Supplementation of Moderate and High Doses of Vitamin E in the Practical Diet of Labeo rohita: Effects on Growth, Proximate Composition, Lipid Peroxidation and Fatty Acid Profile
Supplementation of Moderate and High Doses of Vitamin E in the Practical Diet of Labeo rohita: Effects on Growth, Proximate Composition, Lipid Peroxidation and Fatty Acid Profile
Mahroze Fatima1*, Syed Zakir Hussain Shah2, Muhammad Afzal3, Muhammad Bilal4 and Ayesha Khizar1
1Department of Fisheries and Aquaculture, University of Veterinary and Animal Sciences, Lahore, Pakistan.
2Department of Zoology, University of Gujrat, Gujrat, Pakistan.
3Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad.
4School of Life Science and Food Engineering, Huaiyin Institute of Technology, Huaian, 223003, China
ABSTRACT
Vitamin supplementation beyond the requirement level show therapeutic effect. Vitamin E being an antioxidant has become very attractive to reduce oxidation and improve overall performance of fish. Two adequate (100, 150 mg/kg) and two high levels (1000, 1500 mg/kg) of vitamin E with a control level (0 mg/kg) were analyzed in this study. Results showed that adequate levels of supplementation increased growth performance compared to higher levels. The minimum value of thiobarbituric acid reactive substances (TBARS) was recorded in 100 mg/kg vitamin E supplemented group, which started to increase gradually with increase in vitamin E level. Supplementation of vitamin E lowered the antioxidant enzyme (superoxide dismutase, catalase and peroxidase) activities, however the higher doses did not outperform the moderate doses. Saturated fatty acids and monoenes were more in fish fed the control diet compared to vitamin E fed fish. The percentages of 18:3n-6 (linolenic acid), 20:5n-3 (eicosapentaenoic acid), 22:5n-3 (docosapentaenoic acid), 22:6n-3 (docosahexaenoic acid), total n-3 and n-6 polyunsaturated fatty acids were found higher in diets enriched with vitamin E than in the control group. To conclude, dietary supplementation with vitamin E has been shown to improve the growth and antioxidant status of fish. Nevertheless, high doses of vitamin E showed the pro-oxidative effect that favored lipid peroxidation, resulting in a decreased growth rate.
Article Information
Received 23 April 2022
Revised 15 May 2022
Accepted 06 June 2022
Available online 08 August 2022
(early access)
Published 13 June 2023
Authors’ Contribution
MF planed and conducted the experiment. SZHS helped in experiment planning and analysis. MA supervised the experiment. MB helped in manuscript write up. AK helped in experiment conduction.
Key words
TBARS, α-tocopherol, Antioxidant enzymes, Rohu, Pro-oxidation
DOI: https://dx.doi.org/10.17582/journal.pjz/20220423080405
* Corresponding author: mahroze.fatima@uvas.edu.pk
0030-9923/2023/0004-1743 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Vitamin E is a fat-soluble vitamin with effective antioxidant properties and prevent oxidative damage by terminating oxidation chain reactions (Mourente et al., 2007). However, the vitamin E cannot be synthesized by fish endogenously, so dietary supplementation is needed to fulfil the requirement. Various researchers have reported the requirement of vitamin E of different species of fish such as rohu fry (Sau et al., 2004), cobia (Zhou et al., 2013) channel catfish (Bai and Gatlin, 1993), eel (Bae et al., 2013), juvenile golden pompano (Zhang et al., 2021), Caspian trout (Saheli et al., 2021) and juvenile Sillago sihama (Huang et al., 2020).
High levels of polyunsaturated fatty acids (PUFA) with CC20 and C3 double bonds have been reported in fish, most important of which are docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). According to various reports, these PUFA are highly useful in treating and preventing cancer, autoimmune disorders, inflammation, depression, aggression, and cardiovascular diseases in humans. Nonetheless, the n-3 long chain PUFA (LC-PUFA) are very sensitive to free radical’s attack and degradation, referred to as lipid peroxidation rate (Mourente et al., 2007). The oxidation of LC-PUFA negatively affects fish performance by compromising its metabolism leading to lost antioxidant enzymes activity, growth, and increased mortality (Hamre, 2011). In biological membranes, the lipid peroxidation can be evaded by dietary addition of vitamin E, which helps prevent the free radical formation in fish (Tocher et al., 2003; Gao et al., 2012a). Moreover, dietary vitamin E supplementation in the fish also enhances DHA and EPA contents in fish tissues (Gao et al., 2012b). Hence, being a potent antioxidant, vitamin E is effective in preventing lipid peroxidation and has a significant role in the acceleration of lipids metabolism.
Supplementation of certain vitamins above required levels in diet may be useful in enhancing immunity and disease resistance. Moreover, the supplementation of this dietary antioxidant beyond the required level has shown to reduce the chances of disease outbreaks and severity by stimulating the immune system (Scrimshaw and San Giovanni, 1997). According to Chaiyapechara et al. (2003) the feeding of elevated doses of vitamin E a few weeks before harvest protects the tissues from lipid oxidation.
In turbot, sea bream and halibut, the supplementation of 1000 mg/kg vitamin E lowered the lipid peroxidation rate than that of control and 100 mg/kg vitamin E supplementation (Tocher et al., 2002). Similarly, Fatima et al. (2019) also reported that 1000 mg/kg vitamin E lowered lipid peroxidation and enhanced the growth rate of oxidized oil based practical diet fed L. rohita. In contrast, some old studies revealed that the lipid peroxidation from tissues was induced in the yellow tail and rainbow trout when fed with excessive vitamin E supplemented semi-purified diets (Ito et al., 1999; Tokuda and Takeuchi, 1999).
Rohu (Labeo rohita) is a prevalent fish of the subcontinent region because of its high demand and palatability. Some research has been done on L. rohita for determining the requirement of vitamin E, however, no previous investigation explored the impact of high vitamin E dosages on antioxidant and fish lipid peroxidation status. Therefore, the current study was planned to confirm the behavior of high dose of vitamin E on the growth, lipid peroxidation (antioxidant activity and TBARS), fatty acid profile and α-tocopherol contents of the juveniles of L. rohita, when fed on practical diet. The outcomes of this study will help the feed formalists in the formulation.
MATERIALS AND METHODS
Following approval by the university’s Ethical Review Committee, the current study was executed in the Fish Nutrition Laboratory, University of Agriculture, Faisalabad.
