Synergistic Effect of Entomopathogenic Fungi and Bacteria against Pulse Beetle, Callosobruchus chinensis
Synergistic Effect of Entomopathogenic Fungi and Bacteria against Pulse Beetle, Callosobruchus chinensis
Mohsan Iqbal1, Farid Asif Shaheen1, Rashid Mahmood2,*, Muhammad Khalid Rafique2, Imran Bodlah1, Farah Naz3 and Muhammad Usman Raja3
1Department of Entomology, PMAS-Arid Agriculture University, Rawalpindi
2Honeybee Research Institute, National Agricultural Research Centre, Islamabad
3Department of Plant Pathology, PMAS-Arid Agriculture University, Rawalpindi
ABSTRACT
Callosobruchus chinensis is one of the most destructive insect pests of Cicer arietinum (Chickpea) in storages and renders grains unfit for human consumption. In the present study, synergistic effects of entomopathogenic fungi (EPF), Beauveria bassiana and Metarhizium anisopliae and entomopathogenic bacteria (EPB), Photorhabdus temperata and Xenorhabdus nematophila were studied as bio-control agents to manage this pest. In addition, percent conidial germination and % mortality of C. chinensis were also evaluated when B. bassiana was used with DEBBM. The minimum number of eggs (0.66 grain-1), number of holes (1 grain-1), number of F1 new emerged (5.6 jar-1), days to 100 percent mortality F1 (3), weight loss (4%), damage (5%) whereas the maximum inhibition rate of 85% and percent mortality of C. chinensis were observed in synergistic concentration of EPB as compared to EPF. Synergistic concentration (1×108) of bacteria gave the best results against C. chinensis as compared to that of entomopathogenic fungi. The maximum percent conidial germination (90%) of B. bassiana and percent mortality (50.40±2.20a), (79.70±0.28a) and (90.76±0.56a) of C. chinensis were observed when DEBBM was synergized with B. bassiana after seven, fourteen and twenty one days, respectively.
Article Information
Received 13 June 2018
Revised 22 August 2018
Accepted 16 January 2019
Available online 18 June 2019
Authors’ Contribution
MI Conducted research. FAS conceptualized and supervised research. RM and MKF provided research materials and analysed the data. IB proofread the manuscript. FN and MUR facilitated in conducting pathological aspects of research.
Key words
Cicer arietinum, Callosobruchus chinensis, Entomopathogenic fungi, Entomopathogenic bacteria, DEBBM, Mortality.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.5.1685.1691
* Corresponding author: rashid_ento1@yahoo.com
0030-9923/2019/0005-1685 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
Introduction
Chickpea ranks 2nd in area under cultivation and 3rd in production among pulses in the world (Iqbal et al., 2018). In Pakistan, annual production of 0.5m tonnes of dry seed is obtained from an area of around 1m hectares (Iqbal et al., 2018). In storages, it has severe post harvest losses due to Callosobruchus chinensis (Ahmed et al., 2011), which causes about 10% damage and renders grains unfit for human consumption (Aslam, 2004).
For the control of stored grain insect pests, grain protectants and fumigants have been used for the last many years. However, these chemicals lost effectiveness due to resistance developed in insects and presence of residual effects is the other demerit posing risks to human health and the environment (Rajendran and Sriranjini, 2008). Botanicals have also been used as safe grain protectants against stored grain insect pests (Bakkali et al., 2008) but also faced resistance issues inviting the other safer approaches like entomopathogens to manage insect pests.
Entomopathogenic fungi (EPF) are widely distributed with both restricted and wide host ranges. They have different biocontrol potentials against arthropods insects and plant pathogenic fungi. They are among the first organisms to be used for the biological control of pests. Target insect pests of M. anisopliae are stored grain insect pests namely Callosobruchus sp., and others like cockroaches and locusts (Nabaei et al., 2012). Target insect pests of B. bassiana are the stored insect pests, Callosobruchus sp., Tribolium castaneum, Sitophilus granarius and Oryzaephilus surinamensis and others like mites and white flies (Khashaveh et al., 2008; Nabaei et al., 2012).
Entomopathogenic bacteria (EPB), namely P. temperata and X. nematophila are symbiotic in nature. Significant pathogenicity has been observed between these two bacteria against the stored insect pests. Main target insect pests of these bacteria are stored grain insect pests namely red flour beetle and other insects like Spodoptera litura, diamond back moth (Sony and Kim, 2010; Jung and Kim, 2006).
