The Effect of Organic Wheatgrass Fodders (Triticum aestivum) on Growth Performance, Meat Quality of Japanese Quail (Coturnix japonica)
The Effect of Organic Wheatgrass Fodders (Triticum aestivum) on Growth Performance, Meat Quality of Japanese Quail (Coturnix japonica)
Saranyah Sathiaganeshan1,2, Nur Aina Natasha Yusoff1, Siti Juzailah Zuraimi1, Rukayat Omolara Folarin1,3, Satya Narayana Rao Ramasamy1,4, Asmad Kari1, Enike Dwi Kusumawati⁵, I Wayan Karyasa⁶ and Connie Fay Komilus1*
1School of Animal Science, Aquatic Science, and Environmental, Faculty of Bioresources and Food Industry, University Sultan Zainal Abidin, Besut Campus, 22000, Besut, Terengganu, Malaysia; 2Department of Animal Science, Faculty of Agriculture, Universiti Putra Malaysia, 43000 Seri Kembangan, Selangor; 3Sultan Farm Subsidiary, Folajin Global Consult RC, 3228188 Molaagbo, Ikire, Osun State, Nigeria; 4Pet World Nutrition Sdn. Bhd, No 8, Persiaran Kemajuan, Seksyen 16, 40200, Shah Alam, Selangor, Malaysia; ⁵Departmrnt of Animal Husbandry, Universitas PGRI Kanjuruhan Malang, Jl. S. Supriadi No. 48, Malang 65148, East Java, Indonesia; ⁶Department of Chemistry, FMIPA Universitas Pendidikan Ganesha, Jalan Bisma Utara No. 75X Singaraja, Bali, 81117, Indonesia.
Abstract | A 30-day feeding trial on 45 quails aged 12 days was conducted to evaluate the effects of organic wheatgrass (Triticum aestivum) fodders on growth performance and meat quality of Japanese quail (Coturnix japonica). This research aims to determine nutrient compositions in organic wheatgrass fodder (OWF) and to evaluate the effect of organic wheatgrass on the growth performance and meat quality of quail. Wheatgrass fodder was grown using organic fertilizer which is goat dung. Then, proximate analysis was done on OWF and commercial feed. Five dietary treatments consisting different percentages (%) such as Control (0% OWF+ 100% Commercial Feed), Treatment 1 (5% OWF+ 95% Commercial Feed), Treatment 2 (10% OWF+ 90% Commercial Feed), Treatment 3 (15% OWF+ 85% Commercial Feed), Treatment 4 (20% OWF+ 80% Commercial Feed) were given for feeding experiment in triplicates. Body Weight Gain (BWG), Average Daily Weight Gain (ADWG), Daily Feed Intake (DFI), Feed Conversion Ratio (FCR), survival rates were recorded and calculated. 15 quails aged 42 days were slaughtered on day-30 for carcass yield, pH, color, WHC, shear force and proximate analysis were conducted. Results showed that nutrient composition of OWF for crude protein (CP), crude fibre (CF), Crude Lipid (CL), moisture, nitrogen-free extract (NFE), ash content were 26.80%, 7.09%, 2.49%, 9.89%, 45.35%, 4.30% respectively. In conclusion, Treatment 3 (15% OWF+ 85% Commercial Feed) showed the overall best performance among quails.
Received | February 22, 2024; Accepted | August 30, 2024; Published | November 01, 2024
*Correspondence | Connie Fay Komilus, School of Animal Science, Aquatic Science, and Environmental, Faculty of Bioresources and Food Industry, University Sultan Zainal Abidin, Besut Campus, 22000, Besut, Terengganu, Malaysia; Email: [email protected]
Citation | Sathiaganeshan, S., N.A.N. Yusoff, S.J. Zuraimi, R.O. Folarin, S.N.R. Ramasamy, A. Kari, E.D. Kusumawati, I.W. Karyasa and C.F. Komilus. 2024. The effect of organic wheatgrass fodders (Triticum aestivum) on growth performance, meat quality of Japanese quail (Coturnix japonica). Sarhad Journal of Agriculture, 40(Special issue 1): 186-194.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40/s1.186.194
Keywords | Wheatgrass, Quail, Organic fertiliser, Growth performance, Meat quality, Animal
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Wheatgrass (Triticum aestivum) is a good option for cultivated crops as food and fodder for humans and livestock. It is a tender, small plant grown from wheat seed. It has antioxidant molecules and acts as an antimicrobial agent. Its increased growth rate, the number of harvests taken from a single crop input, and its nutritive quality made wheatgrass worthwhile (Linsha et al., 2018). Quails are small, ground-nesting birds. Depending on the species, they can reach up to 5–10 ounces in weight and 6–12 inches in length. Quail serves as protein source and achieves the self-sufficiency level of white meat in Malaysia. The Japanese quail (Coturnix japonica) was domesticated for its eggs (layer) and meat (broiler) (Mat et al., 2021). The taste and texture of it have led to increasing demand for animal and food output. Quails are fed twice daily and consume varieties like seeds, berries, insects, and leaves. Young quail mainly feed on insects and shaft to grains as they become adults (Mohd, 2018).
