Rumen Fluid Profile, Methane Emission and Nitrogen Excretion of Young and Mature Kacang Goats Under Different Feeding Levels
Research Article
Rumen Fluid Profile, Methane Emission and Nitrogen Excretion of Young and Mature Kacang Goats Under Different Feeding Levels
Nadlirotun Luthfi1, Edy Rianto2*, Endang Purbowati2, Christina Maria Sri Lestari2, Agung Purnomoadi2, Nurul Mukminah3
1Faculty of Animal Husbandry, University of Darul Ulum Islamic Centre Sudirman, Semarang Regency, Indonesia; 2Faculty of Animal and Agricultural Sciences, Universitas Diponegoro, Semarang, Indonesia; 3Subang State Polytechnic, Subang, Indonesia.
Abstract | The objective of this study was to evaluate ruminal fermentation, methane emissions, and nitrogen excretion of young and mature Kacang goats at different feeding levels. Sixteen goats were divided into two age groups (young and mature) and fed at either low feeding level (maintenance) or high feeding level (production). The ration contained 18% crude protein (CP) and 75% total digestible nutrients (TDN). Parameters evaluated included nutrient intake, acetate, propionate, and butyrate concentrations, rumen ammonia levels, microbial protein production, methane emissions, and nitrogen excretion. The study found significant differences in nutrient intake between age and feeding level treatments (p<0.05). Volatile fatty acids (VFA) levels were similar at 0 hours before feeding (p>0.05) for both age and feeding level treatments, but significantly differed at 3 and 6 hours after feeding among feeding levels. Rumen ammonia levels were similar across age groups (p>0.05) but significantly varied at 3 and 6 hours after feeding among feeding levels (p<0.05). Microbial protein production did not differ significantly among treatments (p>0.05). Methane emissions and nitrogen excretions of young and mature Kacang goats were similar (p>0.05) but higher at high feeding levels compared to low feeding levels (p<0.05). Methane emission and total nitrogen excretion per unit of body weight gain (BWG) were lower at high feeding levels in both young and mature goats (p<0.05). In conclusion, young and mature Kacang goats exhibited similar abilities in rumen fermentation. Higher feeding levels resulted in increased concentrations of ruminal VFA and ammonia, and decreased methane emissions and total nitrogen excretion per unit of BWG. Therefore, raising Kacang goats at high feeding levels is recommended for environmental sustainability.
Keywords | Age, Feeding level, Rumen fluid profile, Methane, Nitrogen, Kacang goat
Received | March 30, 2024; Accepted | June 23, 2024; Published | August 20, 2024
*Correspondence | Edy Rianto, Faculty of Animal and Agricultural Sciences, Universitas Diponegoro, Semarang, Indonesia; Email: erianto_05@yahoo.com
Citation | Luthfi N, Rianto E, Purbowati E, Lestari CMS, Purnomoadi A, Mukminah N (2024). Rumen fluid profile, methane emission and nitrogen excretion of young and mature kacang goats under different feeding levels. J. Anim. Health Prod. 12(3): 420-428.
DOI | http://dx.doi.org/10.17582/journal.jahp/2024/12.3.420.428
ISSN (Online) | 2308-2801

Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Rumen fermentation profile is considered an effective indicator to evaluate the nutrient utilization in ruminants (Sun et al., 2020; Li et al., 2021). The results of fermentation in the rumen have an important role in providing most of the energy and protein to meet the needs of ruminant animal, especially in the form of volatile fatty acids and microbial protein (Weimer, 2015). Volatile fatty acids from ruminal fermentation provide not less than 70% of the energy needed for maintenance, growth, and production of the ruminant (Pokharel et al., 2018; Yin et al., 2021). Studies found that a part of protein needed for ruminant was provided by microbial protein synthesized in the rumen (Bach, 2005; Li et al., 2021). The fermentation of feed in the rumen is greatly influenced by the activity of rumen microbes, which require the feed entering the rumen as the source of energy and protein. Therefore, dietary energy and protein also become main factors for the sustainability of microbial activity in the rumen (Wang et al., 2021).
