Some Reproductive Characteristics of Metapenaeus affinis (H. Milne Edwards, 1837) in Izmir Bay (Eastern Aegean Sea, Turkey)
Some Reproductive Characteristics of Metapenaeus affinis (H. Milne Edwards, 1837) in Izmir Bay (Eastern Aegean Sea, Turkey)
Gülnur Metin* and İlker Aydin
Department of Fishing Technology, Ege University Faculty of Fisheries, 35100 Bornova, Izmir, Turkey
ABSTRACT
In this study, some reproductive characteristics (reproductive season, first maturation size (LM50) and sex ratio) of the jinga shrimp, Metapenaeus affinis (H. Milne Edwards, 1837), in Izmir Bay (Aegean Sea, Turkey) were investigated. A total of 2894 M. affinis were obtained from commercial fishermen between November 2008 and January 2011. The total length and weight of the females or males were measured as 7.3–17.5 cm and 2.6-38.9 g or 7.8–15.4 cm and 2.6-41.6 g, respectively. The samples were composed of 56.6% females and 46.6% males, with a female to male ratio of 1:0.77. The spawning period was determined from May to October. The total lengths at first maturity of females and males were 10.64 cm and 10.16 cm, respectively.
Article Information
Received 04 October 2016
Revised 16 December 2016
Accepted 07 January 2017
Available online 25 August 2017
Authors’ Contributions
GM carried out the study and data collection. IA helped in the laboratory work.
Key words
Metapenaeus affinis, The jinga shrimp, Spawning season, LM50, Reproduction.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.5.sc2
* Corresponding author: [email protected]
0030-9923/2017/0005-1913 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
The jinga shrimp, Metapenaeus affinis (H. Milne Edwards, 1837), inhabiting the “Gulf” and the Arabian Sea from the Gulf of Oman to South India. It is also present in Sri Lanka, Philippines and Taiwan Island. It is found in the depths of about 55 m (occasionally in deeper water to 90 m) from the coastline, mainly on mud or sandy-mud (Fischer and Bianchi, 1984).
Endogenous and environmental factors (e.g., temperature, photoperiod, salinity, etc.) affect the reproductive cycle in crustaceans (Knudsen, 1964; Little, 1968; Pillay and Nair, 1971; Wenner et al., 1974; Campbell and Fielder, 1986; Saigusa, 1992; Company and Sarda, 1997; Flores and Negreiros-Fransozo, 1998; Litulo, 2005). Actually, these phenomena may be restricted to decapod’s spawning season for a few months or year-round (Subrahmanyam, 1963; Pillay and Nair, 1971; Ramamurty et al., 1975; George, 1987; Pinheiro and Fransozo, 2002; Gerami et al., 2013).
Metapenaeus affinis was first noted by Aydın et al. (2009) as an alien species in Izmir Bay (Aegean Sea, Turkey). However, Aydın and Metin (2010) and Ünal et al. (2012) reported that the jinga shrimp has attained the status of economic species and it can be intensively cultured between April and October. Aydın and Metin (2010) and Ünal et al. (2012) also reported that catch rates per a boat ranges between 3 and 26 kg. The present study was performed to investigate reproductive season, first maturation size (LM50) and sex ratio of M. affinis in Izmir Bay (Aegean Sea, Turkey).
Material and methods
A total of 2894 individuals of M. affinis were obtained from commercial fishermen in Izmir Bay in the Middle Eastern part of the Aegean Sea. The water depth of the fishing areas varied between 6-16 m. Duplex or single prawn trammel nets were used for the fishing operations. The mesh size of the inner net and the mesh numbers in depth of the gear were 40 mm and 75 or 100 meshes (single or duplex), respectively. After the samplings from November 2008–January 2011, the total lengths (TL), body weights (W) and gonad weights (Wg) of 1656 females and 1238 males were recorded. Spawning period was established from the variations of the monthly values of the gonadosomatic index (GSI) (Ricker, 1975):
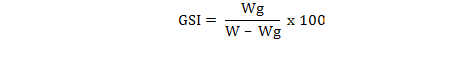
Sex and maturity stages were determined by macroscopic analysis of the gonads. The sex ratio (female:male) and the mean length for sexes were calculated. The maturity stages were assessed according to Lumare and Scordalle (2001) using scale immature, stage I; resting, stage II; developing, stage III; ripe, stage IV and spent, stage V. The chi-square test (χ2) and t-test with a confidence level of 5% were used to detect differences in the sex ratio and to check for differences in mean length of the sexes, respectively.
For calculating the first maturity length (LM50), we used length of the 50% of the population’s gonad size shown in the study by Somerton (1980). Logit model was used for the calculations (İlkyaz et al., 1998).
p(l)=[e(a+bl)/1+e(a+bl)]
LM50= (-a/b)
Where, p(l) is the proportion of matures in each length class (%), l is the shrimp length (cm), LM50 is the mean length at sexual maturity (50%, cm), a is intercept and b is slope.