About 70–80% of the total production cost is for feed costs for quail because of insufficient, expensive sources of protein and high expenses for importation (Komilus et al., 2021). Besides, the quail feed is expensive, which burdens small-scale farmers as their profit will be low (Rahman et al., 2022). Therefore, farmers need to find cheaper, better-nutritive-value, and more sustainable sources of quail feed. The price of fodder seeds ranges from RM6–15 per kg depending on species, and 1kg will yield up to 6 kg of fodder (RM1.00–RM2.50 per kg), while the commercial feed is approximately RM136 for 50 kg (RM2.72 per kg). Other than that, it is challenging to own land and maintain it. Among the different urban agricultural systems, the evolution brings the method of tray cultivation, which is widely chosen now (Shamshiri et al., 2019). However, Malaysia has yet to explore fodder cultivation on a large scale, although the Agriculture Department has tried to cultivate fodder at the experimental stage (Taffesse and Tsakok, 2019).
Materials and Methods
House preparation for quails
Cage setup was done and saw dust was used as bedding. On the day of collecting the chicks, quails were kept in boxes with holes for breathing 45 tails, unsexed 4-days old quails, weighing an average of 29.8g were purchased from a commercial hatchery. Throughout the brooding process (4 to 11-day old), lightbulbs were used to provide constant lighting and heat for chicks. After brooding, for 30 days, quails were randomly placed according to treatment groups with 3 replicate cages each and 3 birds per cage in a safe, enclosed rearing facility.
Germination of wheatgrass fodder
Wheatgrass fodders were grown using tray cultivation method (Linsha et al., 2018). The sprouted seeds of wheatgrass fodder were placed in the trays uniformly. Every day, 4 trays were sown with the rate of 120g/tray. Irrigation was performed manually by filling water underneath the tray where the roots will absorb it. Fertilizer was diluted in ratio of 100g goat dung: 300ml of water. Goat dung was mixed in the water, ratio of 150ml of goat dung: 600ml of water.
Experimental diets
Five treatments were formulated with some modifications to meet all nutritional requirements of a starter diet for quails. The feed will be supplied ad libitum while clean and fresh water will be provided at free access.
Diets will be prepared based on 5 different treatments consisting of:
Control = 0% Organic Wheatgrass fodder (OWF)+ 100% Commercial feed
Treatment 1 = 5% OWF+ 95% Commercial feed
Treatment 2 = 10% OWF+ 90% Commercial feed
Treatment 3 = 15% OWF+ 85% Commercial feed
Treatment 4 = 20% OWF+ 80% Commercial feed
Feeding trial
The feeding trial started after growing wheatgrass fodder for 7 days and fed the one harvested on the eighth day. The quails were fed experimental diets based on 5% of the bird’s body weight. The quails were under feeding trials for 30 days. The experimental diet was given twice daily at 8-9 am and 5-6 pm.
Data collection on parameters for quail growth performance
Data were collected on initial to final weight, amount of feed given and the leftover weekly (Komilus et al., 2021).
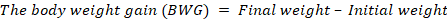
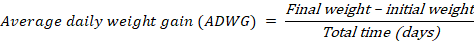
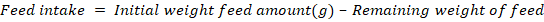
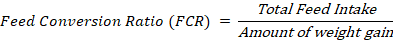
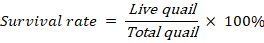
Data collection on parameters for meat quality
Three quails from each treatment were chosen for slaughter when the feeding trial had reached 30 days, according to the Malaysian Protocol for Halal Meat and Poultry Production MS 1500: 2009. Quails were fasted for four hours. After exsanguination, quails were allowed to bleed out for 2 minutes. Quails were plunged into hot water (50 – 55 °C) in the scalding tank and de-feathered (Komilus et al., 2021).
Carcass yield: Post-mortem was done on the slaughtered quails. All parts and organs such as carcass, wing, breast, drumstick, thigh, feet, head, neck, heart, crop, spleen, gizzard, empty gizzard, liver, diaphragm, small intestine, large intestine, duodenum are separated, weighed and recorded.
pH: Breast muscle’s pH were evaluated at 45 minutes and 24 hours after death using a digital pH meter (pH 45min and pH 24h) according to (Ilavarasan et al., 2016).
Colour: Three replications of the meat colour of breast muscle were determined using a chromameter (Konica Minolta CR-400 Chromameter). Colour values were presented as L* (lightness), a* (redness), and b* (yellowness) based on the CIE-LAB system at 45 minutes and 24 hours (Purnama et al., 2021).
Water holding capacity (WHC): WHC values were measured in triplicate, followed by 24-hour post-mortem period as described by (Komilus et al., 2021; Ilavarasan et al., 2016). Quail breast muscles were cut into 5 g cubes and placed between two Whatman No. 1 filter papers and two glass plates for 10 minutes with a 5 kg weight on top of the glass plate.