Several things need to be considered to meet the nutrients requirement of the animal. Nutrients requirement for productivity are highly affected by physiological status of the animal (Riaz et al., 2014; Jayanegara et al., 2017; Luthfi et al., 2022). The finding by Purnami et al. (2021) showed that mature Kejobong goats had higher nutrient intake than the young ones. Lambs raised under high feeding level had higher productivity than those under low feeding level (Choirunnisa et al., 2016; Aluns and Luthfi, 2018; Luthfi et al., 2022).
However, the high feed intake may have impact on the by-product of the fermentation process in the rumen (methane) and nitrogen excretion (Hristov et al., 2013; Luthfi et al., 2018; Tan et al., 2021; Luthfi et al., 2023) because higher feed intake resulted in the increase of the amount of nutrients being fermented in the rumen and reduced feed digestibility (Rianto et al., 2002; Luthfi et al., 2023). A study by Aprilliza et al. (2019) showed that every increase of 100 g BWG resulted to 43.58 liters per day of methane emission in lambs. The excessive of protein intake resulted to high ammonia production in the rumen which is eventually excreted through urine. Nabila et al. (2021) reported that higher crude protein intake in the mature Kejobong goats resulted in a higher nitrogen excretion. Until recently, there are still limited studies on the effect of age and feeding levels on rumen fluid profile, methane mission and nitrogen excretion of Kacang goats. This study was to evaluate the effect of high and low feeding levels on ruminal volatile fatty acids concentration, methane emission and nitrogen excretion in young and mature Kacang goats.
Materials and methods
Animals and feed
The experiment used 16 Kacang goats that were divided into 2 age groups (young and mature). The young Kacang goats aged 5-6 months and weighed 12.75 kg; and the mature Kacang goats aged 12-15 months weighed 17.34 kg. Each age group was divided into high and low feeding levels. Each feeding treatment consisted of 4 replications. The diet contained 18% crude protein (CP) and 75% total digestible nutrients (TDN), and consisted of 25% wheat straw, 32% soybean meal, 39% rice bran, 3% sugarcane molasses and 1% minerals (Table 1).
A completely randomized design (CRD) in a nested structure was applied in this study. The main factor (the nest) was the age of the goat, i.e. young and mature; while the second factor (the nested factor) was feeding levels, i.e. low (maintenance level) and high (twice of maintenance level). All study protocols were carried out in line with the international animal ethical standard.
Table 1: Nutrients content of the diet
| Nutrients | % |
| Dry Matter | 80.1 |
| Organic Matter | 92.3 |
| Crude Protein | 18.8 |
| Ether Extract | 3.1 |
| Crude Fibre | 12.2 |
| Ash | 7.7 |
| Nitrogen Free extract | 58.1 |
| Gross Energy (MJ/kg) | 5.3 |
Procedures
There were three stages in this study. First, the animals were adapted to the environment and feed. In this stage, dry matter intake (DMI) and average daily weight gain were recorded and observed for a month. The data obtained were used in a regression to find DMI required for maintenance. According to this finding, DMI for maintenance was multiplied by 2 to determine DM needed for production. The result of the observation showed that the DMI required by the young goats for maintenance and production was 2.24% BW and 4.8% BW respectively. The DMI required by the mature goats for maintenance and production was 1.87% BW and 3.74% BW respectively (calculated as twice the DMI need for maintenance). The diet was provided 3 times a day, i.e. at 07.00 am, 12.00 noon and 16.00 pm. The residual was weighed in the next morning.
The second stage was preliminary stage. In this stage each age group was randomized to obtain feeding level treatment and eliminated the effect of previous feed from adaptation stage.
The third stage involved data collection. During this stage, the dry matter (DM) content of the diet was determined by drying feed samples in a “Yamato Scientific” oven at 105°C for 24 hours. Faeces and urine from the goats were collected over a week to measure nutrient excretion. Feed samples and faecal sub-samples were dried in an oven at 65°C for 72 hours for chemical analysis. Urine sub-samples, representing 10% of total urine excreted during the collection period, were collected every morning and stored in a “GEA” freezer at -5°C until analysis. A duplicate of 1 ml of urine sub-sample was dried in an oven at 60°C for 4 days until its weight stabilized. Dry matter of the feed and faecal samples were analysed according to AOAC (1995) standard processing. Nitrogen contents of the diet, faeces and urine were analysed by Kjehdahl method.