The objectives of the proposed research were to investigate synergistic effect of EPF (B. bassiana and M. anisopliae) and EPB (P. temperata and X. nematophila). In addition DEBBM (mixture of DE (Diatomaceous Earth) and BBM (Bitterbarkomycin, Chinese plant extract))effectiveness as inert material was also studied.
Materials and methods
The study was carried out at Stored Grain Pests Research Lab of Entomology Department of Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, during 2017. Infested samples of stored chickpea grains were collected from different research stations including National Agriculture Research Council (NARC) Islamabad, Pakistan. C. chinensis culture was maintained in an incubator at temperature of 30±2oC and 70±5% relative humidity in the Department of Entomology, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan and was named as ‘Pulse Beetle Rearing Cell’ during 2017. For bioassays, chickpea cultivar NOOR-2009 was obtained from NARC, Islamabad. Agtoxin was applied for fumigation to kill already existing insect pests (Shaheen et al., 2006). EPF namely B. bassiana isolate (DEBI 005) and M. anisopliae isolate (DEMI 001) and EPB P. temperata isolate (ANU101) and X. nematophila isolate (K1) were obtained from Korean Agricultural Culture Collection (KACC), NAC, RDA, Suwon, 441-707, Korea.
Initially the culture was grown in Potato Dextrose Agar (PDA) at 25oC at 200 rpm for two weeks, and then it was multiplied in Potato Dextrose Broth medium to count the number of conidia/ml. The conidia/ml was grown on PDA medium. Later on then conidia/ml was counted by haemocytometer at 24 h interval (Tuan et al., 2009). Then petri dishes were kept in incubator at a temperature of 28±1°C and relative humidity of 75±5% for fungi mass culturing. After drying the culture in oven, rubber scalpel was used to harvest the conidia from culture. Fungal concentrations of 1×108 of both fungi were synergized by adding the sterile 0.02% (Tween 80) in sterile distilled (purified) water (Atif et al., 2012).
Initially, the culture was streaked on nutrient agar (NA) plates at 25oC for 4-6 days. Culture was purified by re-streaking single colony on NA. Purified culture was multiplied in nutrient broth at 200 rpm for two to three days. To count/optimize the (colony forming units) cfu’s per unit volume serial dilution method was employed for drawing dilution curve between optical density (OD) and cfu’s. Bacterial dilutions of 1×108 of both bacteria were prepared (Atif et al., 2012). Each of the EPF and EPB were applied @ 2ml per jar against C. chinensis.
Experiment No. 1
In each jar, 50g of chickpea grains was put in plastic jars. The jars were covered with muslin cloth tightened and then placed in an incubator at 30ºC. Ten pairs of C. chinensis were released into each jar. Best concentration viz. 1×108 (conidia ml-1) of both bacteria and fungi were prepared for experiment. Parameters of study includes number of eggs, number of holes, number of F1 progeny adults emerged, percent inhibition rate (% IR), days to 100% mortality of F1 emerged, weight loss (%), and percent damage.
The formulae used were (Shaheen et al., 2006) as follows:
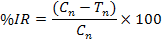
Where, Cn is the number of newly emerged adults in untreated jar (control) and Tn is the number of newly emerged adults in treated jar.

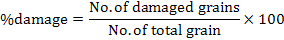
Where, WL is weight loss and wt. is weight
Experiment No. 2
Mortality of C. chinensis was determined in treated and untreated 50g of stored chickpea grains in each petri plate 07cm diameter ((38.5 cm2), which had filter paper (Whatman No. 1). The concentration of bacteria and fungi viz., 1×108 (conidia/ml) were applied @ 2ml on grains. Each treatment had 3 replications. The mortality of C. chinensis due to concentration of both EPF and EPB was observed after 24, 48 and 72 h. Ten beetles of C. chinensis were released. Petri-plates were placed in an incubator at 30ºC.