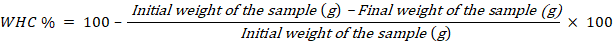
Cooking loss: At 24 h post-mortem, approximately 10 g of breast muscles were kept in plastic bags, cooked in a water bath at 75°C for 20 min and cooled at room temperature (Komilus et al., 2021).
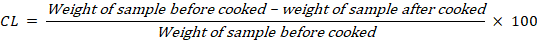
Table 1: Observation of wheatgrass growth.
|
Sprouting time |
Observation |
Description |
|
24 hours |
Wheat seeds was sown |
|
|
48 hours |
Wheat seed starts to germinate |
|
|
72 hours |
More wheat seeds start to germinate |
|
|
96 hours |
The leaves and roots are more visible |
|
|
120 hours |
All seeds were germinated uniformly |
|
|
144 hours |
The leaves start getting darker green color |
|
|
168 hours |
The leaves continue to grow taller |
|
|
192 hours |
The wheatgrass has reached its maximum height and is ready for harvest |
Shear force: According to minor modifications, shear force analysis was carried out by Omar et al. (2018). Cooked breast muscle was cut into two adjacent muscular strips measuring 1 cm in width and 3 cm in length in the direction of muscle fibre before being sheared once. Muscle strips were put in a texture analyser with the fibres directions perpendicular to the Warner Bratzler blade and a downstroke distance of 20.0 mm to determine shear force.
Proximate composition of quail’s breast meat: Breast meat samples were kept in the freezer before being transferred into the containers of a freeze dryer and freeze-dried for 24 hours. Samples were then ground into powder form. Crude protein, crude lipid, ash and moisture contents were measured according to (AOAC, 2006).
Statistical analysis
Data was evaluated using one way analysis (ANOVA) to evaluate the significance differences (p < 0.05) among treatment groups in this study via SPSS version 27.0 (Komilus et al., 2021). Tests were done on post hoc, Tukey, Games-Howell and homogeneity of variance tests with a 95% confidence interval.
Results and Discussion
Observation of wheatgrass growth
Proximate composition in wheatgrass
Wheatgrass fodders were grown using tray cultivation method and fertilized with goat dung with ratio of 150ml of goat dung: 600ml of water.
Growth parameters of quails
The BWG results revealed significantly (P≤0.05) higher body weight gain in T3 (158.70 g) group compared to the control (77.40 g), T1 (98.65 g), T2 (101.75 g), T4 (115.48 g) group. ADWG was highest in T3 (31.74 g/d), while others were significantly different and lower. The control showed lower weight gain than other treatments supplemented with OWF. The weight gain was mainly caused by increasing protein level in the diet with the inclusion of wheatgrass. T4 (20% OWF) can be considered as the level beyond the optimum amount, which is T3 (15% OWF). The result obtained is further supported by the findings of Ekbal et al. (2021), where the final gained body weight was increased in treated groups of rats.; Barbacariu et al. (2021), wherein common carp fed with 2% wheatgrass juice, final weight increased by up to 39% and body length increased by up to 7%. Other than that, Asaduzzaman et al. (2017) found that hydroponic wheatgrass has positive effects at up to 15% of OWF but showed negative effects at 20% of OWF on weight gain of turkeys; also found that wheatgrass can improve the performance of animals by up to 8%.
Table 2: Proximate composition in wheatgrass.
|
Nutrients |
% in wheatgrass |
|
CP |
26.80 ± 0.99 |
|
Lipid |
2.49 ± 0.42 |
|
CF |
7.09 ± 0.30 |
|
Moisture |
9.89 ± 0.08 |
|
Ash |
4.30 ± 0.19 |
|
NFE |
45.35 ± 0.64 |
Proximate composition in commercial feed
Table 3: Proximate composition in commercial feed.
|
Nutrients |
% in commercial feed |
|
CP |
21.0 |
|
Lipid |
4.5 |
|
CF |
5.0 |
|
Moisture |
13.0 |
|
Ash |
8.0 |
|
NFE |
61.5 |
|
Calcium |
0.8 |
|
Phosphorus |
0.4 |
Table 4: Growth parameters of quails.
|
Parameter |
Control |
T1 |
T2 |
T3 |
T4 |
p-value |
|
BWG (g) |
77.40ᵃ±4.49 |
98.65ᵇ±2.94 |
101.75ᵇ±0.93 |
158.70ᵈ±5.76 |
115.48ᶜ±2.39 |
<0.001 |
|
ADWG (g/d) |
15.48ᵃ±0.90 |
29.78ᵇ±1.77 |
20.35ᵇ±0.19 |
31.74ᵈ±1.16 |
23.09ᶜ±0.48 |
0.151 |
|
DFI (g) |
9.59ᵇ±0.20 |
9.84ᶜ±0 |
9.84ᶜ±0 |
9.10ᶜ±0.02 |
9.84ᶜ±0 |
<0.001 |
|
FCR |
3.19ᵈ |
2.53ᶜ |
2.44ᶜ |
1.50ᵃ |
2.16ᵇ |
<0.001 |
|
Survival rate |
100% |
100% |
100% |
100% |
66.67% |
- |
Control=100% commercial feed, T1=5% OWF+ 95% Commercial feed, T2=10% OWF+ 90% Commercial feed, T3=15% OWF+ 85% Commercial feed, T4=20% OWF+ 80% Commercial feed. The presence of subsets on the same row indicates a significant difference between the diets (p < 0.05).