Experimental design and feed formulation
L. rohita juveniles were fed five isocaloric and isonitrogenous experimental diets (Table I) containing α-tocopherol acetate (Vitamin E) at moderate (E100, 100 mg/kg and E150, 150 mg/kg) and high (E1000, 1000 mg/kg and E1500, 1500 mg/kg) levels, whereas one test diet was designed as control diet with no vitamin E (E0) addition. The experiment was performed in a Completely Randomized Design (CRD). Moderate levels of α-tocopherol met the recommended concentration as an optimum requirement for L. rohita fingerlings, i.e., 131.91 mg/kg, based on weight gain% with semi-purified diet (Sau et al., 2004). Firstly, cod liver oil was blended with α-tocopherol acetate (Sigma-Aldrich) and then added to the respective test diets. The ingredients used in test diets were analyzed chemically following AOAC (1995) methods. Dry ingredients were ground in a grinder (FFC-45, JIMO, China) and sieved (0.05 mm) to prepare test diets. The ingredients, mineral mixture, choline chloride and vitamin premix (without vitamin E) were thoroughly blended by using an electric mixer. When all ingredients were mixed evenly, then cod liver oil was added to each respective experimental diet and dough was made by adding 15% distilled water and pelleted. For pelleting, a hand pelletizer was used. The resulting pellets were dried containing up to 10% moisture contents and preserved at -18℃ during the experimental trial. The chemical composition of diet is shown in Table I.
Table I. Composition of experimental diets
|
Experimental diets |
|||||
|
E0 |
E100 |
E150 |
E1000 |
E1500 |
|
|
Ingredients (g/kg) |
|||||
|
Fishmeal |
400 |
400 |
400 |
400 |
400 |
|
Soybean meal |
250 |
250 |
250 |
250 |
250 |
|
Wheat flour |
130 |
129.9 |
129.85 |
129 |
128.5 |
|
Rice polish |
117 |
117 |
117 |
117 |
117 |
|
Mineral mixture* |
10 |
10 |
10 |
10 |
10 |
|
Vitamin premix (E free)† |
10 |
10 |
10 |
10 |
10 |
|
Choline Chloride |
3 |
3 |
3 |
3 |
3 |
|
Cod liver oil‡ |
80 |
80 |
80 |
80 |
80 |
|
Vitamin E (mg/kg)§ |
0 |
100 |
150 |
1000 |
1500 |
|
Chemical composition |
|||||
|
Dry matter (g/kg) |
911 |
909 |
910 |
910 |
909 |
|
Crude protein (g/kg) |
341 |
340 |
344 |
340 |
343 |
|
Crude fat (g/kg) |
109 |
108 |
111 |
111 |
110 |
|
Gross energy (kcal/kg) |
4081 |
4102 |
4079 |
4083.3 |
4087.3 |
|
α-tocopherol (mg/kg) |
17.90 |
109.13 |
160.10 |
943.53 |
1401.43 |
*Mineral premix (kg) comprised of CaCO3 (316); KH2PO4 (479); NaCl (51); CoCl.6H2O (0.0816); MgSO4.7H2O (153); AlCl3.6H2O (0.255); Ammonium molybdate, (0.061); CuSO4.5H2O (210.67); ZnSO4.7H2O (121.33); FeSO4.H2O (100.67); MnSO4.5H2O (116.67); †Vitamin premix (kg) comprises; Vitamin A (5.0 g), Vitamin B1 (0.5 g), Vitamin B2 (3.0 g), Vitamin B3 (5.0 g), Vitamin B6 (1.0 g), Vitamin B7 (0.05 g), Vitamin B9 (0.18 g), Vitamin B12 0.002 g, Choline 100 g, Ascorbic acid 5.0 g, Vitamin D3 0.002 g, Cellulose 815.26 g; ‡Cod liver oil was provided by Poultry-vet Co, Karachi, Pakistan;§Vitamin E was included as DL-α-toopherol acetate (Sigma-Aldrich, St. Louis, Missouri, USA).
Fish culture
The juveniles of L. rohita were purchased from the Govt. Fish Seed Hatchery, Faisalabad. After transportation of juveniles to the laboratory, acclimatization to indoor rearing conditions was done in cemented tanks (1000 L) for 2 weeks. At the initiation of the experimental trial, 25 fish were stocked randomly with uniform biomass (initial body weight of 3.58±0.04 g) into three tanks (90-L) for each dietary treatment. The test diets were given to fish until apparent satiety, 6 days per week. The experimental feeds were given to L. rohita juveniles at 8 am and 4 pm in one day. For feed intake estimation, the orts diet was accumulated and dehydrated. During the complete feeding trial, all the fish culture tanks were aerated through a capillary system. The physicochemical parameters, such as pH, dissolved oxygen and temperature was monitored constant at 7.4-8.6, 5.8-7.3 mg L-1 and 24.9-28.7°C, respectively, throughout the experimental trial of eight weeks.
Sample collection
Upon completion of the experimental trial, starved the juveniles for 24 h, and MS-222 was used to anesthetize the fish and then sacrificed. To determine hepatosomatic index (HSI), liver of fifteen fish were dissected out and weighed individually. In addition, the muscle samples (without skin) were also dissected from the same fifteen fish, pooled, homogenized, and utilized for further evaluation.
Growth performance and chemical analysis
At the initiation, fortnightly, and at completion of the experimental trial, the growth and survival rate of fish were examined. The following indicators were used to evaluate growth performance:
Absolute weight gain (AWG) = Wf (g) - Wi (g)
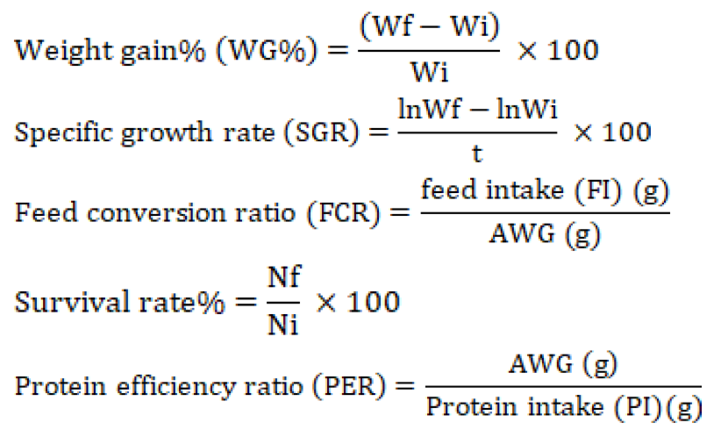
Where the initial and final weight are presented as Wi and Wf, respectively; the number of fish (initial and final) in each tank represented with Ni and Nf; whereas, t represents the duration of experiment in days.
The HSI was determined as:
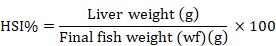
Proximate analysis
The proximate analysis of muscle and diet was analyzed following the standard AOAC (1995) methods. For moisture content analysis, the muscles and diet samples were dried in the hot air-dry oven up to a consistent weight at 105℃. The Kjeldahl apparatus was used for the estimation of nitrogen after digestion in acid (N×6.25) (KjeltecTM 8100, FOSS Analytical A/S). The ether extraction method through Soxhlet apparatus was used for the crude fat analysis (Sr. no. 70861). While Crude ash analysis was performed in a muffle furnace by incinerating the samples for 6 h at 600℃. The calorie values from the test diets were analyzed with an adiabatic oxygen bomb calorimeter.