Experiment No. 3
DE component of DEBBM belongs to a group of freshwater DEs composed of amorphous SiO2 (89%), Al2O3 (4%), Fe2O3 (1.7%), CaO (1.4%), MgO + K2O (less than 1%), H2O (3%). Median particle size is 10 mm, specific gravity 2.2, surface area 35.7 m2g-1, pH 8 and 0.1% crystalline silica. BBM (Bitterbarkomycin, Chinese plant extract) is a polyol ester, extracted from the roots of the plant, Celastrus angulatus. Experiment conducted at temperatures of 25, 30 and 35°C (70% RH). 50 g of grain samples placed in plastic jars. Following concentrations were used: (i) D1 (20 mg of DEBBM), (ii) D2 (40mg of DEBBM), (iii) B1 (6.69×105 conidia/kg), (iv) B2 (6.69×105 conidia/kg), (v) B3 (6.69×105 conidia/kg), (vi) D1+B1, (vii) D1+B2, (viii) D1+B3, (ix) D2+B1, (x) D2+B2 and (xi) D2+B3.
Both B. bassiana and DEBBM were thoroughly mixed and shook manually for 2 min. Twenty adults of C. chinensis were released into each jar. Adult mortality was recorded after 7, 14 and 21 days of treatments application.
Statistical analysis
SPSS 16.0 for Windows program was used for one factor analysis. Duncan’s multiple range test (DMRT) was applied to all the means. Moreover the graphical work was done using Microsoft excel programme.
Table I.- Number of eggs (Mean ± SEM) per grain laid by C. chinensis and number of hols/grain in chickpea grains treated with different synergistic concentrations of B. bassiana+M. anisopliae and P. temperata+X. nematophila.
|
S No. |
Concentrations 1×108 (conidia/ml) |
No. of eggs/grain |
No. of holes/grain |
|
1 |
B. bassiana + M. anisopliae |
1 ± 0.57a |
1.3 ± 0.66a |
|
2 |
P. temperata + X. nematophila |
0.66 ± 0.66a |
1 ± 0.57a |
|
3 |
Control |
18 ± 1.52b |
7 ± 0.57b |
Table II.- Number of holes/grain by new emerged F1 C. chinensis in chickpea grains treated with different synergistic concentrations of B. bassiana+M. anisopliae and P. temperates+X. nematophila.
|
S No. |
Concentrations 1×108 (conidia/ml) |
No. of holes/grain |
|
1 |
B. bassiana + M. anisopliae |
7.6 ± 0.33a |
|
2 |
P. temperata + X. nematophila |
5.6 ± 0.66a |
|
3 |
Control |
41.3 ± 0.33b |
Results and discussion
Experiment No. 1
The lowest number of eggs (0.66) was counted in the synergistic dilution of P. temperata+X. nematophila as compared to synergistic dilution of B. bassiana+M. anisopliae (1) which is not significantly different with each other. Whereas the highest number of eggs (18±1.52) was observed in control where no synergistic dilution was used (Table I). Minimum number of holes were seen in synergistic dilution of P. temperata + X. nematophila and B. bassiana + M. anisopliae which is not significant with reference to each other. Maximum number of holes was recorded in control where no synergistic dilution was used (Table I). More number of holes F1 emerged (7.6±0.33) in synergistic concentration of B. bassiana+M. anisopliae as compared to synergistic concentration of P. temperata+X. nematophila (5.6±0.66) (Table II). Inhibition rate of 81.34% of C. chinensis was observed using B. bassiana in combination with M. anisopliae whereas 86.40% was observed for combined use of P. temperata and X. nematophila. These results were significantly different with each other (Fig. 1).
More number of days (18) recorded to 100% mortality of F1 in control as compared to individual treatments of synergistic concentrations of B. bassiana+ M. anisopliae (4.60) and EPB P. temperata and X. nematophila (3.0) which is significantly different (Fig. 2). The maximum weight loss (82.2%) was recorded in control as compared to individual synergistic concentration used. More weight loss (5.2%) was measured in synergistic concentration of B. bassiana+M. anisopliae as compared to synergistic concentration of P. temperata+X. nematophila (2.2%) which is significantly different from each other (Fig. 3). Minimum damage (7.73%) is measured in synergistic concentration of P. temperata, X. nematophila as compared to synergistic concentration of B. bassiana, M. anisopliae (11.3%) which is significantly different from each other. Maximum damage (91.73%) was noted in control (Fig. 4).
Experiment No. 2
Mortality (%) of C. chinensis after 72 h was found absolute same as compared to 24 h and 48 h after application of synergistic dilutions of entomopathogenic fungi and bacteria.