Daily feed intake was highest in T1, T2, and T4 and lowest in birds that consumed 15% of OWF. Lower FI is preferred as it can save feed costs and have a good impact with a minimal amount of feed, like in T3. As per (Ekbal et al., 2021), a significant decrease in feed intake was also observed in rats who consumed wheatgrass.
FCR is a useful measure for evaluating feeds since it calculates the quantity of feed required per unit of somatic growth (Rana et al., 2020). Lower FCR is preferable in animal diets, where less feed intake provides a higher impact on body weight (Komilus et al., 2021). In this study, the lowest FCR was 1.50 found in T3, which was significantly lower (P < 0.05) than the highest FCR in control (3.19). The presence of diverse bioactive components in OWF that may have a role in increased nutrition utilisation in supplemented birds could explain the higher body weight and reduced FCR in this study. The better FCR can be attributed to improved nutrient absorption and digestibility from gut. Salam et al. (2020) mentioned that adding OWF is cost-effective for feed formulation. The photogenic property may lead to the reduction of FCR, as demonstrated by, Mocanu et al. (2019), who reduced FCR from 1.6 -1.2% by utilising plant extracts like garlic and sea buckthorn in a 4% proportion.
The survival rate of all the groups was recorded as 100% except for T4 (66.67%). The mortality percentage observed in this investigation is not normal, as one quail from each replicate dies due to high percentages of OWF. The percent values of survival rate indicate that OWF beyond optimum level (>15%) affects perniciously on health of Japanese quail.
Carcass yield
Japanese quails didn’t produce much butchery yield. In this study, the crop had a significant difference (P<0.05) where T2 was markedly highest, followed by T1, T3, and T4 and control was the lowest. Without significant difference, carcass yield was slightly higher with the inclusion of OWF for wing, neck, heart, and small intestine. Overall, there’s no significant difference between treatments for carcass yield.
Table 5: Carcass yield of Japanese quails after bleeding.
|
|
Control |
T1 |
T2 |
T3 |
T4 |
p-value |
|
203.5±4.70 |
173.57±3.98 |
170.32±1.32 |
197.33±7.95 |
190.53±3.02 |
0.204 |
|
|
Carcass |
180.70±3.35 |
163.73±4.16 |
153.62±9.81 |
173.65±1.61 |
177.19±4.00 |
0.363 |
|
Wing |
18.03±0.40 |
20.48±1.27 |
22.92±5.77 |
20.72±4.12 |
23.34±5.11 |
0.512 |
|
Breast |
75.02±14.30 |
69.76±6.07 |
79.53±17.82 |
83.02±9.78 |
86.19±2.73 |
0.471 |
|
Drumstick |
20.38±2.27 |
19.30±2.85 |
19.57±3.54 |
19.89±1.11 |
21.20±1.22 |
0.757 |
|
Thigh |
30.14±6.40 |
29.62±3.00 |
26.71±5.62 |
31.03±3.72 |
34.34±2.35 |
0.400 |
|
Feet |
6.98±0.10 |
6.60±0.04 |
6.84±0.25 |
6.52±1.14 |
7.50±0.39 |
0.282 |
|
Head |
14.14±1.96 |
12.69±0.47 |
15.24±4.03 |
13.35±0.62 |
16.61±1.71 |
0.264 |
|
Neck |
8.04±2.25 |
8.62±1.39 |
9.15±1.96 |
8.61±0.81 |
8.29±2.32 |
0.957 |
|
Heart |
2.16±0.21 |
2.67±0.59 |
2.98±0.58 |
2.87±0.19 |
2.90±0.16 |
0.157 |
|
Crop |
1.17ᵃ±0.03 |
1.25ᵃᵇ±0.05 |
1.78ᵇ±0.40 |
1.42ᵃᵇ±0.08 |
1.50ᵃᵇ±0.28 |
0.049 |
|
Spleen |
0.28±0.09 |
0.20±0.09 |
0.35±0.25 |
0.24±0.08 |
0.30±0.03 |
0.705 |
|
Gizzard |
5.93±0.28 |
5.08±0.85 |
5.74±0.64 |
7.14±1.50 |
6.89±1.16 |
0.134 |
|
Empty Gizzard |
5.40±0.12 |
4.91±0.91 |
5.41±0.64 |
6.39±1.25 |
6.14±1.50 |
0.421 |
|
Liver |
7.22±1.15 |
6.11±1.05 |
8.57±1.68 |
7.78±0.83 |
9.21±1.31 |
0.082 |
|
Diaphragm |
18.08±6.18 |
17.35±3.68 |
22.05±4.05 |
17.19±4.55 |
16.11±4.15 |
0.585 |
|
Small intestine |
4.22±0.84 |
4.21±0.83 |
4.92±2.67 |
4.33±1.02 |
4.96±1.25 |
0.935 |
|
Large intestine |
4.40±0.67 |
3.84±0.98 |
4.59±1.28 |
4.33±0.87 |
3.60±0.77 |
0.681 |
|
Duodenum |
4.81±0.84 |
3.85±0.49 |
5.76±1.25 |
3.22±1.56 |
5.05±1.81 |
0.196 |
Control=100% commercial feed, T1=5% OWF+ 95% Commercial feed, T2=10% OWF+ 90% Commercial feed, T3=15% OWF+ 85% Commercial feed, T4=20% OWF+ 80% Commercial feed. The presence of subsets on the same row indicates a significant difference between the diets (p<0.05).