The fats were extracted from the samples of the test diet and liver tissues by the extraction with petroleum ether and used to determine a-tocopherol contents. The α-tocopherol content from the samples were analyzed on HPLC according to a procedure as reported earlier (Anwar et al., 2006). The rate of lipid peroxidation from the samples of liver was examined calorimetrically in the form of TBARS (Gatta et al., 2000). To determine fish antioxidant status, the liver samples were homogenized in the phosphate buffer leading to enzyme extraction. The superoxide dismutase (SOD) enzymatic activity was observed by assessing its capability to stop photoreduction of nitro blue tetrazole as described earlier (Giannopolitis and Ries, 1977). The enzymatic activity of catalase was examined based on its capability to reduce the absorption of H2O2 at 240 nm wavelength (Chance and Maehly, 1955). The peroxidase enzymatic activity was observed by measuring its capacity to decrease H2O2 level at 470 nm wavelength (Civello et al., 1995). The liver fatty acid composition was influenced on gas chromatography (model GC-17A FID, SHIMADZU). The fatty acids were analyzed after formation of fatty acid methyl esters (FAMEs) derived from trans-esterified fats (IUPAC, 1987).
Statistical analysis
Statistical analyses were carried out on the Costat Computer Software (Version 6.303, PMB 320, Monterey, CA, 93,940 USA). The data is represented as mean and pooled standard error (PSE=√MSE/n (where MSE= mean-squared error. One-way ANOVA was applied after completing the pre-assumptions of ANOVA. Multiple comparisons among mean values were performed with the Student Newman–Keul’s test. The differences between treatments were found to be significant at a probability level of p<0.05.
RESULTS
Growth performance
The parameters of growth such as final weight (g), SGR, AWG, WG% showed significant variations among the test diets based on p-value. Regarding SGR, moderate levels of supplementation (E100 and E150) showed better performance (0.21 and 0.22%) compared to higher levels (E1000 and E1500), while a maximum value was recorded at 100 mg/kg α-tocopherol level. The same pattern was found in other growth parameters. Variations among treatments were also recorded for feed intake. Again, the high vitamin E supplemented levels were unsuccessful in improving the FCR. In contrast, its lowest value (0.53% from control and 0.38% from a higher level) was recorded in fingerlings fed 100 mg/kg α-tocopherol diet. The HSI was markedly decreased in fish having E0 diet and increased with vitamin supplementation at E100 and E150; however, it again decreased by at E1000 and E1500 diets. Similarly, maximum PER was also observed in moderate vitamin E supplemented diets while; its lower value was recorded in high vitamin supplementation and control. However, % survival rate remained similar among all treatments (Table II).
Proximate composition
The moderate and high doses of supplemented vitamin E showed non-significant effects on the muscle proximate composition of juveniles of L. rohita (Table III) as moisture content, ash content, CP, crude fat of fish muscles remained the same by feeding control and experimental diets.
α-tocopherol level
A linear increase in the α-tocopherol content was observed in the muscle and liver tissues when vitamin E level was increased in test diets (Table IV). However, it was most distinct in the liver sample as compared to muscle samples.
Table II. Effect of moderate and high vitamin E doses on growth performance of L. rohita fingerlings.
|
Growth |
Experimental diets |
PSE |
p-value |
||||
|
E0 |
E100 |
E150 |
E1000 |
E1500 |
|||
|
Initial weight (g) |
3.57 |
3.57 |
3.57 |
3.58 |
3.59 |
0.087 |
|
|
Final weight (g) |
11.68e |
14.22a |
13.71b |
12.35c |
12.03d |
0.065 |
p<0.05 |
|
AWG (g)* |
8.11e |
10.65a |
10.15b |
8.76c |
8.44d |
0.059 |
p<0.05 |
|
Weight gain% |
227.05e |
298.27a |
284.52b |
244.52c |
235.25d |
1.619 |
p<0.05 |
|
SGR (%/day)† |
1.97e |
2.30a |
2.24b |
2.06c |
2.02d |
0.007 |
p<0.05 |
|
Feed intake (g) |
12.92b |
11.07e |
11.83d |
12.62c |
13.22a |
0.066 |
p<0.05 |
|
FCR‡ |
1.59a |
1.04d |
1.17c |
1.44b |
1.57a |
0.013 |
p<0.05 |
|
Hepatosomatic index |
0.48d |
0.69a |
0.69a |
0.66b |
0.59c |
0.004 |
p<0.05 |
|
PER (Protein efficiency ratio) |
1.84d |
2.83a |
2.49b |
2.04c |
1.86d |
0.018 |
p<0.05 |
|
Survival rate % |
98.67 |
100 |
100 |
98.67 |
97.33 |
1.033 |
NS |
In rows the mean value indicates significantly different (p<0.05) results; NS, non-significant (p>0.05); †AWG, Absolute weight gain, †SGR, Specific growth rate, ‡FCR, Feed conversion ratio.
Table III. Influence of moderate and high vitamin E doses on muscle proximate composition (g/kg) of L. rohita fingerlings.
|
Experimental diets |
PSE |
p-value |
|||||
|
E0 |
E100 |
E150 |
E1000 |
E1500 |
|||
|
Moisture |
748.2 |
743.4 |
748.5 |
746.0 |
745.3 |
0.264 |
NS |
|
Crude protein |
178.6 |
177.5 |
177.9 |
178.5 |
179.0 |
0.189 |
NS |
|
Crude Lipids |
25.3 |
31.1 |
32.2 |
27.2 |
27.6 |
0.242 |
NS |
|
Ash |
11.5 |
11.4 |
11.5 |
11.7 |
11.5 |
0.016 |
NS |
All data are derived from three independent repeats; NS= non-significant (p>0.05). Mean values in the same row with no common superscripts indicate significant difference (p<0.05).
Table IV. Effect of moderate and high doses of vitamin E on α-tocopherol level (µg/g) of L. rohita fingerlings.
|
α tocopherol† |
Experimental diets |
PSE |
p-value |
||||
|
E0 |
E100 |
E150 |
E1000 |
E1500 |
|||
|
Liver |
55.5e |
174.0d |
258.6c |
2217.4b |
2940.9a |
2 |
p<0.05 |
|
Muscle |
10.8e |
13.8d |
14.1c |
15.2b |
15.8a |
0.04 |
p<0.05 |
Mean values within a row having no superscripts in common indicate significant difference (p< 0.05).
Thiobarbituric acid reactive substances (TBARS)
The impact of moderate and high dosages of α-tocopherol supplemented experimental diets on TBARS level from liver samples is presented in Table V. The value of TBARS was noted lower in the liver samples of E150 group and progressively increased as the vitamin E content increased. Nevertheless, its highest value was recorded in the E0 diet (control).
Antioxidant enzyme activities
The antioxidative properties of the antioxidant enzymes in various treatments showed significant improvement by including dietary vitamin (Table V). In the liver, minimum activities of antioxidant enzymes (SOD 3.27 U/mg protein, catalase 65.00 U/mg protein and peroxidase 74.43 m U/mg protein) were observed in L. rohita when fed with the diet containing 100 mg/kg α-tocopherol, which progressively enhanced with increase in dietary vitamin E supplementation at E1000 (peroxidase 101.84 m U/mg protein; catalase and SOD, 80.16 and 4.27 U/mg protein, respectively).
