Salicylic Acid Improves Rice Seed Germination under Induced Drought Stress
Research Article
Ch. Muhammad Rafiq1, Qasim Raza1*, Awais Riaz1, Misbah Hanif2, Wajiha Saeed2, Shawaiz Iqbal1, Tahir Hussain Awan1, Syed Sultan Ali1 and Muhammad Sabar1
1Rice Research Institute, Kala Shah Kaku, Sheikhupura, Punjab, Pakistan; 2Department of Plant Breeding and Genetics, University of Agriculture Faisalabad, Pakistan.
Ch. Muhammad Rafiq and Qasim Raza contributed equally to this study.
Abstract | Rice is the staple food of more than 50% of the world’s population, and water scarcity is threating its sustainable production. Dry seeded rice (DSR) technology has water and labour saving advantages over conventional transplanting culture, however, poor crop establishment due to reduced germination in variable field conditions greatly hampers its large-scale adaption. To address subordinate germination issues, we investigated the effects of five salicylic acid (SA) concentrations (0, 75, 150, 225 and 300 ppm) on polyethylene glycol (PEG) induced drought stress conditions (0, -0.2, -0.4, -0.6 and -0.8 MPa). Highly significant (p < 0.01) effects of drought, SA and their interactions were observed on seed germination. Interestingly, varying SA concentrations imparted more pronounced effects under higher osmotic stress levels. Day-wise germination data indicated that the SA treatments alleviated inhibitory effects of different osmotic stress levels after 2–4 days of stress applications. Under medium to high osmotic stress levels, mean germination time, germination index and seed vigour index (SVI) of SA primed seeds were better than non-primed seeds. Remarkably, SVI of all SA concentrations under -0.2 MPa osmotic stress was surprisingly improved as compared with control and other osmotic stress levels, indicating a ‘drought-escape strategy’ in rice seeds under low osmotic stress level. Overall, our results indicated that seed priming with 225 ppm SA concentration is ideal under all environments and should be recommended in DSR system to improve early crop establishment and sustainable production.
Received | February 21, 2021; Accepted | March 28, 2021; Published | June 05, 2021
*Correspondence | Qasim Raza, Rice Research Institute, Kala Shah Kaku, Sheikhupura, Punjab, Pakistan; Email: [email protected]
Citation | Rafiq, C.M., Q. Raza, A. Riaz, M. Hanif, W. Saeed, S. Iqbal, T.H. Awan, S.S. Ali and M. Sabar. 2021. Salicylic acid improves rice seed germination under induced drought stress. Journal of Innovative Sciences, 7(1): 152-160.
DOI | https://dx.doi.org/10.17582/journal.jis/2021/7.1.152.160
Keywords | Salicylic acid, DSR, Germination, PEG, Seed treatment, Sustainable production
1. Introduction
Rice is one of the most important food commodities and provides major portion of daily calories requirement, especially in under-developed and developing countries (Fukagawa and Ziska, 2019). The world population is increasing at an exponential rate, demanding approximately 70% increase in food production to meet the food security targets by 2050 (Fróna et al., 2014). Water scarcity or drought is one of the biggest threats to food security worldwide and could reduce crop production by 50% (Budak et al., 2015). Therefore, management of drought stress is crucial for successfully achieving the desired production targets. About 77% of the total cultivated rice is conventionally transplanted globally (Rao et al., 2017). Nevertheless, the flood irrigation culture requires large amount of water, hence, this conventional establishment method is no longer suitable for sustainable rice production. Dry seeded rice (DSR) technology has emerged as an alternate to conventional transplanting culture (Liu et al., 2015a). The main advantages of DSR includes resource conservation (water saving) and economical, making it more suitable for sustainable rice production (Iqbal et al., 2019, 2021). However, DSR confronts with poor crop establishment due to unequal water distribution in variable field conditions, which greatly hampers large-scale adoption of this technology. Thus, an intervention in current DSR technology is essentially needed to accelerate its large-scale adoption among farming community.
