthong A (2020). Roughage to Concentrate Ratio and Saccharomyces cerevisiae Inclusion Could Modulate Feed Digestion and In Vitro Ruminal Fermentation. Vet. Sci., 7(4): 151. https://doi.org/10.3390/vetsci7040151
Polyorach S, Wanapat M, Cherdthong A (2014). Influence of Yeast Fermented Cassava Chip Protein (YEFECAP) and Roughage to Concentrate Ratio on Ruminal Fermentation and Microorganisms Using In vitro Gas Production Technique. Asian-Aus. J. Anim. Sci., 27(1): 6-45. https://doi.org/10.5713/ajas.2013.13298
Ramanzin M, Bailoni L, Schiavon S (1997). Effect of forage to concentrate ratio on comparative digestion in sheep, goats and fallow deer. Anim. Sci., 64: 163-170. https://doi.org/10.1017/S1357729800015678
Rostini T, Abdullah L, Wiryawan KG, Karti PDMH (2014). Utilization of Swamp Forages from South Kalimantan on Local Goat Performances. Media Peternakan, 37(1). https://doi.org/10.5398/medpet.2014.37.1.50
Rostini T, Biyatmoko D, Zakir I, Hidayatullah A (2019). Physical and Chemical Quality Profile of Lamb Meat Which Was Swamp Buffalo’s Rumen Liquid Based Fodder-Fed. J. Agric. Sci., https://doi.org/10.5539/jas.v11n5p547
Rostini T, Zakir I, Biyatmoko D (2018). Different in Quantity of Microbial Rumen Fluid of River Buffalo and Swamp Bufallo, https://doi.org/10.2991/icase-18.2018.32
Samuel M, Sagatheman S, Thomas J, Mathen G (1997). An HPLC method for estimation of volatile fatty acids of ruminal fluid. Indian J. Anim. Res., 67.
Santra A, Pathak NN (2001). The effect of dietary concentrate level on rumen enzyme profile and ciliate protozoa population in cattle fed wheat straw diet. J. Anim. Feed Sci.,10(4): 589-604. https://doi.org/10.22358/jafs/68011/2001
Sauer M, Marx H, Mattanovich D (2012). From rumen to industry. Microb. Cell Factories, 11: 121-121. https://doi.org/10.1186/1475-2859-11-121
Smith SB, Blackmon TL, Sawyer JE, Miller RK, Baber JR, Morrill JC, Cabral AR, Wickersham TA (2018). Glucose and acetate metabolism in bovine intramuscular and subcutaneous adipose tissues from steers infused with glucose, propionate, or acetate. J. Anim. Sci., 96(3): 921-929. https://doi.org/10.1093/jas/sky017
Steel RGD, Torrie JH (1980). Principles and Procedures of Statistics: A Biometerial Approach, 2nd ed. McGraw-Hill.
Suriyapha C, Cherdthong Az Suntara C, Polyorach S (2021). Utilization of Yeast Waste Fermented Citric Waste as a Protein Source to Replace Soybean Meal and Various Roughage to Concentrate Ratios on In Vitro Rumen Fermentation, Gas Kinetic, and Feed Digestion. Fermentation, 7(3): 120. https://doi.org/10.3390/fermentation7030120
Tajima K, Nagamine T, Matsui H, Nakamura M, Aminov RI (2001). Phylogenetic analysis of archaeal 16S rRNA libraries from the rumen suggests the existence of a novel group of archaea not associated with known methanogens. FEMS Microbiol. Lett., 200(1):67-72. https://doi.org/10.1111/j.1574-6968.2001.tb10694.x
Thanh VTK (2014). Differences in protein nutrition in swamp buffaloes compared to yellow cattle. Buffalo Bull., 33: 362-369.
Van Soest P, Robertson JB (1985). A Laboratory Manual for Animal Science. Cornell University Press.
Van Soest PJ (1994). Nutritional Ecology of The Ruminant. Cornell University. https://doi.org/10.7591/9781501732355
Wanapat M, Gunun P, Anantasook N, Kang S (2014). Changes of rumen pH, fermentation and microbial population as influenced by different ratios of roughage (rice straw) to concentrate in dairy steers. The J. Agric. Sci., 152(4): 675-685. https://doi.org/10.1017/S0021859613000658
Wanapat M, Nontaso N, Yuangklang C, Wora-anu S, Ngarmsang A, Wachirapakorn C, Rowlinson P (2003). Comparative Study between Swamp Buffalo and Native Cattle in Feed Digestibility and Potential Transfer of Buffalo Rumen Digesta into Cattle. Asian-aus. J. Anim. Sci., 16: 504-510. https://doi.org/10.5713/ajas.2003.504
Wang R, He S, Huang D, He S, Guo T, Chen T, Peng H, Jiaka L, He M, Chen C, Song D, Huang X, Wu D, Mao H (2023). Differences in composition and diversity of rumen fungi in buffalo fed different diets. Anim. Biotechnol.,34(9): 5075-5086. https://doi.org/10.1080/10495398.2023.2276974
Xing BS, Cao S, Han Y, Wang XC, Wen J, Zhang K (2020). A comparative study of artificial cow and sheep rumen fermentation of corn straw and food waste: Batch and continuous operation. Science of The Total Environment,745: 140731. https://doi.org/10.1016/j.scitotenv.2020.140731
Xu Y, Feng T, Ding Z, Li L, Li Z, Cui K, Chen W, Pan H, Zhu P, Liu Q (2024). Age-related compositional and functional changes in the adult and breastfed buffalo rumen microbiome (Original Research). Front. Microbiol.,15. https://doi.org/10.3389/fmicb.2024.1342804
Yang Z, Liu S, Xie T, Wang Q, Wang Z, Yang H, Li S, Wang W (2022). Effect of Unsaturated Fatty Acid Ratio In Vitro on Rumen Fermentation, Methane Concentration, and Microbial Profile. Fermentation, 8(10): 540. https://doi.org/10.3390/fermentation8100540
Yu Z, Morrison M (2004). Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques, 36(5): 808-812. https://doi.org/10.2144/04365ST04
Zhang Y, Li F, Chen Y, Guan LL (2022). The Effects of Breed and Residual Feed Intake Divergence on the Abundance and Active Population of Rumen Microbiota in Beef Cattle. Animals, 12(15): 1966. https://doi.org/10.3390/ani12151966
Zhang YK, Zhang XX, Li FD, Li C, Li GZ, Zhang DY, Song Q, Li XL, Zhao Y, Wang WM (2021). Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal : Int. J. Anim. Biosci.,15 3: 100161. https://doi.org/10.1016/j.animal.2020.100161
Zurak D, Kljak K, I aladrovic J (2023). Metabolism and utilisation of non-protein nitrogen compounds in ruminants: a review. J. Cen. Eur. Agric., 24(1): 1-14. https://doi.org/10.5513/JCEA01/24.1.3645
To share on other social networks, click on any share button. What are these?