Table IV.- Percent mortality (Mean±SE) of adults of C. chinensis when chickpea grains were treated with entomopathogenic Beauveria bassiana and DEBBM alone and in combination at different temperatures for 7, 14 and 21 days.
|
Treatments |
Temperature |
||
|
25oC |
30oC |
35oC |
|
|
Exposed for 7 days |
|||
|
D1+B1 |
21.67±1.88 bc |
28.67±1.20cde |
37.60±0.25abc |
|
D1+B2 |
26.55±1.33 ab |
34.67±0.88bcd |
44.77±0.46 a |
|
D1+B1 |
15.55±1.90 c |
21.40±1.56 e |
28.56±1.42 e |
|
D2+B2 |
22.77±1.45 bc |
27.35±2.50 de |
29.95±1.50cde |
|
D2+B3 |
25.45±1.20 ab |
35.67±1.78 bc |
37.28±1.68abc |
|
DEB1+BB1 |
17.15±1.15 c |
28.58±0.56de |
23.48±0.58 e |
|
DEB1+BB2 |
23.45±1.96abc |
36.88±0.86bcd |
30.67±1.22cde |
|
DEB1+BB3 |
27.66± 1.40 ab |
40.30±1.34 b |
34.68±2.08bcd |
|
DEB2+BB1 |
20.33±0.85 bc |
30.52±2.05 cd |
25.58±1.54 de |
|
DEB2+BB2 |
25.10±1.20 ab |
39.55±1.55 b |
35.36±2.10abcd |
|
D2+B2+BB3 |
31.67±1.67 a |
50.40±2.20 a |
43.55±1.90 ab |
|
Exposed for 14 days |
|||
|
DEB1 |
24.80±0.92 cd |
41.87±0.96 de |
52.96±0.33 bc |
|
DEB2 |
32.58±1.48bcd |
47.62±0.78 de |
59.37±0.43 ab |
|
BB1 |
22.85±1.70 d |
35.45±1.51 e |
35.52±0.49 c |
|
BB2 |
30.72±1.46bcd |
45.35±0.50 de |
47.92±1.55 bc |
|
BB3 |
37.25±1.28 ab |
51.60±1.58 cd |
55.29±1.62 b |
|
DEB1+BB1 |
22.19±1.15 d |
41.51±0.52 de |
36.18±0.88 c |
|
DEB1+BB2 |
31.44±1.96bcd |
55.82±0.96bcd |
49.60±1.20 bc |
|
DEB1+BB3 |
41.67± 1.42 ab |
62.38±1.64 bc |
57.67±0.08 ab |
|
DEB2+BB1 |
34.33±0.25 bc |
52.22±1.07 cd |
55.54±1.44 bc |
|
DEB2+BB2 |
40.16±1.28 ab |
67.50±1.53 ab |
61.86±1.10 ab |
|
DEB2+BB3 |
48.53±1.58 a |
79.70±0.28 a |
73.58±1.98 a |
|
Exposed for 21 days |
|||
|
DEB1 |
30.33±1.98bc |
49.80±0.33 de |
59.80±1.20abc |
|
DEB2 |
43.50±1.48abc |
66.69±0.68de |
71.37±0.45 ab |
|
BB1 |
28.45±1.75c |
42.45±1.59 e |
43.52±0.89 c |
|
BB2 |
37.76±1.49abc |
48.38±1.50 de |
49.92±1.45 bc |
|
BB3 |
45.23±1.68abc |
57.63±1.08cd |
61.29±1.68abc |
|
DEB1+BB1 |
31.10±1.12 bc |
56.24±0.51de |
51.18±0.82 bc |
|
DEB1+BB2 |
40.64±1.96abc |
71.89±0.96bcd |
60.60±1.28abc |
|
DEB1+BB3 |
48.60± 1.43 ab |
75.35±1.04 bc |
67.67±0.78 ab |
|
DEB2+BB1 |
36.35±0.25 bc |
66.29±1.05 cd |
61.54±1.42 abc |
|
DEB2+BB2 |
47.18±1.20 ab |
80.30±1.58 ab |
74.86±1.17 ab |
|
DEB2+BB3 |
55.55±1.32 a |
90.76±0.56 a |
85.58±1.66 a |
Means followed by the same alphabets within the columns and rows are significantly similar (P ≤ 0.05); Duncan, 1951 (DMRT). D1, 20g of DEBBM; D2, 40g of DEBBM ; B1, 6.69×105 (conidia/kg); B2 6.69×107 (conidia/kg); B3, 6.69×109 (conidia/kg).
Experiment No. 3
A large number of conidia were germinated when B. bassiana was used with DEBBM at 30oC as compared to individual treatments at other temperatures.