Seed germination starts after imbibition with water, initiation of embryo growth, changes in seed water content and completes with radicle emergence and elongation (Ali and Elozeiri, 2017). Among different abiotic factors which effect seed germination, temperature, drought, salinity and phytohormones have been reported to have massive influence on rice seed germination (Kurniasih et al., 2013; El-Mokhtar et al., 2015; Jan et al., 2015; Liu et al., 2015b; Wei et al., 2015; Zheng et al., 2016). El-Mokhtar et al. (2015) studied the response of three rice cultivars to different salinity stress levels and reported highly significant effects of salt stress on rice seed germination. Similarly, Liu et al. (2015b) also reported highly significant effects of high temperature stress on rice seed germination. Water deficiency or drought significantly affects germination and seedling emergence in rice (Hussain et al., 2017; Islam et al., 2018). Water stress ceases metabolic activities in seeds, delays germination process and reduces the biomass production in germinating seeds, ultimately leading to poor crop establishment. To overcome these issues, farmers in Asia prefer to pre-treat seed before sowing to obtain uniform germination in DSR system (Zheng et al., 2016). Nevertheless, such interventions exhibited no beneficial effects under drought conditions (Matsushima and Sakagami, 2013).
Pre-treatment of crop seed with different agents could improve its germination. Among different pre-treatment approaches, seed priming is the most potent approach for improving synchronization of germination (Hussain et al., 2017). In rice, seed priming improves germination and seedling growth under abiotic stresses such as chilling, drought and salinity (Zheng et al., 2016; Sheteiwy et al., 2017; Shatpathy et al., 2018; Sarfraz et al., 2019; Sharma and Parikh, 2020). Salicylic acid (SA) is a naturally occurring phenolic compound which exists in plants at a very low concentration. This hormone like compound plays pivotal role in regulating the growth and development of plants. Earlier evidences also suggest that SA improves tolerance against biotic and abiotic stresses (Rivas-san and Plasencia, 2011; Khan et al., 2019; Mohamed et al., 2020). In rice, SA promotes seed germination under abiotic stresses such as salinity, cold and heavy metal stresses (Guo et al., 2007; Tavares et al., 2014; Wang et al., 2016). However, effect of seed priming with salicylic acid (SA) under drought stress is somewhat unclear in rice. In this study, we investigated effect of different SA concentrations on rice seed germination under induced osmotic stress conditions. Furthermore, we also examined effect of SA concentrations on seed vigour index.
2. Materials and Methods
2.1 Experiment location and plant material
This study was conducted under controlled laboratory condition at Rice Research Institute, Kala Shah Kaku (RRI, KSK), Pakistan to investigate the effects of drought and salicylic acid on rice seed germination. Seed of PK 1121 aromatic (a long grain and short stature aromatic rice variety) was collected from RRI, KSK and used for germination experiment.
2.2 Pre-treatments and experiment setup
Homogenous seeds with uniform colour, shape and size were selected and experiment was carried out with healthy seeds only. The petri dishes and seeds were disinfected with 10% Sodium Hypochlorite (NaOCl, Sigma Aldrich) solution for ten minutes, and then thoroughly rinsed with distilled water to remove all chlorine traces. The sterilized seeds were divided into five sets and separately soaked overnight in 0 (control), 75, 150, 225 and 300 ppm SA solutions (acetylsalicylic acid/Aspirin) at 35 ºC, respectively. Afterwards, hydrated seeds were kept between two layers of blotting paper for surface drying. Then, surface dried seeds were transferred to sterilized petri dishes.