|
|
Control |
T1 |
T2 |
T3 |
T4 |
p-value |
|
pH 45 min |
6.86±1.57 |
6.46±0.39 |
6.52±0.16 |
6.82±0.31 |
7.93±0.19 |
0.186 |
|
pH 24 hr |
6.62±0.29 |
6.33±0.14 |
6.52±0.37 |
6.91±0.14 |
6.61±0.34 |
0.213 |
Control=100% commercial feed, T1=5% OWF+ 95% Commercial feed, T2=10% OWF+ 90% Commercial feed, T3=15% OWF+ 85% Commercial feed, T4=20% OWF+ 80% Commercial feed.
Table 7: Colour of Japanese quails.
|
|
Control |
T1 |
T2 |
T3 |
T4 |
p-value |
|
L* 45min |
51.82ᵇ±3.22 |
54.26ᵇ±3.19 |
52.39ᵇ±2.17 |
48.08ᵃᵇ±0.87 |
44.77ᵃ±1.25 |
0.004 |
|
a* 45min |
12.00±.5.00 |
9.53±4.50 |
11.60±5.43 |
10.45±1.97 |
8.35±1.47 |
0.793 |
|
b* 45min |
7.64±0.98 |
8.06±1.74 |
9.26±1.42 |
7.96±2.86 |
6.09±0.30 |
0.314 |
|
L* 24hr |
54.56±6.51 |
54.58±1.65 |
52.37±4.73 |
49.90±3.17 |
46.92±5.70 |
0.284 |
|
a* 24hr |
12.04±5.06 |
12.44±4.06 |
13.62±6.83 |
10.87±2.09 |
13.59±3.42 |
0.938 |
|
b* 24hr |
9.03±2.93 |
12.49±0.89 |
10.55±2.72 |
7.33±3.61 |
9.26±3.95 |
0.363 |
Control=100% commercial feed, T1= 5% OWF+ 95% Commercial feed, T2=10% OWF+ 90% Commercial feed, T3=15% OWF+ 85% Commercial feed, T4=20% OWF+ 80% Commercial feed. The presence of subsets on the same row indicates a significant difference between the diets (p < 0.05).
pH
In this study, breast meat of quail showed no significant differences (p < 0.05) among control and all treatments in pH45min and pH24h values. Age, sex, husbandry practices, feed additives, pre-slaughtering stress, muscle conformation, and glycogen content are all factors that influence pH levels. As for the chicken meat, pH drops 7 to 5.5 after death due to accumulation of lactic acid in the muscle (Warner, 2017). A few studies examined the correlation between the nutritional value and meat quality features of Nandanam Quail-III that were slaughtered at different ages. The findings were pH value of 6.65 for 5-week old quail as per Ilavarasan et al. (2016) and 5.94 according to (Komilus et al., 2021). Unlike turkey and chicken, Japanese quails have dark muscle fibers that are made up of oxidative type. This type of muscle is known to contribute to higher pH values in their meat (El-Tahawy, 2022). In this study, pH value after 24 hours post-mortem falls within 6.33 to 6.91 (good pH for quail).
However, pH 45min value for all treatments was in the normal range except T4, which was higher (7.93). According to Boubekeur et al. (2021), higher pH values could indicate reduced glycogen levels in quail birds before slaughter. Excessive starvation or malnutrition has been linked with increased pH levels in animals after death. This study found no evidence of low pH. Low pH values (<5.4) in poultry meat have been related to decreased water-holding ability, which leads to greater thawing and cooking losses (Masenya et al., 2021). In addition, low pH influences meat storability.
The pH at 24hr was better than 45min. The variations in pH for 45 min and 24 hrs could potentially have been caused by the way the quail muscles were stored. According to Damaziak et al. (2016), who noted a favorable influence of cold storage duration on pH value in chicken meat quality, where an extended storage period within 24 hrs after slaughter may cause the pH to fall within the normal range.