It was observed that significant mortality of pulse beetle (90.76%) was recorded after 21 days, 14 days (79.70%) and after 07 days (50.40%) at 30oc temperature. when B. bassiana was used synergistically with DEBBM as compared to individual treatments(Table IV).
Discussion
EPF and bacteria have been used very effectively against different insect pests both in field and storages. They have been used singly as well as in combination. Kryukov et al. (2009) were of the view that a synchronous coinfection of the Colorado potato beetle Leptinotarsa decemlineata (Say) with the EPF Bacillus thuringiensis spp. morrisoni Bonnifoi & de Barjak var. tenebrionis and hyphomycete M. anisopliae (Metsch.) Sorokin or B. bassiana (Bals.) Vuill leads to the rapid death of 95–100% of larvae. The synergistic effect of two pathogens is recorded at a relatively low hyphomycete titer (1–5×106 conidia/ml) and is evident in the mortality dynamics at all larval ages. These bacterial and fungal pathogens display no antagonism on artificial nutrient media. This microbial complex is highly efficient under natural conditions (80–90% larval mortality rate and no plant defoliation).
Similarly, Shaheen (2016) used EPF Beauveria bassiana as biological control agent to control pulse beetle C. chinensis in chickpea grains at different temperatures. Five concentrations (1×106, 1×107, 1×108, 1×109 and 1×1010 spores ml-1) of commercially available conidia of B. bassiana were prepared. Mortality of pulse beetle was directly proportional to concentrations of B. bassiana. B. bassiana was less effective at 25ºC as compared to 30ºC. At 25ºC, highest mortality was recorded at concentration of 1×1010 spores ml-1 after 5, 10 and 15 days and vice versa. At 30ºC, B. bassiana showed better results and all pulse beetles died after 15 days at each concentration. This effective control strategy has significant contribution towards development of commercial microbial formulations of B. bassiana and is recommended to be a part of integrated pest management of pulse beetle.
According to Radha (2012), the efficacy of two EPF M. anisopliae (Deuteromycotina: Hyphomycetes) and B. bassiana (Ascomycota: Hypocreales) formulations were assessed against cowpea bruchid, C. maculatus. Five different concentrations of each formulation were used against each pest under investigation and compared with control insects. In liquid formulation of B. bassiana against C. maculatus, the percentage of adult mortality was 96 % in 5 x 106 conidial concentrations at 96 h interval and LT50 value was only 1.24%. Comparison of LC50, LT50 values and mortalities indicated that in both assays, B. bassiana was consistently more virulent to bruchids than M. anisopliae because it had lower LC50 and LT50 and caused the highest mortality (96%) in treatment by suspensions containing 5×106 conidia/ml. B. bassiana had higher virulence than M. anisopliae against adult of cowpea weevil.
Sony and Kim (2010) described that two EPF, P. temperata subsp. Temperata (Ptt) and X. nematophila (Xn.) are symbiotically associated with the nematodes, Heterorhabdis megidis and Steinernema carpocapsae, respectively. There is little information on natural host ranges of the nematodes, but a significant difference in pathogenicity was observed between these two bacteria against the red flour beetle, Tribolium castaneum, in which Photorhabdus temperata exhibited more than six times higher pathogenicity than Xn. The pathogenic difference was not due to their inhibitory effect on phospholipase A2 activity that is required for expression of immune response of T. castaneum. The culture broths were fractionated into aqueous and organic extracts, most insecticidal activity remained in the aqueous extracts. The aqueous extracts of two bacteria contained proteins which showed different profiles.
Mburu et al. (2011) observed that isolates of the fungus B. bassiana have different levels of virulence and repellency against the termite Macrotermes michaelseni. They compared the volatile profiles and gene sequences of two isolates of the fungus with different levels of virulence and repellence to the termite. Subtractive bioassays showed that the repellency of each isolate was due to synergistic effects of a few constituents. As previously reported for isolates of M. anisopliae, some differences also were found in the nucleotide sequences of the two isolates of B. bassiana, suggesting a genetic basis for the observed intra-specific differences in their repellency and virulence against the termite.