Fatty acid profile
Vitamin E supplementation at adequate and high levels resulted in a lower value in monoene’s and saturated fatty acids (SFA) while improving PUFA as compared to control group (Table VI). Similarly, EPA and DHA were increased in response to vitamin E supplementation while arachidonic acid (ARA), DPA, linoleic acid and alpha-linolenic acid were not affected. Besides, n-3/n-6 ratios and EPA/DHA were augmented while the monoene/polyene ratio was decreased in the liver of fish. However, ARA/EPA ratio remained non-significant in the liver. Nonetheless, the alterations between vitamin E at high and adequate doses were insignificant for most individual fatty acids.
DISCUSSION
In the current study, 100 and 150 mg/kg (moderate levels), 1000 and 1500 mg/kg (high levels) and 0 mg/kg vitamin E (control level) were added and fed to fish in CRD. The maximum growth performance was recorded in the 100 mg/kg dietary vitamin E supplemented group. A dose-dependent reduction was detected in other groups with vitamin E supplement. Nevertheless, the control group had a minimal increase in growth. These results demonstrate that the supplementation of vitamin E had a significant influence on fish growth. Nevertheless, depressed growth performance was noted in the test diet containing vitamin E at high dose level, which can result from the radical imbalance of vitamin E and their accumulation. These radicals might play a pro-oxidative role (Hamre et al., 1997). Similarly, from the reports, higher vitamin E doses reduce the phagocytic activity, compromising the immune system (Prasad, 1980). In agreement with our study, an increase in growth performance followed by a decrease at higher supplementation levels have been stated in rohu (Sau et al., 2004), catfish (Abdel-Hameid et al., 2012), beluga (Amlashi et al., 2011), eel (Bae et al., 2013) Ctenopharyngodon idella (Li et al., 2014) juvenile golden pompano (Zhang et al., 2021), Caspian trout (Saheli et al., 2021) and juvenile Sillago sihama (Huang et al., 2020). In contrast to our results, the growth performance showed no effect when fed with vitamin E supplemented diet in Sparus aurata (Ortuno et al., 2000) juvenile Dicentrarchus labrax (Gatta et al., 2000) and haliburt (Tocher et al., 2002). Differences in the observations may be due to variations in size, species, environment in which the fish was cultured, and the presence of other nutrients which interact with vitamin E, such as vitamin C and selenium (Se) (Li et al., 2014). Like our findings, Wang et al. (2009) examined that the survival of sea cucumber did not differ between the supplementation of variable levels of vitamin E.
As can be seen from Table III, the lower HSI was detected in fish when given E0 (Control) diet and increased with the supplementation of moderate level of vitamin (E100 and E150), however, again reduced by high dosage of vitamin E such as E1000 and E1500. The liver is the primary accumulation site of fat-soluble vitamins (vitamin E) and fats. Amlashi et al. (2011) described that the diets without α-tocopherol supplementation might have resulted in the utilization of hepatic fat and deposited vitamin E to overcome the deficiency of dietary vitamin E required for metabolism and growth improvement in fish. Another possibility of lower HSI as a result of E0 feeding may be because of certain liver tissue degeneration by lipid peroxidation. This type of degeneration was also observed during histological investigations in salmonids (Smith, 1979). Similar to our work, some studies have reported increased HSI by adding vitamin E in the beluga (Amlashi et al., 2011) and Sparus aurata L. (Tocher et al., 2002). Meanwhile, a reduction in HSI of sea bream was recorded due to the supplementation of the high (1000 mg/kg) α-tocopherol concentration (Tocher et al., 2002).
Table V. Effect of moderate and high doses of vitamin E on liver TBARS and antioxidant enzymatic activity of L. rohita fingerlings.
|
|
Experimental diets |
PSE |
p value |
||||
|
E0 |
E100 |
E150 |
E1000 |
E1500 |
|||
|
TBARS* |
3.29a |
2.95c |
2.64e |
2.77d |
3.09b |
0.019 |
p<0.05 |
|
SOD† |
5.27a |
3.27e |
3.66d |
4.27c |
4.84b |
0.113 |
p<0.05 |
|
Catalase |
90.48a |
65.00e |
72.49d |
80.16c |
83.30b |
0.436 |
p<0.05 |
|
Peroxidase |
109.58a |
74.43d |
82.91c |
101.84b |
103.94b |
0.884 |
p<0.05 |
NS, non-significant (p>0.05). within rows mean values indicate significantly different results (p<0.05); *TBARS, Thiobarbituric acid-reactive substances (mg g-1 protein); †SOD, Superoxide dismutase (Units/min/mg protein).
Table VI. Effect of moderate and high vitamin E doses on liver fatty acid composition of L. rohita fingerlings.
|
Fatty acids* |
Experimental diets |
||||||
|
E0 |
E100 |
E150 |
E1000 |
E1500 |
PSE |
p value |
|
|
14:0 n-0 |
4.12 |
4.07 |
4.04 |
3.94 |
4.15 |
0.08 |
NS |
|
16:0 n-0 |
8.98 |
8.88 |
8.7 |
9.08 |
9.02 |
0.16 |
NS |
|
18:0 n-0 |
3.68a |
3.13b |
2.95b |
2.96b |
3.11b |
0.06 |
p<0.05 |
|
16:1 n-7 |
7.97a |
7.45b |
7.35b |
7.42b |
7.46b |
0.06 |
p<0.05 |
|
18:1 n-7 |
9.77 |
10.02 |
9.87 |
9.89 |
9.89 |
0.09 |
NS |
|
18:1 n-9 |
14.65a |
14.00b |
14.06b |
14.65a |
14.63a |
0.05 |
p<0.05 |
|
18:2 n-6 |
3.45 |
3.25 |
3.5 |
3.34 |
3.29 |
0.07 |
NS |
|
20:4 n-6 |
6.73 |
6.51 |
6.73 |
6.58 |
6.6 |
0.08 |
NS |
|
18:3 n-3 |
3.11 |
3.21 |
3.33 |
3.16 |
2.96 |
0.12 |
NS |
|
20:5 n-3 |
10.53b |
10.92a |
10.99a |
11.02a |
10.95a |
0.07 |
p<0.05 |
|
22:5 n-3 |
6.85 |
7.18 |
6.92 |
6.98 |
6.93 |
0.08 |
NS |
|
22:6 n-3 |
14.96d |
15.78a |
15.84a |
15.54b |
15.35c |
0.06 |
p<0.05 |
|
Others† |
5.19 |
5.59 |
5.74 |
5.44 |
5.65 |
0.18 |
|
|
Total |
100 |
100 |
100 |
100 |
100 |
||
|
Saturated |
16.78a |
16.08ab |
15.68b |
15.98b |
16.28ab |
0.18 |
p<0.05 |
|
MUS |
32.39a |
31.47c |
31.27c |
31.96b |
31.98b |
0.11 |
p<0.05 |
|
n-3 |
35.46c |
37.10a |
37.08a |
36.70a |
36.19b |
0.12 |
p<0.05 |
|
n-6 |
10.18a |
9.76b |
10.23a |
9.92ab |
9.90ab |
0.08 |
p<0.05 |
|
n-9 |
14.65a |
14.00b |
14.06b |
14.65a |
14.63a |
0.05 |
p<0.05 |
|
ARA/EPA |
0.64 |
0.6 |
0.61 |
0.6 |
0.6 |
0.01 |
NS |
|
EPA/DHA |
0.704ab |
0.692b |
0.693b |
0.708ab |
0.713a |
0.005 |
p<0.05 |
|
n-3/n-6 |
2.42c |
2.65a |
2.64a |
2.51b |
2.47b |
0.02 |
p<0.05 |
|
Monoenes/Polyenes |
0.537a |
0.517bc |
0.509c |
0.521b |
0.526b |
0.003 |
p<0.05 |
MUS, Monounsaturated, NS, non-significant (p>0.05); Mean values in the row indicate significant difference (p<0.05); *Fatty acid denotes the detection of total fatty acids (%); †Others, Sum of 15:0, 15:1, 16:1 n-9, 16:2 n-7, 17:0, 17:1 n-7, 18:2 n-3, 20:1 n-9, 21:5 n-3, 22:1 n-9, 22:2 n-6, 22:4 n-6.