Colour
L* indicates lightness (0=black, 100=diffuse white). a* indicates redness (negative values=green, whereas positive values=red). b* indicates yellowness (negative values=blue, positive values=yellow) (Zhuang and Savage, 2013). Lightness is an indicator of meat freshness and has a direct impact on consumers’ final buying decisions (Masenya et al., 2021).
The results of colour for the breast meat of quail showed there were significant differences (p<0.05) in L*45min only. In broiler chickens, the standard L* value falls between 46 and 53. Meat with below than 46 is Dark Firm Dry (DFD), which has a high water holding capacity and short shelf life. For L* 45min, control, T2, T3, and T4 were in the ideal range showing the best lightness on the meat except T1 (54.26).
Results for L* 24hr were in the range for T2, T3, and T4, while control (54.56) and T1 (54.58) values indicate PSE (Pale Soft Exudative). PSE meat is defined as meat having a lightness value greater than 53 (Purnama et al., 2021). Protein denaturation in muscle and post-mortem pH has an impact on PSE meat. No DFD meat was observed. The results also have a pattern of decreasing lightness as the percentages of OWF increase but still in range. No significant difference in a* 45 min, b* 45 min, L* 24 hr, a* 24 hr, b* 24 hr. The redox reactions of myoglobin, haemoglobin, and heme pigment determine the redness (a*) of meat (Calnan et al., 2016). Genetics, carotenoid pigments in feed, liver biochemistry and meat preparation influence the yellowness (b*) of meat (Purnama et al., 2021).
Water holding capacity (WHC), cooking loss and shear force
The result of water holding capacity for breast meat of quail showed significant different (p>0.05) among control and treated quails. When the muscle’s pH falls after death, some of the muscle’s capacity to store water is lost (Warner, 2017). The best WHC was in T3.
The result for breast meat of quail showed that there was no significant difference among all treatments in cooking loss value, but T2 showed the highest percentage of cooking loss, and T3 was the lowest. The moisture, juiciness in meat can be retained even after cooking in all treatments.
Shear force didn’t differ statistically among treatments. Shear force (toughness) was the force needed to cut off the meat sample. Lower values indicated the most tender meat (Bowker and Zhuang, 2019). The shear force range of all treatments falls within 2.15 to 3.15. T3 recorded the least value, 2.15, indicating it as the most tender meat among treatments. This result can be supported by the findings in cooking loss.
Proximate composition of breast meat
Proximate analysis of the breast meat of quail showed that there were significant differences among treatments in crude protein, ether extract, ash and moisture contents. For crude protein, control has the lowest value and treatments with the inclusion of OWF have a higher value. The findings didn’t exhibit any trend with the inclusion of wheatgrass and similar to findings of (Barbacariu et al., 2021; Islam et al., 2017), where the result did not have a trend with the inclusion of OWF but higher than control. For lipids, the value drops as the level of OWF increases. This result was parallel to a previous study (Barbacariu et al., 2021; Rana et al., 2020; Islam et al., 2017), where lipid content dropped in carp as the level of OWF increased. For ash, control has the highest value, followed by T1, T3, T4, and T2, showing decreasing pattern with the inclusion of OWF. For moisture, the result showed T3 significantly has the highest moisture and value scattered among control and treatment, which shows the inclusion of wheatgrass didn’t affect much moisture, similar to Islam et al. (2017) result on stinging catfish fry fed on OWF.
Table 8: Water holding capacity (WHC), cooking loss and shear force of Japanese quails.
|
|
Control |
T1 |
T2 |
T3 |
T4 |
p-value |
|
WHC |
77.74ᵃᵇ±2.77 |
77.13ᵃ±7.09 |
83.73ᵃᵇ±1.97 |
88.48ᵇ±4.58 |
80.08ᵃᵇ±2.35 |
0.040 |
|
CL |
25.72±4.43 |
27.57±2.88 |
27.96±2.93 |
23.52±1.03 |
24.28±2.52 |
0.023 |
|
SF |
2.80±0.58 |
3.15±1.00 |
2.64±0.91 |
2.15±0.18 |
2.46±0.39 |
0.507 |
Control=100% commercial feed, T1=5% OWF+ 95% Commercial feed, T2=10% OWF+ 90% Commercial feed, T3=15% OWF+ 85% Commercial feed, T4=20% OWF+ 80% Commercial feed. The presence of subsets on the same row indicates a significant difference between the diets (p<0.05).