Five osmotic stress solutions (0, -0.2, -0.4, -0.6 and -0.8 MPa) were prepared by dissolving 118, 175, 212 and 254 g of polyethylene glycol (PEG) 6000 in 1000 mL of distilled water, respectively. Thirty pre-treated seeds in each treatment were allowed to germinate on saturated tissue papers in petri dishes. Each treatment contained equal volume of different osmotic stress solutions and distilled water as control treatment. Factorial completely randomized design (CRD) with three replications was adopted for experiment setup. Petri dishes were transferred to a controlled room (25±3ºC; relative humidity 60-70%; 12 hours day light) and germination data was recorded on daily basis for five days. A seed was considered to have germinated, when its radicle elongated by at least 2 mm. For dry weight calculation, roots and shoots of 10 representative seedlings from each treatment were oven dried at 50 ºC for 10 days.
2.3 Calculation of germination parameters
Five germination parameters were calculated as previously reported in literature. The calculation methodology for parameters 1 to 4 followed Anaya et al. (2018) and for parameter 5 Abdul-Baki and Aderson (1973).

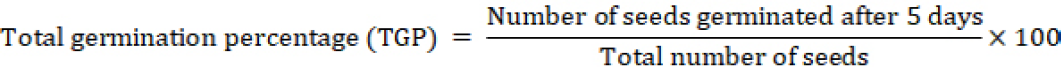
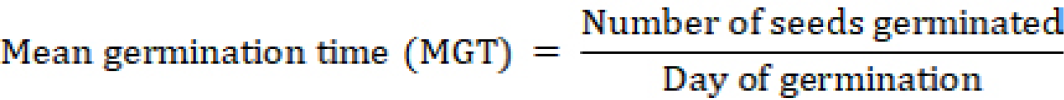

Seed Vigour index (SVI)= TGP × Dry weight
2.4 Statistical analyses
Two-way analysis of variance (ANOVA) was performed to compare means of respective treatments by using Statistix 10.1. Statistical analyses were conducted on mean data of three replicates with α < 0.05 probability level.
3. Results and Discussion
3.1 Effect of drought and SA on total seed germination
To investigate possible effects of drought and SA treatments on germination, we pre-treated rice seeds with different concentrations of SA and then allowed these to germinate under different osmotic stress levels. Highly significant effects of drought, SA and their interactions were observed on total seed germination (Table 1). In absence of SA, seed germination was significantly reduced with an increase in osmotic stress level, whereas increasing SA concentration had non-significant effects on germination (Figure 1). Interestingly, varying SA concentrations imparted more pronounced effects under higher osmotic stress levels. For example, under -0.6 MPa osmotic stress, all SA treatments showed 90–100% germination as compared to 70% germination in control treatment (Figure 2). Similarly, 75 ppm and 225 ppm SA treatments also showed higher germination i.e. 93% and 97%, respectively under -0.8 MPa osmotic stress than respective control treatments (70%). Whereas, less pronounced effects of different SA concentrations were observed under lower osmotic stress conditions (-0.2 and -0.4 MPa). Overall, 225 ppm SA treatment showed best results under control and all osmotic stress conditions (Figure 1). Likewise, 75 ppm SA treatment demonstrated good results under medium to high stress conditions, whereas 150 ppm SA treatments showed better germination under -0.6 MPa osmotic stress. These results strongly suggest a potential role for SA in germination enhancement under water deficient conditions.
Table 1: ANOVA table summarizing two-way effect of treatments on total germination percentage of rice seeds.
|
Source of variation |
Degree of freedom |
Mean sum of square |
|
Drought |
4 |
834.953** |
|
Salicylic Acid |
4 |
297.653** |
|
Drought x Salicylic Acid |
16 |
219.445** |
|
Error |
48 |
0.963 |
** Highly significant at p < 0.01.
3.2 Effect of SA on germination process under water deficient conditions
To determine at which time point significant differences occur between germination of SA treated and non-treated seeds, we collected germination data
from all osmotic stress and control treatments on daily basis and generated germination speed curve using line graphs (Figure 3). Under normal condition, germination started after 1st day (3%) and reached maximum after 4th day (100%) in control treatment (Figure 3A). In SA treatments, germination also started after 1st day (0-3%) but reached maximum (97-100%) after 5th day. Interestingly, germination rate was lower in all SA treatments after 2nd, 3rd and 4th day as compared with control treatment. Under osmotic stress conditions, germination was delayed and significantly reduced (p < 0.01) with an increase in the stress level (Figure 3B). Significant reductions were observed after 2–4 days among germination rates of different osmotic stress treatments. These results indicate that 2nd, 3rd and 4th days are critical for germination process, as during this period any differences due to favourable or harmful conditions become more apparent between experimental treatments.