Rumen fluid samples were taken using a pump and a tube. The tube was inserted into the goat’s mouth to rumen cavity. The pump was turned into suck up 20 ml of rumen fluid. The pH value of the rumen fluid was measured to determine H2SO4 (20% concentration) which was mixed with the rumen fluid so that the pH reached 3 or less. Then, sample was filtered to extract the liquid and stored in freezer before analysis. Rumen fluid was taken in the last day of treatment as many as 3 times, i.e before feeding, 3 h after feeding and 6 h after feeding.
The methane emission was measured using the facemask method as described by Luthfi et al. (2018). The mask was connected to a methane analyser (Horiba Ltd., Japan) which was equipped with an airflow meter to measure gas volume (Litres) to calculate total methane production. Methane emission data was recorded on the IBM PC for 10 minutes at 3 hour intervals. The volume of methane obtained was converted into units of grams with a conversion factor of 1 litre of CH4 equal to 0.714 g (Kawashima, 2001).
Parameters
The parameters measured in this study were DMI, organic matter intake (OMI), carbohydrate intake (CHI), protein intake, the concentration of ruminal volatile fatty acids (VFA), concentrations of acetic, propionic and butyric acids, the concentration of ruminal ammonia (NH3), microbial N production, methane emission, and nitrogen excretion. VFA concentration was analysed using chromatography (Ahmad et al., 2020), while rumen fluid ammonia was analysed spectrophotometrically (Weatherburn, 1967). The nutrient intake and OM digestibility were measured using following equations:
Nutrients intake (g/d) = Dry matter intake x % nutrient of feed
OM digestibility 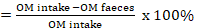
The calculation of microbial N production in small ruminants was based on the recommendation of Chen and Gomes, (1992) as follows:
Y = 0.84 X+(0.150 BW0.75- e -0.25X)
microbial N production (g N/ d)
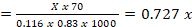
microbial N production efficiency (gN/kg OM)
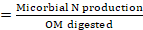
while,
X (mmol/d): the amount of microbial purine absorbed
Y (mmol/d): the amount of microbial purine derivatives excreted in the urine
BW0,75: metabolic body weight
e : 2.71828
0.83 : Digestibility coefficients for microbial purines
70 : Amount of N in purines (mg/mmol)
0.116 : the ratio between purines and total N in the microbial mixture
Data Analysis
The data obtained was analysed using analysis of variance (ANOVA). Significance level was considered at 5%.
Results and Discussions
Nutrients intake
The data on nutrients intake (Table 2) showed significant (p<0.01) differences both in age and feeding levels. The difference in dry matter intake (DMI) observed in this study varied between age groups and feeding levels, resulting in higher nutrient intake among older animals and those in the higher feeding level. Specifically, older animals and those on a higher feeding regimen exhibited increased intake of organic matter, crude protein, carbohydrates, and crude fiber. This finding aligns with previous studies by Aluns and Luthfi (2018), Luthfi et al. (2022), and Luthfi et al. (2023), which similarly reported that higher feeding levels enhance nutrient intake and utilization by animals.
The dry matter intake (DMI) and nutrient intake in this study were similar to the findings of Mariam et al. (2023), who reported DMI of local goats ranging from 318 g/d to 494 g/d, with organic matter intake (OMI) between 285 g/d and 436 g/d. The nutrient requirements of animals are significantly influenced by age and physiological status, particularly for maintenance and growth across different body weights (Jayanegara et al., 2017; Kenny et al., 2018). Young animals have higher energy requirements for metabolism compared to mature animals, with metabolic rates generally decreasing as animals mature (Luo et al., 2004). Consequently, this leads to changes in nutritional requirements for both maintenance and growth in young and mature goats.