Results
The total lengths and weights of the females and males were measured as 7.3–17.5 cm and 2.6-38.9 g or 7.8–15.4 cm and 2.6-41.6 g, respectively. In both sexes the smallest individuals were found in November and the largest ones were found in September. The most abundance lengths were determined as 12 cm with 27.23% for the females and 11 cm with 28.27% for the males (Fig. 1). Mean lengths were calculated for the females and the males as 12.1±0.05 cm and 11.2±0.04 cm, respectively. In the population, the male individuals were usually smaller than the females. Up to 11 cm male specimens were found dominant; above this length the the females became dominant.
The female population was 56.6%, whereas the males constituted 46.6% of the population. The sex ratio of the jinga shrimps was estimated 1:0.77. It was determined that the females were dominant in November (1:0.30) and males were dominant in September (1:2.22). Both the sexes were equally distributed in June (1:1.24). Figure 2 shows month wise GSI values. The reproduction period extended from May to October. The gonad maturity started in May and the most mature gonads (Stage 4) were observed in August (Fig. 3).
Females (643) and males (897) were examined for the minimum spawning size during the spawning period. First mature females and males had total length of 9.8 cm and 9 cm, respectively. LM50 values of the females and the males were detected as 10.64 cm (a=-16.124, b= 1.515, R2=0.98) and 10.16 cm (a=-14.247, b=1.393, R2=0.98) in total length, respectively (Fig. 4).
Discussion
Maximum total length of the M. affinis ranges 17.1-18.6 cm in the females and 14.6-16.5 cm in the males (Pillay and Nair, 1971; Fischer and Bianchi, 1984; George, 1987). The present total length values of M. affinis agreed with those of the previous studies. Furthermore, the fluctuations of sex ratio in the jinga shrimp may be related to the differences in mortality rates between the two sexes or because of differences in behavioral characteristics such as migration (Kim, 2005), and/or the higher natural mortality of males may deviate in favor of females in most years (Cha et al., 2004; Gerami et al., 2013), and/or the greater vulnerability of females to fishing due to their size (Da Costa et al., 2010).
Penaeidae are able to have one or two peaks of spawning in their life (Garcia, 1977; O’Connor, 1979) and that M. affinis had at least two spawning seasons in life (Treece, 2000). Likewise, the current results demonstrate that the reproductive season of M. affinis occurred from May to October with two peaks (June and September) and the reproductive stages 3 and 4 were the dominant stages. Conversely, Mathews (1989) indicated two peaks of spawning seasons in spring and autumn for this species in Kuwaiti waters. Rao (1968) reported spawning season from October to March or April. For Malabar Coast it has been reported to be between January and March (Subrahmanyam, 1963). Pillay and Nair (1971) reported period of the breeding of M. affinis from August to April, with major and minor peaks in December and April, respectively, along the south-west coast of India. For Pakistan’s inshore waters, Ayub and Ahmed (2002) showed two spawning peaks, the first one in winter-spring (February-May) and second one in July, September and/or October. Meanwhile, Gerami et al. (2013) notified that the peak spawning season occurs in late winter and early spring (March-April) in M. affinis, and it has continuous spawning over the year. The differences in the spawning period are probably because of the various regional and ecological factors.
Jinga shrimp prefers to reproduce within 5-15 meters of coastal areas. In Bombay waters there are indications that the species prefers soft muddy, rich in plankton and shallow coastal areas for mating and spawning (Shaikmahmud and Tembe, 1960). Hall (1962) observed that in Malaysian waters the species breed very close to inshore. Subrahmanyam (1963) showed that a sizable proportion of M. affinis start appearing in the coastal waters and is mature in January. Also studying the breeding of the species, it has been observed in Calicut and on the Southwest Coast of India, the species moves to offshore areas for spawning (25-45 m). In the present study, M. affinis prefers coastal areas to reproduce since the specimens are found in 6-16 meters in İzmir Bay during the spawning season. These findings are similar to those of Shaikmahmud and Tembe (1960), Hall (1962) and Subrahmanyam (1963). Our study shows that, the peak recruitment of M. affinis to fishing grounds occurred in November because of the smallest individuals in the both sexes. On the other hand, George (1987) reported that recruitment of bigger M. affinis specimens into the fishery starts in October, and the smaller individuals get recruited from December to January. Mohammed (1995) stressed that peak recruitment periods of both sexes in M. affinis to fishing grounds occurs at different times; males: from February-March to April-May and from September to October; females: from March-June, and from July to August. It is thought that these differences may stem from the various regional and ecological factors.
First maturity sizes in M. affinis found as 120 mm and 88.6 mm by Subrahmanyam (1963) and Rao (1968), respectively. Ramamurthy et al. (1975) recorded that the mature female and male individuals of M. affinis seen after 93 mm and 98 mm, respectively, and the first spawning size calculated as 116 mm. In the present study, first mature females and males were found as 9.8 cm and 9 cm in total length, respectively. LM50 values of the females and the males were detected as 10.64 cm and 10.16 cm in total length, respectively. So, it is thought that the present results are similar to the previous findings.
Conclusion
This is the first study on reproductive biology of M. affinis (H. Milne Edwards, 1837) for Mediterranean Region (Izmir Bay in Aegean Sea, Turkey). The current study has demonstrated that M. affinis reproduces between May and October in İzmir Bay, and is able to adapt to unfamiliar conditions successively.