Mean germination time (A) and germination index (B) of rice seeds after five days of drought, SA and their interactive treatments. Data are means (% ± S.E) of three replicates.
The combination of SA treatments with osmotic stress levels imparted beneficial effects on germination process. Under low osmotic stress level (-0.2 MPa), all SA treatments showed high germination rates after 1–4 days than respective control treatment (Figure 3C). Under medium osmotic stress levels (-0.4–0.6 MPa), at least two SA treatments (225 ppm and 300 ppm) showed enhanced germination during 2–4 days as compared to respective control treatments (Figure 3D and 3E). Similarly, under high osmotic stress level (-0.8 MPa), 75 ppm and 225 ppm SA treatments resulted in improved germination during 2–5 days as compared with control treatment (Figure 3F). Remarkably, all SA treatments exhibited highly significant beneficial effects on germination process after 2–5 days under -0.6 MPa osmotic stress. Likewise, 75 ppm and 225 ppm SA treatments also demonstrated highly significant effects under -0.8 MPa osmotic stress. These results strongly indicate that beneficial effects of SA treatments alleviated inhibitory effects of different osmotic stress levels after 2–4 days of stress applications.
3.3 Effect of SA on mean germination time and germination index
Increase in osmotic stress levels delayed the germination process, as MGT and GI were increased and decreased, respectively. Under normal conditions, control treatment had lowest MGT (2.57 days) and reached maximum (3.48 days) under -0.8 MPa osmotic stress (Figure 4A). All SA treatments exhibited higher MGT in absence of osmotic stress as compared with control treatment. Whereas, under stress conditions, three SA treatments (75 ppm, 225 ppm and 300 ppm) showed less MGT as compared to respective control treatments. Interestingly, MGT for 225 ppm and 300 ppm SA treatments were significantly lower than control treatments under all stress levels. Furthermore, MGT ranged between 2–4 days during control and stress treatments. In contrast to MGT, GI demonstrated decreasing trend with an increase in osmotic stress levels (Figure 4B). Under normal conditions, control treatment had high GI than all SA treatments. Under -0.2 MPa and -0.6 MPa osmotic stresses, all SA treatments exhibited higher GI than respective controls. Likewise, under all stress conditions, 75 ppm, 225 ppm and 300 ppm SA treatments showed higher GI as compared to respective controls. Collectively, these results strongly support our data and indicate advantageous influence of SA treatments on germination process under stressful conditions.
3.4 Effect of drought and SA treatments on seed vigour index
To examine physiological effects of drought and SA treatments on SVI, we also recorded dry matter weight data from 10 representative seedlings as mentioned in material and method section. The SVI first significantly increased and then decreased as compared to control with an increase in osmotic stress levels (Figure 5). Under normal conditions, 300 ppm SA treatment exhibited higher SVI than control treatment. Likewise, 225 ppm SA treatment also showed comparable SVI with control treatment. Interestingly, under all osmotic stress levels, 75 ppm, 225 ppm and 300 ppm SA treatments demonstrated significantly high or comparable SVI when compared with respective controls. Whereas, at medium to high osmotic stress levels (-0.6 MPa and -0.8 MPa), all SA treatments showed significantly higher SVI than controls (Figure 5). All these results collectively indicate a strong beneficial effect of SA treatments on seed germination process and crop establishment under water deficient environments.