In a contemporary report, vitamin E use in diet presented no effect on the muscle proximate analysis. Similar observations were also recorded in sea bass (Gatta et al., 2000), beluga (Amlashi et al., 2011), Japanese eel (Bae et al., 2013) and Ctenopharyngodon idella (Li et al., 2014). Nonetheless, Abdel-Hameid et al. (2012) demonstrated that in Channa punctatus up to 140 mg/kg vitamin E level enhanced the whole-body protein contents, but at higher levels a significant decrease was observed. They also observed that an increase in vitamin E level decreased the level of fat in fish body, indicating that vitamin E tends to increase the assimilation and transportation of fats, thus contributing to lower the deposition in the storage sites of the fish body (Mourente et al., 2007).
In the existing experimental study, the samples of muscle and liver showed increased α-tocopherol concentration with an increase in vitamin E. A similar increment of vitamin E concentration in tissues was observed by Tocher et al. (2002) in turbot, Gatta et al. (2000) in sea bass, Mourente et al. (2000) in gilthead sea bream, Huang and Huang (2004) in hybrid tilapia, Peng et al. (2009) in black sea bream and Zhong et al. (2008) in Atlantic cod. In addition, a dose-response relationship was found between the vitamin E supplementation in diet and its deposition in fish tissues. This relationship was more noticeable in liver samples than muscle samples (Olsen et al., 1999; Zhang et al., 2007). This shows that dietary vitamin E addition might have altered the resistance of tissues to oxidative stress which ultimately resulted in enhance tissue deposition of α-tocopherol contents.
Due to natural biological antioxidant property, α-tocopherol protects unsaturated fatty acid-rich diets and biological membranes from free radical damage (Huang and Huang, 2004). These unsaturated fatty acids are broken down into malnodialdehyde (MDA) and other aldehydes in the diet or tissues. Measurement of MDA is of great interest as it is an indicator of the peroxidation of lipids. The commonly used analysis for the measurement of MDA is TBARS. The vitamin E supplementation at 100 mg/kg showed the lowest TBARS value which began to improve with the further rise of vitamin E in the diet. However, the control group showed maximum TBARS values. Like previous reports in sea cucumber and sea bream, the TBARS activity was higher when the vitamin E level was high as compared to a low level in diet (Tocher et al., 2002; Wang et al., 2015; Huang et al., 2020; Saheli et al., 2021; Zhang et al., 2021), which shows that high α-tocopherol levels aid in lipid peroxidation. This mechanism can be explained by describing its antioxidant behavior. Lipid peroxidation is a common process involving a chain reaction of free radicals in which lipid peroxyl radicals act as a chain carrier. Vitamin E inhibits the peroxyl radicals from oxidizing the lipids by absorbing hydrogen ions and converting them into α-tocopheroxyl radical (Tappel, 1992). Therefore, vitamin E has a scavenging property because it captures lipid peroxyl radicals before their attack on any other lipid-based substrates. Generally, α-tocopheroxyl radical forms a stable product by reacting with another α-tocopheroxyl radical or is stabilized in tissues with vitamin C and glutathione (Packer et al., 1979; Sato et al., 1990). Nevertheless, this is not the case when vitamin E is supplemented beyond the required doses. In such cases, vitamin C becomes inadequate to lower the radicles of α-tocopheroxyl, being generated in physiological processes. These radicals of α-tocopheroxyl start a free radical chain reaction by attacking the lipids (Mukai and Okauchi, 1989). The more radicals of lipid peroxyl may be generated due to pro-oxidative impact, resulting in an increase in tissue TBARS levels.
Reactive oxygen species are produced even during normal metabolic activities, and their elimination is indispensable for adequate functioning and survival of the organism. Catalase, SOD, and glutathione peroxidase perform this enzymatic activity and form the antioxidant defense system of the organism. The SOD and catalase are superoxide and hydrogen peroxide scavengers. The activity of glutathione peroxidases acts on lipid hydroperoxides and hydrogen peroxide (H2O2). While, in this study, the reduced enzymatic activities (catalase, peroxidase and SOD) were observed at a 100 mg/kg supplementation. The decreased SOD activities following treatment of vitamin E could be due to a reduction in the O-2 substrate and production. Likewise, in our results, Palace et al. (1993) also observed decreased activities of these enzymes by feeding a vitamin E supplemented diet to rainbow trout. Puangkaew et al. (2005) demonstrated that the dietary vitamin E supplementation lowered the enzyme activities in the kidney and plasma of rainbow trout. Nevertheless, this study also revealed that with the increase in the supplemental level of vitamin E these enzyme activities were also increased. Furthermore, these enzyme activities showed a more profound increase at a high dosage of vitamin E. The results of these parameters were in favor of the hypothesis that adding excessive vitamin E in the biological entities accelerates the rate of lipid peroxidation (Kaewsrithong et al., 2001). Therefore, Dandapat et al. (2000) explained that the induction of antioxidant enzymes might be an adaptive physiological response for minimizing the oxidative stress. Comparable findings have been reported by Puangkaew et al. (2004) in Oncorhynchus mykiss, Wang et al. (2015) in Apostichopus japonicus, Dandapat et al. (2000) in freshwater prawn and Zhang et al. (2007) in Sparus microcephalus, Zhang et al., (2021) in juvenile golden pompano, Saheli et al. (2021) in Caspian trout and Huang et al. (2020) in juvenile Sillago sihama.
This clearly shows that vitamin E addition at adequate levels in the fish diet may provide sufficient protection from oxidative stress, compared to excessive vitamin E added diets. On the other hand, Tocher et al. (2002) noted that the vitamin E doses at 1000 mg kg-1 supplementation decreased the enzymatic activity (e.g. glutathione peroxidase and catalase) in halibut than 100 mg/kg α-tocopherol.