The current findings of the study are in conformity with those of Correa et al. (2016). They reported that P. luminescens inhibited the growth of B. bassiana and M. anisopliae up to 40% by the secretion of secondary metabolites, whereas fungal extracts did not inhibit P. luminescens; this explains the in vivo interactions of these biological control agents. They established that on days 0, 2 and 4 there was an antagonistic interaction, while a synergistic interaction occurred on day 6. Therefore, the use of the interaction between H. bacteriophora HNI0100 with M. anisopliae Ma9236 and B. bassiana Bb9205 is an innovative alternative for the control of P. xylostella.
Similar results were observed by Jung and Kim (2006) when they revealed that Xenorhabdus sp. and P. temperata subsp. temperata (Ptt) are the symbiotic bacteria of the entomopathogenic nematodes, Steinernema monticolum and Heterorhabditis megidis, respectively. To increase their pathogenicity in the fifth instar, the bacteria should be delivered into the hemocoel. To this end, Bacillus thuringiensis aizawai (Bt) as a synergist was used to facilitate entry of the bacteria from the gut lumen into the hemocoel of S. exigua by its disruption of the insect gut epithelium. The bacterial mixture treatment was highly synergistic against the fifth-instar larvae of S. exigua. The synergistic effects were shown by the successful infection of X. sp. or Ptt in the insect haemocoel. This research shows a possibility that Xenorhabdus or Photorhabdus can be applied to kill S. exigua by oral treatment in a mixture with Bt.
Park et al. (2016) tested a hypothesis that bacterial immunosuppresants could enhance the susceptibility of mosquitoes (Aedes albopictus and Culex pipiens pallens) to Bt. Bacillus thuringiensis israelensis (BtI) was highly toxic to both culicid mosquitoes with median lethal concentration (LC50, spores/ml) of 2.9105 and 2.2105 at 16 h after treatment, respectively. Addition of each bacteria-cultured broth enhanced BtI toxicity to these mosquito larvae. The LC50 values of BtI to Ae. albopictus larvae were reduced to 1.5105 in Xn mixture, 1.7105 in Xh mixture, and 1.9105 in Ptt mixture. The LC50 values of Bacillus thuringensis to Cx. pipiens pallens larvae were also reduced to 1.2105 in Xn mixture, 1.3105 in Xh mixture, and 1.5105 in Ptt mixture. Adding benzylideneacetone or oxindole produced from Xn and Ptt also enhanced BtI toxicities to these mosquito larvae. Based on these results, they developed a new mosquitocidal Bt formulation called “DipKill” consisting of 80% Xn-cultured broth, 10% BtI (1010 spores ml-1), and 10% preservative. Dip-Kill at 1,000ppm was superior to a commercial BtI product at its recommended dose.
Acknowledgments
We are grateful to Entomology and Plant Pathology Department of PMAS-Arid Agriculture University, Rawalpindi, Pakistan and Entomology and Nematology Department of University of Florida, USA for their facilitation to conduct these experiments.
Statement of conflict of interest
The authors declare no conflict of interest.
References
Aslam, M., 2004. Pest status of stored chickpea beetle, Callosobruchus chinensis Linnaeus on chickpea. J. Ent., 1: 28-33. https://doi.org/10.3923/je.2004.28.33
Ahmed, Z., Aslam, M., Falak, N. and Illyas, M., 2011. Bio-efficacy of some plant extracts against chickpea beetle, Callosobruchus chinensis Linneaus (Bruchidae: Coleoptera) against chickpea. Pakistan J. Zool., 43: 733-737.
Atif, H.M., Javed, N., Khan, S.A. and Ahmed, S., 2012. Virulence of Xenorhabdus and Photorhabdus bacteria and their toxins against juvenile’s immobilization of Meloidogyne incognita. Pak. J. Phytopathol., 24: 170-174.
Bakkali, F., Averbeck, S., Averbeck D. and Idaomar, M., 2008. Biological effects of essential oils-A review. Fd. Chem. Toxicol., 46: 446-475. https://doi.org/10.1016/j.fct.2007.09.106
Correa, J.P., Sáenz-Aponte, A. and Rodríguez-Bocanegra, M.X., 2016. In vitro interaction of Metarhizium anisopliae Ma9236 and Beauveria bassiana Bb9205 with Heterorhabditis bacteriophora HNI0100 for the control of Plutella xylostella. SpringerPlus, 5: 2068.
Iqbal, M., Shaheen, F.A., Bhatti, A.R., Zia, A., Bodlah, I., Naz, F. and Fiaz, M., 2018. Effectiveness of Photorhabdus temperata and Xenorhabdus nematophila against Callosobruchus chinensis attacking stored chickpea grains. Pak. Entomol., 40:95-103.