New interventions in DSR system are required for enhancement of seed germination and early plant growth to ensure rice production on sustainable basis. The beneficial effects of SA in biotic and abiotic stress tolerance in crop plants have been well documented. In this study, we investigated possible effects of SA on rice seed germination under induced drought stress conditions.
Germination process starts after changes in seed water contents and completes with radicle elongation (Ali and Elozeiri, 2017). During germination, drought stress causes reduction in water availability and subsequently reduces germination indices (Islam et al., 2018). In this study, germination was also reduced significantly with an increase in water stress level (Figures 1 and 2). In presence of SA, germination was significantly improved under medium to high drought conditions. However, no significant differences in germination indices occurred between control and SA treatments in absence of water stress. These results are in accordance with Anaya et al. (2018) and indicate that inhibitory effects of drought stress on germination indices are reversed by SA treatments. Thus, a clear evidence between SA based seed priming and germination enhancement under drought stress have been established in rice which could address poor crop establishment issues in DSR system.
Increasing evidences have suggested that drought stress delays the germination process (Pandey and Shukla, 2015) and 2–4 days after sowing are most critical of radicle elongation (Rice Knowledge Bank, 2021; Ella et al., 2011) because any differences in germination indices due to experimental conditions are more clear during this period. This hypothesis prompted us to investigate drought and SA effects on daily germination rates. Here, germination was delayed in presence of water stress and absence of SA treatments, whereas maximum germination was achieved after 4 days in control treatment (Figure 3A and 3B). In drought, SA and their interactive treatments, germination differences were more prominent after 2–4 days (Figure 3A-F and 4A). These results strongly suggest that 2–4 days after sowing are critical and a quick germination report is available after this period which could help in subsequent decision making, if poor germination is observed.
Recent studies have reported drought and SA mediated regulation of growth and development in crop plants. Chen et al. (2020) showed that drought stress intensity significantly effects rice seedlings growth as compared with drought duration. Similarly, Sohag et al. (2020) demonstrated that exogenous hydrogen peroxide (H2O2) and SA applications significantly improve rice seedlings growth under drought conditions. In this research, varying SA concentrations also had significant effects on SVI (Figure 5). Under medium to high osmotic stress levels, SVI of SA treated seeds was at par than non-treated seeds. Interestingly, SVI of all treatments under -0.2 MPa osmotic stress was surprisingly improved as compared with control and other osmotic stress levels. The unexpected increase in SVI might indicate a ‘drought-escape strategy’ in rice seeds under low osmotic stress level. This kind of escape strategy is also reported during different abiotic stresses such as chilling, drought, salinity and high temperature in crop plants (Franks, 2011; Kooyers, 2015; Gull et al., 2019). Collectively, these results demonstrate a positive effect of SA on seed germination and seedlings health and provide new insights into drought response mechanism at early growth stage.
Different SA concentrations impart dissimilar effects on germination indices under varying stress levels and a lack of harmony exists in literature regarding ideal dose of SA for optimal plant growth under normal and stress conditions (Tavares et al., 2014; Shatpathy et al., 2018; Ahmed et al., 2020). In absence of stress, SA reveals non-significant effects on germination (Figure 2). On the contrary, it gives optimal results under stressful conditions. However, change in SA concentration is often linked with arbitrary change in germination indices, thus making it very difficult to identify an optimal dose which equally performs under changing environments. In this study, we identified osmotic stress level specific SA doses for germination improvement. Seed priming with 225 ppm SA exhibited significantly high germination indices under all osmotic stress levels and it also showed non-significant difference when compared with control treatment (Figures 2, 3, 4 and 5). Similarly, 75 ppm SA treatment demonstrated high germination under medium to high osmotic stress levels and 150 ppm SA treatments showed best results under medium osmotic stress level. Collectively, these results indicate that seed priming with 225 ppm SA concentration is ideal under all environments and should be recommended in DSR system to improve crop establishment and sustainable production.