Table 9: Proximate composition of quails’ breast meat.
|
|
Control |
T1 |
T2 |
T3 |
T4 |
p-value |
|
CP |
57.09±0.47ᵃ |
61.12±1.58ᵇ |
62.26±0.06ᵇ |
59.64±0.17ᵃᵇ |
60.15±0.21ᵇ |
<0.001 |
|
Lipid |
4.08±0.05ᶜ |
4.00±0.07ᶜ |
3.45±0.07ᵇ |
3.18±0.04ᵃ |
2.99±0.01ᵃ |
<0.001 |
|
Ash |
5.21±0.04ᶜ |
4.92±0.12ᵇ |
4.57±0.02ᵃ |
4.68±0.02ᵃ |
4.59±0.01ᵃ |
<0.001 |
|
Moisture |
71.48±0.01ᵇ |
71.95±0.01ᵈ |
70.26±0.01ᵃ |
72.33±0.02ᵉ |
71.79±0.01ᶜ |
<0.001 |
Control=100% commercial feed, T1=5% OWF+ 95% Commercial feed, T2=10% OWF+ 90% Commercial feed, T3=15% OWF+ 85% Commercial feed, T4=20% OWF+ 80% Commercial feed. The presence of subsets on the same row indicates a significant difference between the diets (p<0.05).
Conclusions and Recommendations
In conclusion, Treatment 3 (15% OWF+ 85% Commercial Feed) showed overall best performance among treatments. The study suggests that inclusion of OWF up to 15% dietary levels can be a growth and performance enhancer for Japanese quail without detrimental impact. As a recommendation, the wheatgrass seed can be treated first during soaking process to avoid fungus and reduce risk of diseases. For organic production, apple cider vinegar and for chemical, sodium chloride or hydrogen peroxide can be used.
Acknowledgements
Acknowledgements to the top management of the Faculty of Bioresources and Food Industry for granting permission to conduct the research. The authors are also thankful to the faculty staff who assisted the team during the research.
Novelty Statement
The study presents wheatgrass as the potential feed substitute that can reduce farmers feed expenses and dependability on commercial feeds for Japanese Quail (Coturnix japonica).
Author’s Contribution
All authors contributed to the conceptualisation, methodology, data collection and analysis, interpretation of results and writing of the original draft.
Ethical approval
The study was conducted at Lab Agrostology and poultry house, Universiti Sultan Zainal Abidin (UniSZA) Kampus Besut, Tembila, 22200, Besut, Terengganu. Application for ethical approval was applied to Universiti Sultan Zainal Abidin Animal and Plant Research Ethics Committee (UAPREC) under UAPREC/07/013 before the feeding trial commences.
Conflicts of interest
The authors have declared no conflict of interest.
References
AOAC, 2006. Official methods of analysis of the association of official analytical chemists (AOAC). Kenneth, H. (Editor), volume 1(15th edition), 69–88. https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf
Asaduzzaman, M., U. Salma, H.S. Ali, M.A. Hamid, A.G. Miah and S.H. Sabuz. 2017. Dietary effects of hydroponic wheat sprouted fodder on growth performance of Turkey. Res. Agric. Livest. Fish., 4(1): 101–110.
Bakshi, Wadhwa, M., P.S. Harinder and Makkar. 2017. Hydroponic fodder production: A critical assessment. December, 1–10. https://doi.org/10.1079/PAVSNNR201712006
Barbacariu, C.A., M. Burducea, L. Dîrvariu, E. Oprea, A.C. Lupu, G.C. Teliban, A.L. Agapie, V. Stoleru and A. Lobiuc. 2021. Evaluation of diet supplementation with wheat grass juice on growth performance, body composition and blood biochemical profile of carp (Cyprinus carpio L). Animals, 11(9). https://doi.org/10.3390/ani11092589
Boubekeur, F., R. Arbouche, Y. Arbouche and F. Arbouche. 2021. By-products of apricot processing in quail feed: Effects on growth performance, carcass characteristics, and meat physicochemical quality. Vet. World, 14(4): 878–883. https://doi.org/10.14202/vetworld.2021.878-883
Bowker, B. and H. Zhuang. 2019. Detection of razor shear force differences in broiler breast meat due to the woody breast condition depends on measurement technique and meat state. Poult. Sci., 98(11): 6170–6176. https://doi.org/10.3382/ps/pez334
Calnan, H., R.H. Jacob, D.W. Pethick and G.E. Gardner. 2016. Production factors influence fresh lamb longissimus colour more than muscle traits such as myoglobin concentration and pH. Meat Sci., 119: 41–50. https://doi.org/10.1016/j.meatsci.2016.04.009
Damaziak, K., A. Stelmasiak, M. Michalczuk, J. Wyrwisz, M. Moczkowska, M.M. Marcinkowska-Lesiak, J. Niemiec and A. Wierzbicka. 2016. Analysis of storage possibility of raw and smoked breast meat of oat-fattened White Kołuda® goose based on their quality characteristics. Poult. Sci., 95(9): 2186–2197. https://doi.org/10.3382/ps/pew138
Ekbal, M.S., G. El-Sayeda and S.E. Rahma. 2021. A study on the effect of wheatgrass on nutritional status and some blood parameters in rats with suppressed immune system. Angewandte Chemie Int. Ed., 6(11): 951–952.