The current study found that the monoenes and SFA were present in reduced amount when the fish was provided with vitamin E in their diets while the PUFA were maximum. In fish, vitamin E level supplemented at high level, on the other hand, showed no response against the percentage of these fatty acids. The current findings indicate that the deficiency of vitamin E influences fatty acid profile, and the subsequent addition of dietary vitamin E is sufficient to fulfil the fatty acids requirement of fish. In this case, the same dietary lipid source, cod liver oil, is also responsible for the identical profile of fatty acids in all supplemented diets having graded levels of vitamin E. The dietary oil dependent fatty acid composition in fish muscle was also recorded by Pirini et al. (2000). According to Watanabe et al. (1977), vitamin E supplementation alters the composition of fatty acids; however, supplementation above requirement level has a minimal impact on fatty acid profile. The ability of freshwater organisms to bio-transform linolenic acid (18:3 n-3) to 20:5 and 22:6 HUFA by using desaturases as well as elongases (Sargent et al., 1989) can also explain the existence of higher levels of HUFA (20:5 and 22:6) in the current research. Since the maximum concentration of lipids, SFA and MUFA were present in the processed feed, but they are inadequate in n-3 HUFA, the n-3 HUFA percentage was reduced in farmed fish than that of wild fish (Ackman and Takeuchi, 1986). However, the present research work unveiled that the presence of fatty acid (%) in tissues of the liver proved that the test diets containing cod liver oil (marine fish) and an adequate concentration of vitamin E level could ensure a fatty acids pattern, especially n-3 HUFA, in cultured fish that is very close to that observed in wild aquatic animals (Sharma et al., 2010).
Tocher et al. (2002) observed similar modifications in the composition of fatty acid of halibut when fed with low dose of vitamin E combined with oxidized oil. Furthermore, Bai and Lee (1998) performed an experiment on Korean rockfish and reduced PUFA levels and decreased PUFA/SFA ratio was observed when vitamin E containing low level was supplemented in their diets. Meanwhile, the Atlantic halibut showed decreased n-3 fatty acid levels in muscles and liver samples when fed with diet having no supplementation of vitamin E (Lewis-McCrea and Lall, 2007). However, in turbot liver, the PUFA had no significant effect when dietary α-tocopheryl was added (Tocher et al., 2002). Similarly, the Atlantic salmon’s α-tocopheryl acetate level had no impact on fatty acids of liver tissues and fillet quality, as reported by Scaife et al. (2000).
CONCLUSION
In conclusion, the inclusion of vitamin E in juveniles of L. rohita improved the growth, antioxidant status and fatty acid profile of fish while reducing lipid peroxidation. Meanwhile, vitamin E addition at high doses exhibited the effect of pro-oxidative, which enhanced the rate of lipid peroxidation, hence, lead to a decreased growth rate.
ACKNOWLEDGEMENT
This study received no particular support from funding agencies in the public, commercial, or non-profit sectors.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdel-Hameid, N.A., Abidi, S.F. and Khan, M.A., 2012. Dietary vitamin E requirement for maximizing the growth, conversion efficiency, biochemical composition and haematological status of fingerling (Channa punctatus) Aquacult. Res., 43: 226-238. https://doi.org/10.1111/j.1365-2109.2011.02819.x
Ackman, R.G. and Takeuchi, T., 1986. Comparison of fatty acids and lipids of smolting hatchery-fed and wild Atlantic salmon (Salmo salar). Lipids, 21: 117-120. https://doi.org/10.1007/BF02534431
Amlashi, A.S., Falahatkar, B., Sattari, M. and Gilani, M.T., 2011. Effect of dietary vitamin E on growth, muscle composition, hematological and immunological parameters of sub-yearling beluga (Huso huso L). Fish. Shellfish Immunol., 30: 807-814. https://doi.org/10.1016/j.fsi.2011.01.002
Anwar, F., Hussain, A.I., Ashraf, M., Jamail, A. and Iqbal, S., 2006. Effect of salinity on yield and quality of Moringa oleifera seed oil. Grasas. Y Aceites., 57: 394-401. https://doi.org/10.3989/gya.2006.v57.i4.65
AOAC, 1995. Official methods of analysis. 15th Ed., Association of Official Analytical Chemist, Washington, D.C. USA., pp. 1094.
Bae, J.Y., Park, G.H., Yoo, K.Y., Lee, J.Y., Kim, D.J. and Bai, S.C., 2013. Evaluation of optimum dietary vitamin E requirements using DL-α-tocopheryl acetate in the juvenile eel (Anguilla japonica). J. appl. Ichthyol., 29: 213-217. https://doi.org/10.1111/jai.12001
Bai, S.C. and Gatlin III, D.M., 1993. Dietary vitamin E concentration and duration of feeding affect tissue α-tocopherol concentrations of channel catfish (Ictalurus punctatus). Aquaculture, 113: 129-135. https://doi.org/10.1016/0044-8486(93)90346-Z
Bai, S.C. and Lee, K.J., 1998. Different levels of dietary DL-α-tocopheryl acetate affect the vitamin E status of juvenile Korean rockfish (Sebastes schlegeli). Aquaculture., 161: 405-414. https://doi.org/10.1016/S0044-8486(97)00288-3
Chaiyapechara, S., Casten, M.T., Hardy, R.W. and Dong, F.M., 2003. Fish performance, fillet characteristics, and health assessment index of rainbow trout (Oncorhynchus mykiss) feed diets containing adequate and high concentrations of lipid and vitamin E. Aquaculture, 219: 715-738. https://doi.org/10.1016/S0044-8486(03)00025-5
Chance, B. and Maehly, A.C., 1955. Assay of catalases and peroxidases. Meth. Enzymol., 2: 764-775. https://doi.org/10.1016/S0076-6879(55)02300-8
Civello, P.M., Martinez, G.A., Chaves, A.R. and Anon, M.C., 1995. Peroxidase from strawberry fruit (Fragaria ananassa Duch.), partial purification and determination of some properties. J. Agric. Fd. Chem., 43: 2596-2601. https://doi.org/10.1021/jf00058a008
Dandapat, J., Chainy, G.B. and Rao, K.J., 2000. Dietary vitamin-E modulates antioxidant defence system in giant freshwater prawn, Macrobrachium rosenbergii. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol., 127: 101-115. https://doi.org/10.1016/S0742-8413(00)00132-8
Fatima, M., Afzal, M. and Shah, S.Z.H., 2019. Effect of dietary oxidized oil and vitamin E on growth performance, lipid peroxidation and fatty acid profile of (Labeo rohita) fingerlings. Aquacult. Nutr., 25: 281-291. https://doi.org/10.1111/anu.12851
Gao, J., Koshio, S., Ishikawa, M., Yokoyama, S., Mamauag, R.E. and Han, Y., 2012a. Effects of dietary oxidized fish oil with vitamin E supplementation on growth performance and reduction of lipid peroxidation in tissues and blood of red sea bream (Pagrus major). Aquaculture, 356: 73-79. https://doi.org/10.1016/j.aquaculture.2012.05.034