Conclusion and Recommendations
Cumulative results of this study demonstrated inhibitory effects of induced drought stress on rice seed germination. However, pre-treatment of seed with different SA concentrations alleviated inhibitory effects of drought stress. Varying SA concentrations had beneficial effects on seed germination process and seedlings vigour index. Overall, 225 ppm SA concentration demonstrated better germination indices under all stress and respective control treatments. Based on the results presented in this study, pre-treatment of rice seed with 225 ppm SA concentration is recommended for enhanced germination and early crop establishment in DSR system.
Novelty Statement
To address poor germination issues in DSR system, we investigated the beneficial effects of seed priming with salicylic acid under induced drought stress conditions. Results demonstrated that 225 ppm concentration of SA was ideal and should be recommended for enhanced germination under DSR system.
Author’s Contribution
CMR, QR, AR and MS conceived and designed the experiment. QR, MH and WS performed the experiment. AR, MH and WS data collection. CMR, QR, AR, SSA and MS data analysis. QR and AR writing original draft. CMR, SI, THA, SSA and MS review and editing. The final manuscript was ultimately perused, scrutinized and approved for final submission by all the authors.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdul-Baki, A.A., and Anderson, J.D., 1973. Vigor determination in soybean seed by multiple criteria 1. Crop Science, 13(6): 630-633. https://doi.org/10.2135/cropsci1973.0011183X001300060013x
Ahmed, W., Imran, M., Yaseen, M., Haq, T., Jamshaid, M.U., Rukh, S., and Khan, M.A., 2020. Role of salicylic acid in regulating ethylene and physiological characteristics for alleviating salinity stress on germination, growth and yield of sweet pepper. PeerJ., 8: e8475. https://doi.org/10.7717/peerj.8475
Ali, A.S., and Elozeiri, A.A., 2017. Metabolic processes during seed germination. Advances in Seed Biology, pp. 141-166. https://doi.org/10.5772/intechopen.70653
Anaya, F., Fghire, R., Wahbi, S., and Loutfi, K., 2018. Influence of salicylic acid on seed germination of Vicia faba L. under salt stress. Journal of the Saudi Society of Agricultural Sciences, 17(1): 1-8. https://doi.org/10.1016/j.jssas.2015.10.002
Budak, H., Hussain, B., Khan, Z., Ozturk, N.Z., and Ullah, N., 2015. From genetics to functional genomics: Improvement in drought signaling and tolerance in wheat. Frontiers in Plant Science, 6: 1012. https://doi.org/10.3389/fpls.2015.01012
Chen, Z.K., Xu, W.W., Nie, J., Khan, A., Cao, C.G., and Li, P., 2020. Drought stress intensity, duration and its resistance impact on rice (Oryza sativa L.) seedling. Applied Ecology and Environmental Research, 18(1): 469-486. https://doi.org/10.15666/aeer/1801_469486
El-Mokhtar, S.M., Samb, A., Moufid, A.O., Boukhary, A.O.M.S., and Djeh, T.K.O., 2015. Effect of different levels of salinity on germination and early seedling growth of three rice varieties cultivated in Mauritania. International Journal of Agriculture and Crop Sciences, 8(3): 346-349.