Rumen Fluid Profile
Volatile fatty acids: The rumen fluid profile presented in Table 3 revealed that acetic, propionic and butyric acid in 0 h before feeding were not significantly (p>0.05) different between ages of the goat and between feeding levels. This could be attributed to animals that have not eaten yet at 0 h, so that fermented feed materials produces similar conc-
Table 2: Nutrients intake of young and mature Kacang goats
| Parameters | Young | Mature | Sig | |||
| Low | High | Low | High | Age | Level of Feed | |
| DMI, g |
245.14 ± 28.46xa |
467.52 ± 70.21xb |
301.12 ± 52.34ya |
651.13 ± 81.90yb |
0.00 | 0.00 |
| OM Intake, g/d |
218.34 ± 33.21xa |
341.13 ± 25.83xb |
254.82 ± 33.04ya |
542.32 ± 29.35yb |
0.01 | 0.00 |
| CP intake, g/d |
44.51 ± 6.77xa |
69.54 ± 5.65xb |
51.95 ± 6.73ya |
110.55 ± 5.98yb |
0.00 | 0.00 |
| Carbohydrate intake, g/d |
166.51 ± 25.32xa |
260.15 ± 18.63xb |
194.33 ± 23.27ya |
413.57 ± 20.67yb |
0.01 | 0.00 |
| Crude fiber intake, g/d |
12.72 ± 1.93xa |
19.87 ± 1.32xb |
14.84 ± 1.92ya |
31.58 ± 1.71yb |
0.01 |
0.00 |
The superscripts of x, y showing significant differences between age groups (p < 0.05)
The superscript of a, b showing significant differences between feeding levels (p < 0.05)
Table 3: Volatile fatty acids, A: P ratio, ammonia, pH and microbial N production of young and mature Kacang goats
| Parameters | Young | Mature | Sig | |||
| Low | High | Low | High | Age | Level of Feed | |
| Acetate 0 h, Mmol | 16.87 ± 7.54 | 24.60 ± 3.94 | 27.14 ± 8.82 | 24.79 ± 5.16 | 0.54 | 0.08 |
| Propionate 0 h, Mmol | 6.21 ± 2.05 | 9.18 ± 1.60 | 11.19 ± 4.72 | 9.76 ± 1.80 | 0.12 | 0.65 |
| Butyrate 0 h, Mmol | 5.58 ± 1.87 | 15.95 ± 6.16 | 10.63 ± 3.59 | 10.16 ± 3.62 | 0.88 | 0.08 |
|
A/P 0 h |
2.72 ± 0.11 | 2.68 ± 0.62 | 2.43 ± 0.53 | 2.54 ± 0.17 | 0.43 | 0.67 |
| Acetate 3 h, Mmol |
26.38 ± 5.43b |
35.61 ± 9.34a |
24.53 ± 4.32b |
35.50 ± 7.46a |
0.81 | 0.03 |
|
Propionate 3 h, Mmol |
9.92 ± 2.19b |
18.03 ± 3.02a |
9.80 ± 2.13b |
11.40 ± 0.82a |
0.15 | 0.05 |
| Butyrate 3 h, Mmol |
7.85 ± 3.01b |
21.91 ± 6.72a |
7.84 ± 1.38b |
9.55 ± 1.55a |
0.06 | 0.02 |
| A/P 3 h | 2.66 ± 0.09 | 2.97 ± 0.12 | 2.50 ± 0.21 | 3.11 ± 0.01 | 0.81 | 0.37 |
| Acetate 6h, Mmol |
17.45 ± 3.44b |
23.95 ± 0.98a |
20.78 ± 5.05b |
23.68 ± 3.59a |
0.47 | 0.04 |
| Propionate 6h, Mmol |
5.70 ± 1.17 b |
11.94 ± 1.08 a |
6.55 ± 1.49 b |
8.07 ± 2.08 a |
0.11 | 0.00 |
| Butyrate 6h, Mmol |
4.33 ± 0.63 b |
12.54 ± 3.37 a |
5.62 ± 1.09 b |
6.25 ± 0.61 a |
0.06 | 0.00 |
| A/P 6 h | 3.06 ± 0.31 | 2.01 ± 0.47 | 3.17 ± 0.12 | 2.93 ± 0.17 | 0.26 | 0.14 |
| NH3 0 h, mg/l | 46.50 ± 13.64 | 49.15 ± 4.75 | 48.13 ± 10.00 | 42.98 ± 7.91 | 0.69 | 0.83 |
| NH3 3 h, mg/l | 48.60 ± 9.05 | 49.69 ± 2.81 | 58.59 ± 0.82 | 43.41 ± 5.26 | 0.79 | 0.32 |
| NH3 6 h, mg/l | 47.10 ± 3.26 | 39.73 ± 7.00 | 45.48 ± 4.46 | 39.69 ± 7.75 | 0.85 | 0.16 |
|
pH 0 h |
6.24 ± 0.40 | 6.63 ± 0.12 | 6.24 ± 0.17 | 6.05 ± 0.23 | 0.20 | 0.41 |
| pH 3 h | 5.70 ± 0.36 | 5.56 ± 0.02 | 5.77 ± 0.19 | 5.60 ± 0.29 | 0.38 | 0.26 |
| pH 6 h | 5.93 ± 0.57 | 5.83 ± 0.71 | 5.97 ± 0.81 | 5.90 ± 0.16 | 0.38 | 0.29 |
|
Microbial protein production, g/d |
75.09 ± 8.29 | 165.63 ± 8.63 | 96.21 ± 9.51 | 75.97 ± 5.94 | 0.49 | 0.48 |
| OM Digested, g/d |
155.80 ± 11.93xb |
311.37 ± 17.01xa |
201.65 ± 14.11yb |
430.16 ± 17.31ya |
0.01 | 0.00 |
| Efficiency of microbial protein production, g protein produced / g OM digested | 0.49 ± 0.04 | 0.62 ± 0.05 | 0.57 ± 0.05 | 0.17 ± 0.01 | 0.45 |