Gao, J., Koshio, S., Ishikawa, M., Yokoyama, S., Ren, T., Komilus, C.F. and Han, Y., 2012b. Effects of dietary palm oil supplements with oxidized and non-oxidized fish oil on growth performances and fatty acid compositions of juvenile Japanese sea bass (Lateolabrax japonicas). Aquaculture, 324: 97-103. https://doi.org/10.1016/j.aquaculture.2011.10.031
Gatta, P.P., Pirini, M., Testi, S., Vignola, G. and Monetti, P.G., 2000. The influence of different levels of dietary vitamin E on sea bass (Dicentrarchus labrax) flesh quality. Aquacult. Nutr., 6: 47-52. https://doi.org/10.1046/j.1365-2095.2000.00127.x
Giannopolitis, C.N. and Ries, S.K., 1977. Superoxide dismutases: I. Occurrence in higher plants. Pl. Physiol., 59: 309-314. https://doi.org/10.1104/pp.59.2.309
Hamre, K., 2011. Metabolism, interactions, requirements and functions of vitamin E in fish. Aquacult. Nutr., 17: 98-115. https://doi.org/10.1111/j.1365-2095.2010.00806.x
Hamre, K., Waagbo, R., Berge, R.K. and Lie, O., 1997. Vitamins C and E interact in juvenile Atlantic salmon (Salmo salar L.). Free Rad. Biol. Med., 22: 137-149. https://doi.org/10.1016/S0891-5849(96)00281-X
Huang, C.H. and Huang, S.L., 2004. Effect of dietary vitamin E on growth, tissue lipid peroxidation, and liver glutathione level of juvenile hybrid tilapia (Oreochromis niloticus × O. aureus) fed oxidized oil. Aquaculture, 237: 381-389. https://doi.org/10.1016/j.aquaculture.2004.04.002
Huang, Q.C., Zhang, S., Du, T., Yang, Q.H., Chi, S.Y., Liu, H.Y., Yang, Y.Z., Dong, X.H. and Tan, B.P., 2020. Effects of dietary vitamin E on growth, immunity and oxidation resistance related to the Nrf2/Keap1 signalling pathway in juvenile Sillago sihama. Anim. Feed Sci. Technol., 262: 114403. https://doi.org/10.1016/j.anifeedsci.2020.114403
Ito, T., Murata, H., Tsuda, T., Yamada, T., Yamauchi, K., Ukawa, M., Yamaguchi, T., Yoshida, T. and Sakai, T., 1999. Effects of α-tocopherol levels in extrusion pellets on in vivo lipid peroxidation levels and antioxidant activities in cultured yellowtail (Seriola quinqueradiata) injected with the causative bacteria of fish jaundice. Fish. Sci., 65: 679-683. https://doi.org/10.2331/fishsci.65.679
IUPAC (International Union of Pure and Applied Chemistry), 1987. Standard methods for the analysis of oils, fats and derivatives, 7th Revision, enlarged Ed., (eds. C. Paquot and A. Hautfenne). Blackwell Scientific, London.
Kaewsrithong, J., Ohshima, T., Ushio, H., Nagasaka, R., Maita, M. and Sawada, M., 2001. Effects of an excess dose of dietary α-tocopherol on hydroperoxide accumulation and erythrocyte osmotic fragility of sweet smelt (Plecoglossus altivelis) (Temminck et Schlegel). Aquacult. Res., 32: 191-198. https://doi.org/10.1046/j.1355-557x.2001.00056.x
Lewis-McCrea, L.M. and Lall, S.P., 2007. Effects of moderately oxidized dietary lipid and the role of vitamin E on the development of skeletal abnormalities in juvenile Atlantic halibut (Hippoglossus hippoglossus). Aquaculture, 262: 142-155. https://doi.org/10.1016/j.aquaculture.2006.09.024
Li, J., Liang, X.F., Tan, Q., Yuan, X., Liu, L., Zhou, Y. and Li, B., 2014. Effects of vitamin E on growth performance and antioxidant status in juvenile grass carp (Ctenopharyngodon idellus). Aquaculture, 430: 21-27. https://doi.org/10.1016/j.aquaculture.2014.03.019
Mourente, G., Bell, J.G. and Tocher, D.R., 2007. Does dietary tocopherol level affect fatty acid metabolism in fish? Fish. Physiol. Biochem., 33: 269-280. https://doi.org/10.1007/s10695-007-9139-4
Mourente, G., Diaz-Salvago, E., Tocher, D.R. and Bell, J.G., 2000. Effects of dietary polyunsaturated fatty acid/vitamin E (PUFA/tocopherol ratio on antioxidant defence mechanisms of juvenile gilthead sea bream (Sparus aurata L., Osteichthyes, Sparidae). Fish. Physiol. Biochem., 23: 337-351.
Mukai, K. and Okauchi, Y., 1989. Kinetic study of the reaction between tocopheroxyl radical and unsaturated fatty acid esters in benzene. Lipids, 24: 936-939. https://doi.org/10.1007/BF02544537
Olsen, R.E., Lovaas, E. and Lie, O., 1999. The influence of temperature, dietary polyunsaturated fatty acids, α-tocopherol and spermine on fatty acid composition and indices of oxidative stress in juvenile Arctic char (Salvelinus alpinus L.). Fish Physiol. Biochem., 20: 13-29.
Ortuno, J., Esteban, M.A. and Meseguer, J., 2000. High dietary intake of α-tocopherol acetate enhances the non-specific immune response of gilthead seabream (Sparus aurata L.). Fish. Shellfish Immunol., 10: 293-307. https://doi.org/10.1006/fsim.1999.0238
Packer, J.E., Slater, T. and Willson, R.L., 1979. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature, 278: 737-738. https://doi.org/10.1038/278737a0
Palace, V.P., Majewski, H.S. and Klaverkamp, J.F., 1993. Interactions among antioxidant defenses in liver of rainbow trout (Oncorhynchus mykiss) exposed to cadmium. Can. J. Fish. aquacult. Sci., 50: 156-162. https://doi.org/10.1139/f93-018
Peng, S., Chen, L., Qin, J.G., Hou, J., Yu, N., Long, Z., Li, E. and Ye, J., 2009. Effects of dietary vitamin E supplementation on growth performance, lipid peroxidation and tissue fatty acid composition of black sea bream (Acanthopagrus schlegeli) fed oxidized fish oil. Aquacult. Nutr., 15: 329-337. https://doi.org/10.1111/j.1365-2095.2009.00657.x
Pirini, M., Gatta, P.P., Testi, S., Trigari, G. and Monetti, P.G., 2000. Effect of refrigerated storage on muscle lipid quality of sea bass (Dicentrarchus labrax) fed on diets containing different levels of vitamin E. Fd. Chem., 68: 289-293. https://doi.org/10.1016/S0308-8146(99)00190-9
Prasad, J.S., 1980. Effect of vitamin E supplementation on leukocyte function. Am. J. clin. Nutr., 33: 606-608. https://doi.org/10.1093/ajcn/33.3.606
Puangkaew, J., Kiron, V., Satoh, S. and Watanabe, T., 2005. Antioxidant defense of rainbow trout (Oncorhynchus mykiss) in relation to dietary n-3 highly unsaturated fatty acids and vitamin E contents. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 140: 187-196. https://doi.org/10.1016/j.cca.2005.01.016
Puangkaew, J., Kiron, V., Somamoto, T., Okamoto, N., Satoh, S., Takeuchi, T. and Watanabe, T., 2004. Nonspecific immune response of rainbow trout (Oncorhynchus mykiss Walbaum) in relation to different status of vitamin E and highly unsaturated fatty acids. Fish. Shellfish. Immunol., 16: 25-39. https://doi.org/10.1016/S1050-4648(03)00028-7
Saheli, M., Islami, H.R., Mohseni, M. and Soltani, M., 2021. Effects of dietary vitamin E on growth performance, body composition, antioxidant capacity, and some immune responses in Caspian trout (Salmo caspius). Aquacult. Rep., 21: 100857. https://doi.org/10.1016/j.aqrep.2021.100857
Sargent, J., Henderson, R.J. and Tocher, D.R., 1989. The lipids. In: Fish nutrition (ed. J.E. Halver). Academic Press, San Diego. pp. 153-218.