Ella, E.S., Dionisio-Sese, M.L., and Ismail, A.M., 2011. Seed pre-treatment in rice reduces damage, enhances carbohydrate mobilization and improves emergence and seedling establishment under flooded conditions. AoB Plants, 2011. https://doi.org/10.1093/aobpla/plr007
Franks, S.J., 2011. Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytologist, 190(1): 249-257. https://doi.org/10.1111/j.1469-8137.2010.03603.x
Fróna, D., Szenderák, J., and Harangi-Rákos, M., 2019. The challenge of feeding the world. Sustainability, 11(20): 5816. https://doi.org/10.3390/su11205816
Fukagawa, N.K., and Ziska, L.H., 2019. Rice: Importance for global nutrition. Journal of nutritional science and vitaminology, 65(Supplement): S2-S3. https://doi.org/10.3177/jnsv.65.S2
Gull, A., Lone, A.A., and Wani, N.U.I., 2019. Biotic and abiotic stresses in plants. Abiotic and Biotic Stress in Plants, pp. 1-19. https://doi.org/10.5772/intechopen.85832
Guo, B., Liang, Y.C., Zhu, Y. G., and Zhao, F.J., 2007. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environmental Pollution, 147(3): 743-749. https://doi.org/10.1016/j.envpol.2006.09.007
Hussain, M., Farooq, M., and Lee, D.J., 2017. Evaluating the role of seed priming in improving drought tolerance of pigmented and non‐pigmented rice. Journal of Agronomy and Crop Science, 203(4): 269-276. https://doi.org/10.1111/jac.12195
Islam, M.M., Kayesh, E., Zaman, E., Urmi, T.A., and Haque, M.M., 2018. Evaluation of rice (Oryza sativa L.) genotypes for drought tolerance at germination and early seedling stage. The Agriculturists, 16(1): 44-54. https://doi.org/10.3329/agric.v16i1.37533
Iqbal, S., Khalid, U.B., Awan, T.H., Iqbal, N., Saleem, M.U., Iram, A., and Ahmad, N., 2019. Qualitative response of super basmati rice to different nitrogen levels under varying rice ecosystem. African Journal of Agricultural Research, 14(9): 548-558.
Iqbal, S., Iqbal, N., Khalid, U.B., Saleem, M.U., Iram, A., Rizwan, M., and Awan, T.H., 2021. Growth, yield and economic analysis of dry-seeded basmati rice. Sarhad Journal of Agriculture, 37(1): 200-208. https://doi.org/10.17582/journal.sja/2021/37.1.200.208
Jan, M., Shinwari, K.I., Shah, G., Khan, M.H.U., Ullah, S., Hameed, A., and Malook, I., 2015. Consequences of short-term low temperature stress on physiological and biochemical aspects of rice (Oryza sativa L.). Scientia Agriculturae, 10(1): 1-14. https://doi.org/10.15192/PSCP.SA.2015.10.1.114
Khan, M.S., Akther, T., Ali, D.M., and Hemalatha, S., 2019. An investigation on the role of salicylic acid alleviate the saline stress in rice crop (Oryza sativa L). Biocatalysis and Agricultural Biotechnology, 18: 101027. https://doi.org/10.1016/j.bcab.2019.101027
Kooyers, N.J., 2015. The evolution of drought escape and avoidance in natural herbaceous populations. Plant Science, 234: 155-162. https://doi.org/10.1016/j.plantsci.2015.02.012
Kurniasih, B., Greenway, H., and Colmer, T.D., 2013. Tolerance of submerged germinating rice to 50–200 mM NaCl in aerated solution. Physiologia Plantarum, 149(2): 222-233. https://doi.org/10.1111/ppl.12029
Liu, H., Hussain, S., Zheng, M., Peng, S., Huang, J., Cui, K., and Nie, L., 2015a. Dry direct-seeded rice as an alternative to transplanted-flooded rice in Central China. Agronomy for Sustainable Development, 35(1): 285-294. https://doi.org/10.1007/s13593-014-0239-0
Liu, S.J., Xu, H.H., Wang, W.Q., Li, N., Wang, W.P., Møller, I.M., and Song, S.Q., 2015b. A proteomic analysis of rice seed germination as affected by high temperature and ABA treatment. Physiologia Plantarum, 154(1): 142-161. https://doi.org/10.1111/ppl.12292
Matsushima, K.I., and Sakagami, J.I., 2013. Effects of seed hydropriming on germination and seedling vigor during emergence of rice under different soil moisture conditions. American Journal of Plant Sciences, 2013. https://doi.org/10.4236/ajps.2013.48191
Mohamed, H.I., El-Shazly, H.H., and Badr, A., 2020. Role of salicylic acid in biotic and abiotic stress tolerance in plants. Plant Phenolics in Sustainable Agriculture Springer, Singapore. pp. 533-554. https://doi.org/10.1007/978-981-15-4890-1_23
Pandey, V., and Shukla, A., 2015. Acclimation and tolerance strategies of rice under drought stress. Rice Science, 22(4): 147-161. https://doi.org/10.1016/j.rsci.2015.04.001
Rao, A.N., Wani, S.P., Ramesha, M.S., and Ladha, J.K., 2017. Rice production systems. Rice Production Worldwide Springer, Cham. pp. 185-205. https://doi.org/10.1007/978-3-319-47516-5_8
Rice Knowledge Bank, 2021. Available at http://www.knowledgebank.irri.org/training/fact-sheets/management-of-other-crop-problems-fact-sheet-category/measuring-seed-germination-fact-sheet (accessed 15 February 2021).