0.59 |
The superscripts of x, y showing significant differences between age groups (p < 0.05)
The superscript of a, b showing significant differences between feeding levels (p < 0.05)
-entration of acetate, propionate and butyric acid among treatments. The average of acetic, propionic and butyric acids in this study were 23.35 Mmol, 9.06 Mmol and 10.58 Mmol, respectively.
In this study, the concentration of acetic, propionic and butyric acids at 3 h after feeding increased and was higher than those at 0 h (before feeding). However, the concentration of acetic, propionic and butyric acids at 3 h after feeding were not significantly different (p >0.05) between the ages. This indicates that the rumen of the young goat had developed to be similar to that of the mature goat, so they had the similar fermentation capacity, ability and resulted in similar concentrations of acetic, propionic and butyric acids at 3 h after feeding. Wu et al. (2024) and Abdelsattar et al. (2023) found that volumes of the rumen, reticulum, omasum and abomasum as well as the digestive tract gradually grows with age (birth, 3 and 8 weeks). After that, the digestive tract matures in proportion to body weight especially in adulthood (1 year). However, The concentration of acetic, propionic and butyric acids in 3 h after feeding were significantly (p<0.05) different between low and high feeding levels, both in young and mature Kacang goats. The concentration of a acetic, propionic and butyric acids at 3 h after feeding at high feeding level was higher than those in low feeding level. It was due to there were more nutrients in high feeding level (Table 2) to be fermented in the rumen and resulted in higher VFA concentration, in comparison to that in low feeding level.
The concentrations of acetic, propionic and butyric acids in 6 h after feeding decreased and lower than those at 3 h after feeding (Table 3). It indicated that there had been an absorption of VFA from the rumen walls and a limited amount of nutrients to be fermented at 6 h after feeding. Dieho et al. (2016) stated that concentrations of volatile fatty acids in rumen fluid mainly reflects the production and absorption rate and also the volume of rumen fluid. In this study, There was no significant (p>0.05) difference in the concentration of acetic, propionic and butyric acids between the young and mature goats. On the other hand, the higher feeding level resulted in higher (p<0.05) concentration of acetic, propionic and butyric acids at 6 h after feeding, as compared to the low feeding level, both in young and mature Kacang goats. The high feeding level resulted in higher feed intake, and in turn, increased concentration of volatile fatty acids in the rumen (Table 3).
The fermentation of feed by microbes in the rumen produced volatile fatty acids. The production of VFA in the rumen varies with the amount of feed intake. The concentration of ruminal VFA in this study was lower compared to values reported by Liang et al. (2023) who fermented corn 215 stalk mixed with rumen fluid and artificial saliva in reactor and found the concentrations of 61.3% acetic, 25.5% propionic and 25.5% butyric acids. There are several factors that influence the speed of VFA absorption, namely the length of the acid chain absorbed, acid concentration, pH, and osmotic pressure (Dieho et al., 2017; Dijkstra et al., 2020).