Jung, S. and Kim, Y., 2006. Synergistic effect of entomopathogenic bacteria (Xenorhabdus sp. and Photorhabdus temperata ssp. temperata) on the pathogenicity of Bacillus thuringensis ssp. aizawai against Spodoptera exigua (Lepidoptera: Noctuidae). Environ. Ent., 35: 1584-1589.
Khashaveh, A., Safaralizade, M.H. and Ghosta, Y., 2008. Pathogenicity of three Iranian isolates of the fungus, Metarhizium anisopliae (Metsch.) Sorokin (Deuteromycotina: Hyphomycetes) against granary weevil, Sitophilus granarius L. (Coleoptera: Curculionidae). J. biol. Sci., 8: 804-808. https://doi.org/10.3923/jbs.2008.804.808
Kryukov, Y., Khodyrev, V.P., Yaroslavtseva, O.N., Kamenova, A.S., Duisembekov, B.A. and Glupov, V.V., 2009. Synergistic action of entomopathogenic hyphomycetes and the bacteria Bacillus thuringiensis ssp. morrisoni in the infection of Colorado potato beetle. Appl. Biochem. Microbiol., 45: 511-516. https://doi.org/10.1134/S000368380905010X
Mburu, D.M., Maniania, N.K., Hassanali, A. and Ndungu, M., 2011. Comparison of volatile blends and gene sequences of two isolates of Metarhizium anisopliae of different virulence and repellency toward the termite Macrotermes michaelseni. J. exp. Biol., 214: 956-962. https://doi.org/10.1242/jeb.050419
Nabaei, N.A., Mehrvar, S.M. and Bagheri, M., 2012. Efficacy of entomopathogenic fungi in combination with diatomaceous earth against Callosobruchus maculatus (Coleoptera: Bruchidae). Acta Ent. Sin., 55: 1282-1288.
Park, Y., Jung, J.K. and Kim, Y., 2016. A mixture of Bacillus thuringiensis subsp israelensis with Xenorhabdus nematophila-cultured broth enhances toxicity against mosquitoes Aedes albopictus and Culex pipiens pallens (Diptera: Culicidae). J. econ. Ent., 109: 1086-1093.
Radha, R., 2012. Insecticidal efficacy of entomopathogenic fungi in stored cowpea against cowpea bruchid, Callosobruchus maculates (F) (Coleoptera: Bruchidae). Asian Acad. Res. J. Multidiscip., 1: 117-130.
Rajendran, S. and Srinanjini, V., 2008. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res., 44: 126-135. https://doi.org/10.1016/j.jspr.2007.08.003
Shaheen, F.A., Akram, M.W., Rashid, M.A., Nadeem, M., Saeed, M., Husain, M. and Khalid, M., 2016. Biological control of pulse beetle Callosobruchus chinensis L. (Bruchidae: Coleoptera) in stored chickpea grains using entomopathogenic fungus Beauveria bassiana Balsamo. J. Ent. Zool., 4: 1076-1083.
Shaheen, F.A., Akram, M.W., Rashid, M.A., Nadeem, M., Saeed, M., Husain, M. and Khalid, M., 2006. Resistance of chickpea (Cicer arietinum L.) cultivars against pulse beetle. Pak. J. Bot., 38: 1237-1244.
Sony, S. and Kim, Y., 2010. Differential pathogenicity of two entomopathogenic bacteria, Photorhabdus temperata subsp. temperata and Xenorhabdus nematophila against the red flour beetle, Tribolium castaneum. J. Asia-Pac. Ent. 13: 209-213.
Seogchae, J. and Yonggyun, K., 2006. Synergistic effect of entomopathogenic bacteria (Xenorhabdus sp. and Photorhabdus temperata ssp. temperata) on the pathogenicity of Bacillus thuringiensis ssp. aizawai against Spodoptera exigua (Lepidoptera: Noctuidae). Environ. Ent., 35: 1584-1589. https://doi.org/10.1603/0046-225X(2006)35[1584:SEOEBX]2.0.CO;2
Tuan, A.N., Jeong, J.K., Seon, G.K. and Keun, K., 2009. Production of blastospore of entomopathogenic Beauveria bassiana in a submerged batch culture. Mycobiology, 37: 218-224. https://doi.org/10.4489/MYCO.2009.37.3.218
To share on other social networks, click on any share button. What are these?