Rivas-San Vicente, M., and Plasencia, J., 2011. Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany, 62(10): 3321-3338. https://doi.org/10.1093/jxb/err031
Sarfraz, M., Hussain, S., Ijaz, M., Nawaz, A., Yasir, T.A., Sher, A., and Ahmad, S., 2019. Abiotic stress tolerance in plants by priming and pretreatments with phytohormones. Priming and Pretreatment of Seeds and Seedlings Springer, Singapore. pp. 447-457. https://doi.org/10.1007/978-981-13-8625-1_22
Sharma, B., and Parikh, M., 2020. Seed priming: An emerging technology to impart abiotic stress tolerance in rice. International Journal of Communication Systems, 8(2): 619-623. https://doi.org/10.22271/chemi.2020.v8.i2j.8837
Shatpathy, P., Kar, M., Dwibedi, S.K., and Dash, A., 2018. Seed priming with salicylic acid improves germination and seedling growth of rice (Oryza sativa L.) under PEG-6000 induced water stress. Int. J. Curr. Microbiol. Appl. Sci., 7(10): 907-924. https://doi.org/10.20546/ijcmas.2018.710.101
Sheteiwy, M., Shen, H., Xu, J., Guan, Y., Song, W., and Hu, J., 2017. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environmental and Experimental Botany, 137: 58-72. https://doi.org/10.1016/j.envexpbot.2017.02.007
Sohag, A.A.M., Tahjib-Ul-Arif, M., Brestic, M., Afrin, S., Sakil, M.A., Hossain, M.T., and Hossain, M.A., 2020. Exogenous salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil and Environment, 66(1): 7-13. https://doi.org/10.17221/472/2019-PSE
Tavares, L.C., Rufino, C.A., Oliveira, S.D., Brunes, A.P., and Villela, F.A., 2014. Treatment of rice seeds with salicylic acid: seed physiological quality and yield. Journal of Seed Science, 36(3): 352-356. https://doi.org/10.1590/2317-1545v36n3636
Wang, W., Peng, S., Chen, Q., Mei, J., Dong, H., and Nie, L., 2016. Effects of pre-sowing seed treatments on establishment of dry direct-seeded early rice under chilling stress. AoB Plants, pp. 8. https://doi.org/10.1093/aobpla/plw074
Wei, L.X., Lv, B.S., Wang, M.M., Ma, H.Y., Yang, H.Y., Liu, X.L., and Liang, Z.W., 2015. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiology and Biochemistry, 90: 50-57. https://doi.org/10.1016/j.plaphy.2015.03.002
Zheng, M., Tao, Y., Hussain, S., Jiang, Q., Peng, S., Huang, J., and Nie, L., 2016. Seed priming in dry direct-seeded rice: consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regulation, 78(2): 167-178. https://doi.org/10.1007/s10725-015-0083-5
To share on other social networks, click on any share button. What are these?