Statement of conflict of interest
The authors declare no conflict of interest regarding this paper.
References
Aydin, İ., Bakır, K. and Galil., B.S., 2009. Crustaceana, 82: 1091-1095. https://doi.org/10.1163/156854008X380264
Aydın, İ. and Metin, C., 2010. Int. Mer. Medit., 39: 434.
Ayub, Z. and Ahmed, M., 2002. Indian J. mar. Sci., 31: 119-124.
Campbell, G.R. and Fielder, D.R., 1986. Proc. R. Soc. Queensl., 97: 79-87.
Cha, H.K., Choi, J.H. and Oh, C.W., 2004. J. Crust. Biol., 24: 93-100. https://doi.org/10.1651/C-2399
Company, J.B. and Sarda, F., 1997. Mar. Ecol. Prog. Ser., 148: 49-58. https://doi.org/10.3354/meps150049
Da Costa, R.C., Branco, J.O. and Machado, I.F., 2010. J. Mar. boil. Assoc. U.K., 90: 1-7.
Fischer, W. and Bianchi, G., 1984. FAO species identification sheets for fisheries purposes Western Indian Ocean (Fishing area 51), Volume 5, FAO, Rome.
Flores, A.A.V. and Negreiros-Fransozo, M.L., 1998. Inverteb. Reprod. Develop., 34: 149-155. https://doi.org/10.1080/07924259.1998.9652647
Garcia, S., 1977. Biologie et dynamique des populations de crevettes roses (Penaeus duorarum notialis Perez-Farfante, 1967), Travauxet Documents de l’ORSTOM, pp. 271.
George, M.J., 1987. Synopsis of biological data on the Penaeid prawn Metapenaeus affinis (H. M. Edwards, 1837), FAO Fisheries Synopsis, No: 98.
Gerami, M.H., Ghorbani, R., Paighmabari, S.Y. and Momeni, M., 2013. Int. J. aquat. Biol., 1: 48-54.
Hall, D.N.F., 1962. Fish. Publs colon. off., 17: 229.
İlkyaz, A.T., Metin, C. and Kınacıgil, H.T., 1998. E.U. J. Fish. aquat. Sci., 15: 305-314.
Kim, S. 2005. J. Crust. Biol., 25: 226-232. https://doi.org/10.1651/C-2527
Knudsen, J.W., 1964. Pacif. Sci., 18: 3-33.
Little, G., 1968. Crustaceana, 2: 19-26.
Litulo, C., 2005. Raffles Bull. Zool., 53: 115-118.
Lumare, F. and Scordella, G., 2001. In: Proc. İnt. Works. La Pesca di Paneaus (Melicertus) kerathurus nella costa orientale Italiana e nella lagune, stato attuale, problemi e prospettive, March 16, 2001, Lecce, Italy, pp. 2-14.
Mathews, C.P., 1989. Kuwait Bull. Mar. Sci., 10: 3-36.
Mohammed, H.M., 1995. NAGA, the ICLARM Quart., 60: 38-41.
O’Connor, C., 1979. Aquaculture, 16: 153-162. https://doi.org/10.1016/0044-8486(79)90146-7
Pillay, K.K. and Nair, N.B., 1971. Mar. Biol., 11: 152-166.
Pinheiro, M.A.A. and Fransozo, A., 2002. J. Crust. Biol., 22: 416-428. https://doi.org/10.1651/0278-0372(2002)022[0416:ROTSSC]2.0.CO;2
Ramamuthy, S., Kurup, N.S. and Annigeri, G.G., 1975. Indian J. Fish., 22: 243-254.
Rao, P.V., 1968. FAO Fish. Rep., 2: 285–302
Ricker, W.E., 1975. Bull. Fish. Res. Bd. Can., 191: 1-382.
Saigusa, M., 1992. Biol. Bull., 182: 257-264. https://doi.org/10.2307/1542119
Shaikmahmud, F.S. and Tembe, V.B., 1960. Indian J. Fish., 7: 69-81.
Subrahmanyam, C.B., 1963. Indian J. Fish., 10: 11-22.
Somerton, D.A., 1980. Canadian J. Fish. aquat. Sci., 37: 1488-1494. https://doi.org/10.1139/f80-192
Treece, G.D., 2000. In: Proceedings of the twenty-eighth US-Japan Natural Resources Aquaculture Panel, Kihei, Hawaii, USA, pp. 121-133.
Ünal, V., Aydın, İ., Göncüoğlu, H., Metin, C., 2012. Socio-economic profile of the jınga shrimp Metapenaeus affinis (H. Milne edwards, 1837) (Crustacea: Decapoda: Penaeidae) Fıshery in Izmir Bay (Aegean Sea), Turkey. The Crustacean Society Summer Meeting and the 10th Colloquium Crustacea Decapoda Mediterranean. 3-7 June, Athens, Greece.
Wenner, A.M., Fusaro, C. and Oaten, A., 1974. Canadian J. Zool., 52: 1095-1106. https://doi.org/10.1139/z74-147
To share on other social networks, click on any share button. What are these?