Acetate-propionate ratio of young and mature Kacang goats under different feeding levels was the not significantly (p>0.05) different at 0 h, 3 h and 6 h after feeding. The average of acetate-propionate ratio at 0, 3, and 6 h after feeding was 2.78 (Table 3). It indicated that the rate of production and absorption from the rumen wall of the two acids was similar. It was due to the composition of the feed used in this study was the same, so the concentrations of acetic and propionic acid were also similar in all treatments. Rumen condition is influenced by the ratio of forage to concentrate, degradation and molecular structure of the feed. Forage-dominated in diet stimulate acetate formation, whereas concentrate-dominated in diet encourage propionate formation (Cheng et al., 2021). Acetate-propionate ratio was also highly affected by nutrients contents of feed. The pattern of VFA composition is very important to consider as it will influence changes in the excess hydrogen available for methane formation (Khiaosa-ard and Zebeli, 2014; Luthfi et al., 2018). Luthfi et al. (2018) found that the optimum acetate-propionate ratio in animals being raised for fattening was 3 or less.
Rumen Ammonia: The concentration of rumen ammonia in young and mature Kacang goats under different feeding level was not significantly (p>0.05) different at all time measurements (Table 3). Protein of the diet in this study was degraded in a stable and slow rate in the rumen, both in young and mature Kacang goats, resulting in relatively stable ammonia productions. Ruminal ammonia concentration was highly affected by degradation crude protein content of feed (Li et al. 2019; Wang et al., 2023). The high DMI reduced the rate of protein fermentation, so that ammonia production decreases. Fernandez et al. (2012). Li et al. (2019) found that ammonia concentration is not always directly proportional to the amount of DMI. High NDF intake reduced protein degradation rates. It was due to cellulose and lignin in the plant cell walls that bind together with some feed proteins trapped in feed fibre. Li (2021) stated that lignin is based on its relative solubility and ability to combine with proteins to form a membrane-like layer that is resistant to protease enzymes or microbes. High intake of NDF inhibits microbial proteases from degrading protein thereby reducing ammonia production. Manoni et al. (2023) stated that a stable concentration of rumen ammonia in the rumen provides an advantage to livestock for reducing emissions of rumen ammonia which can cause bloating. A range of optimal ruminal ammonia concentration for microbial growth varies from 2.5 mM to 18.0 mM (Wanapat dan Pimpa, 1999, Wang dan Tan, 2013).
Rumen fluid pH value: As shown in Table 3, the pH of rumen fluid of young and mature Kacang goats under low and high feeding levels was not significantly (p>0.05) different. The initial pH of rumen fluid at 0 h before feeding was 6.26 then slowly decreased and reached 5.6 of pH. After 6 h feeding, the value of pH slightly returned up to be 5.9. The rumen fluid pH was highly affected by the concentration of VFA and ammonia in the rumen. The concentrations of ruminal VFA and ammonia were influence by dietary carbohydrate fermentation and protein degradation in the rumen (Humer et al., 2018; Li et al., 2019). The VFA and ammonia accumulated from carbohydrate fermentation and protein degradation influence rumen pH. The rumen pH is influenced by the concentration
Table 4: Methane emission, and energy and protein excretions of faeces and urine in young and mature Kacang goats
| Parameters | Young | Mature | Sig | |||
| Low | High | Low | Low | High | Low | |
|
CH4, g/d |
6.07 ± 1.07b |
12.34 ± 1.67a |
10.77 ± 2.86b |
12.50 ± 3.04a |
0.65 | 0.04 |
|
CH4, g/kgDMI |
25.44 ± 8.05 | 27.12 ± 6.80 | 33.39 ± 9.01 | 19.57 ± 5.36 | 0.61 | 0.66 |
|
CH4, g/ gBWG |
0.40 ± 0.11b |
0.30 ± 0.06a |
0.56 ± 0.13b |
0.22 ± 0.04a |
0.91 | 0.01 |
| Energy feces, MJ/d | 0.89 ± 0.17 | 1.27 ± 0.07 | 0.91 ± 0.12 | 1.69 ± 0.17 | 0.43 | 0.06 |
| Energy urine, MJ/d | 0.17 ± 0.01 | 0.20 ± 0.04 | 0.19 ± 0.03 | 0.25 ± 0.01 | 0.36 | 0.19 |
| N faeces, g/d |
0.54 ± 0.12xb |
0.75 ± 0.23xa |
0.52 ± 0.11yb |
1.36 ± 0.15ya |
0.01 | 0.00 |
| N urine, g/d | 5.48 ± 1.22 | 5.41 ± 2.06 | 0.52 ± 1.59 | 9.84 ± 2.59 | 0.08 | 0.16 |
| N Total/BWG |
0.41 ± 0.03b |
0.15 ± 0.01a |
0.35 ± 0.02b |
0.20 ± 0.01a |
0.87 |
0.00 |
The superscripts of x, y showing significant differences between age groups (p < 0.05)
The superscript of a, b showing significant differences between feeding levels (p < 0.05)
of VFA and ammonia (Meng et al., 2016; Djikstra et al., 2020). The rumen pH should ranges between 5.6 and 6.3 in order to maintain the rumen microbial activity (Amin and Mao, 2021). The VFA produced from carbohydrate fermentation in the rumen was not only for maintaining the microbial activity in the rumen, but productions were also absorbed from the rumen walls and sent to the liver and converted into glucose and used as a source of energy of the ruminant. About 75% of energy in the ruminant comes from VFA (Li et al., 2019).