El-Tahawy, W.A., 2022. Quail meat characteristics: Chemical constituent and quality. Asia Afr. J. Agric., 1: 95–113.
Ilavarasan, R., R.J. Abraham and V.A. Rao. 2016. The relationship between meat quality characteristics and nutritional composition of nandanam quail-III slaughtered at different ages. J. Anim. Res., 6(2): 275. https://doi.org/10.5958/2277-940X.2015.00174.6
Islam, T., K.M.S. Rana and M.A. Salam. 2017. Potential of wheatgrass powder based feed for stinging catfish fry nursing in laboratory condition. Int. J. Fish. Aquat. Stud., 5(6): 179-184.
Komilus, C.F., N.F. Mokhtar, N.A. Rosli and L.A. Oluodo. 2021. Efficacy of detoxified rubber seed meal (Hevea brasiliensis) on growth performance, meat quality and carcass of Japanese quail (Coturnix japonica). Biosci. Res., 18(SI-2): 272–283.
Linsha, C., R. George and S. Sreeduth. 2018. Development and evaluation of a tray type protected cultivation system for fodder crops. Fac. Agric. Eng. Technol. Kerala Agric. Univ., pp. 4-19.
Masenya, T.I., V. Mlambo and C.M. Mnisi. 2021. Complete replacement of maize grain with sorghum and pearl millet grains in Jumbo quail diets: Feed intake, physiological parameters, and meat quality traits. PLoS One, 16(3 March): 8–15. https://doi.org/10.1371/journal.pone.0249371
Mat, K., N.A.S. Mohamad, N.D. Rusli, M.M. Rahman, H.C. Harun, S.M. Al-Amsyar and M. Mahmud. 2021. Preliminary study on the effect of feeding black soldier fly larvae (BSFL) on growth and laying performance of Japanese quail (Cortunix japonica). 17: 977–986.
Mocanu, E., L. Athanasopoulos, N. Patriche, M. Tenciu and E. Jecu. 2019. Effect of phyto-addictives diets on growth parameters and biochemical composition of carp species (Cyprinus carpio) in recirculating system.
Mohd, A.A.S., 2018. The occurrence of coccidiosis and OPG (oocyst per gram of faeces) in Japanese quail (Coturnix japonica) under two different housing systems.
Omar, N., A.S. Kamarudin and N. Huda. 2018. Effect of probiotics (EM-1) addition on quality characteristics of quail meat effect of probiotics (EM-1) addition on quality characteristics of quail meat.
Purnama, M.T.E., E.P. Ernanda, F. Fikri, A. Purnomo, S. Khairani and S. Chhetri. 2021. Effects of dietary supplementation with breadfruit leaf powder on growth performance, meat quality, and antioxidative activity in japanese quail. Vet. World, 14(7): 1946–1953. https://doi.org/10.14202/vetworld.2021.1946-1953
Rahman, S.U., Z. Ullah, A. Ali, M. Ahmad, H. Sher, Z.K. Shinwari and A. Nazir. 2022. Ethnoecological knowledge of wild fodder plant resources of district Buner Pakistan. Pak. J. Bot., 54(2): 645–652. https://doi.org/10.30848/PJB2022-2(27)
Rana, K.S., M. Salam, M.R. Ahmmed and A.M. Noor. 2020. Dietary supplementation of wheatgrass powder to assess somatic response of juvenile grass carp (Ctenopharyngodon idella). Asian J. Med. Biol. Res., 6(3): 482–490. https://doi.org/10.3329/ajmbr.v6i3.49797
Salam, M.A., K.S. Rana, M.R. Ahmmed and A.M. Noor. 2020. Growth response of Juvenile Rohu (Labeo rohita) to wheatgrass powder supplemented diet. Res. Agric. Livest. Fish., 7(3): 533–543. https://doi.org/10.3329/ralf.v7i3.51372
Shamshiri, R.R., B. Ibrahim, S.K. Balasundram, S. Taheri and C. Weltzien. 2019. Evaluating system of rice intensification using a modified transplanter: A smart farming solution toward sustainability of paddy fields in Malaysia. Int. J. Agric. Biol. Eng., 12(2): 54–67. https://doi.org/10.25165/j.ijabe.20191202.2999
Taffesse, S. and I. Tsakok. 2019. Agricultural transformation and inclusive growth: The Malaysian experience. Agric. Trans. Inclusive Growth, pp. 1–140.
Warner, R.D., 2017. The eating quality of meat IV water-holding capacity and juiciness. In Lawrie´s meat science (Issue May, pp. 419–459). Elsevier. https://doi.org/10.1016/B978-0-08-100694-8.00014-5
Zhuang, H. and E.M. Savage. 2013. Comparison of cook loss, shear force, and sensory descriptive profiles of boneless skinless white meat cooked from a frozen or thawed state. Poult. Sci., 92(11): 3003–3009. https://doi.org/10.3382/ps.2012-02801
To share on other social networks, click on any share button. What are these?