Sato, K., Niki, E. and Shimasaki, H., 1990. Free radical-mediated chain oxidation of low-density lipoprotein and its synergistic inhibition by vitamin E and vitamin C. Arch. Biochem. Biophys., 279: 402-405. https://doi.org/10.1016/0003-9861(90)90508-V
Sau, S.K., Paul, B.N., Mohanta, K.N. and Mohanty, S.N., 2004. Dietary vitamin E requirement, fish performance and carcass composition of rohu (Labeo rohita) fry. Aquaculture, 240: 359-368. https://doi.org/10.1016/j.aquaculture.2004.02.008
Scaife, J.R., Onibi, G.E., Murray, G.I., Fletcher, T.C. and Houlihan, D.F., 2000. Influence of α-tocopherol acetate on the short-and long-term storage properties of fillets from Atlantic salmon (Salmo salar) fed a high lipid diet. Aquacult. Nutr., 6: 65-71. https://doi.org/10.1046/j.1365-2095.2000.00128.x
Scrimshaw, N.S. and San Giovanni, J.P., 1997. Synergism of nutrition, infection, and immunity: An overview. Am. J. clin. Nutr., 66: 464S-477S. https://doi.org/10.1093/ajcn/66.2.464S
Sharma, P., Kumar, V., Sinha, A.K., Ranjan, J., Kithsiri, H.M. and Venkateshwarlu, G., 2010. Comparative fatty acid profiles of wild and farmed tropical freshwater fish rohu (Labeo rohita). Fish. Physiol. Biochem., 36: 411-417. https://doi.org/10.1007/s10695-009-9309-7
Smith, C.E., 1979. The prevention of liver lipoid degeneration (ceroidosis) and microcytic anaemia in rainbow trout Salmo gairdneri Richardson fed rancid diets: A preliminary report. J. Fish Dis., 2: 429-437. https://doi.org/10.1111/j.1365-2761.1979.tb00394.x
Tappel, A.L., 1992. Vitamin E. In: The vitamins (ed. G. F. Combs), Academic Press, New York. pp. 179-203.
Tocher, D.R., Mourente, G., Van der Eecken, A., Evjemo, J.O., Diaz, E., Bell, J.G., Geurden, I., Lavens, P. and Olsen, Y., 2002. Effects of dietary vitamin E on antioxidant defense mechanisms of juvenile turbot (Scophthalmus maximus L.), halibut (Hippoglossus hippoglossus L.) and sea bream (Sparus aurata L.). Aquacult. Nutr., 8: 195-207. https://doi.org/10.1046/j.1365-2095.2002.00205.x
Tocher, D.R., Mourente, G., Van der Eecken, A., Evjemo, J.O., Diaz, E., Wille, M., Bell, J.G. and Olsen, Y., 2003. Comparative study of antioxidant defence mechanisms in marine fish fed variable levels of oxidized oil and vitamin E. Aquacult. Int., 11: 195-216. https://doi.org/10.1046/j.1365-2095.2002.00205.x
Tokuda, M. and Takeuchi, M., 1999. Effects of excess doses of α-tocopherol on lipid in serum and muscle of rainbow trout. Fish. Sci., 65: 496-497. https://doi.org/10.2331/fishsci.65.496
Wang, J., Xu, Y., Li, X., Li, J., Bao, P., Che, J., Li, S. and Jin, L., 2015. Vitamin E requirement of sea cucumber (Apostichopus japonicus) and its’ effects on nonspecific immune responses. Aquacult. Res., 46: 1628-1637. https://doi.org/10.1111/are.12324
Wang, T., Sun, Y., Jin, L., Xu, Y., Wang, L., Ren, T. and Wang, K., 2009. Enhancement of non-specific immune response in sea cucumber (Apostichopus japonicus) by (Astragalus membranaceus) and its polysaccharides. Fish. Shellfish Immunol., 27: 757-762. https://doi.org/10.1016/j.fsi.2009.09.002
Watanabe, T., Takeuchi, T., Matsui, M., Ogino, C. and Kawabata, T., 1977. Effect of a-tocopherol deficiency on carp: VII. The relationship between dietary levels of linoleate and a tocopherol requirement. Bull. Jap. Soc. Sci. Fish., 43: 935-946. https://doi.org/10.2331/suisan.43.935
Zhang, G.R., Xu, C., You, C.H., Wang, S.Q., Xie, D.Z., Hasan, A.M., Ma, Y.C. and Li, Y.Y., 2021. Effects of dietary vitamin E on growth performance, antioxidant capacity and lipid metabolism of juvenile golden pompano Trachinotus ovatus. Aquacult. Nutr., 27: 2205-2217. https://doi.org/10.1111/anu.13355
Zhang, X.D., Wu, T.X., Cai, L.S. and Zhu, Y.F., 2007. Effects of α-tocopheryl acetate supplementation in preslaughter diet on antioxidant enzyme activities and fillet quality of commercial-size (Sparus macrocephalus). J. Zhejiang Univ. Sci. B., 8: 680-685. https://doi.org/10.1631/jzus.2007.B0680
Zhong, Y., Lall, S.P. and Shahidi. F., 2008. Effects of dietary oxidized oil and vitamin E on the growth, blood parameters and body composition of juvenile Atlantic cod (Gadus morhua) (Linnaeus, 1758). Aquacult. Res., 39: 1647-1657. https://doi.org/10.1111/j.1365-2109.2008.02038.x
Zhou, Q.C., Wang, L.G., Wang, H.L., Wang, T., Elmada, C.Z. and Xie, F.J., 2013. Dietary vitamin E could improve growth performance, lipid peroxidation and non-specific immune responses for juvenile cobia (Rachycentron canadum). Aquacult. Nutr., 19: 421-429. https://doi.org/10.1111/j.1365-2095.2012.00977.x
To share on other social networks, click on any share button. What are these?