Microbial Protein Production: Microbial protein production in this study was not significantly (p>0.05) different among the treatments (Table 3). The nonsignificant difference between the age of goats indicated that the rumen of the young goat had developed to be similar to that of the mature goat. Han et al. (2015) found that rumen microbial protein in goats was related to the rumen function maturity and rumen maturity in goats generally occurs at the age of 80 -110 days. The nonsignificant difference between feeding levels in microbial protein production was due to the rumen ammonia concentration at low and high feeding levels was similar (Table 3). Ammonia is the main nitrogen supply for microbial protein synthesis in the rumen (Wu et al., 2020) This study also indicated that at the low feeding level, the microbe population in the rumen had reached its optimum level, so that the increase of protein supply from the diet at the high feeding level did not increase rumen microbe population, and the amount of microbial protein being absorbed in the intestine was not altered.
Methane emission and nitrogen excretion
Methane emissions and nitrogen excretion trough the faeces and urine are presented in Table 4. Methane emission was not significantly (p>0.05) different between the ages of goat (p>0.05). The average methane emission in young and mature Kacang goats was 10.41 g/d. The methane emission of the high feeding level was significantly (p<0.05) higher than that of the low feeding level (12.42 g/d vs 8.42 g/d). Methane emission per feed intake was not significantly different among treatments either in age and feeding level (p>0.05). The average methane emission per feed intake was 26.38 g/kg DMI. Methane per BWG was not significantly (p>0.05) different between the ages of goat (averaged 0.35 g/ g BWG). The study showed that high feeding level resulted in lower methane emission per unit BWG (0.26 g/ g BWG vs 0.48 g/ g BWG). Carbohydrates fermentation results in volatile fatty acids (VFA). This process is accompanied by the release of gas output such as carbon dioxide (CO2) and methane (CH4) (Min et al., 2020). It indicated that high feeding level had high correlation with the methane emission. A higher methane emissions was produced by fermentation of high nutrient intake (Rianto et al., 2002, Luthfi et al., 2023).
Faecal energy in this study was not significantly (p>0.05) different between the age of goats and feeding levels. The average of energy in faeces was 1.19 MJ/d. On the other hand, nitrogen excreted through faeces of was significantly (p<0.05) different between the age of goats and between feeding levels (Table 4). The data showed the mature goat excreted more nitrogen than the young goat; the high feeding level resulted in higher nitrogen excretion through the faeces than the low feeding level. On the other hand, energy and nitrogen excretion via urine were not significantly (p>0.05) different, either between age of goats or between feeding levels. The average of energy and nitrogen excretion though urine were 20.25 MJ/d and 5.85 g/d, respectively. The metabolism of protein by rumen microorganisms is a key factor affecting nitrogen (N) excretion in ruminant (Tan et al., 2021). Nitrogen concentrations that was excreted trough faeces and urine are greatly affected by crude protein intake and are closely related to undegradable nitrogen (Huhtanen et al., 2015).
This study showed that N excretion per unit BWG in young and mature Kacang goats was not significantly (p>0.05) different, but it was significantly (p<0.05) different between low and high level feeding (Table 4). High feeding had lower N excretion per unit BWG than those of low feeding levels (0.17 g/BWG vs 0.38 g/BWG). It indicated that at high level feeding, the utilization of feed was more efficient in term of environmental cost, as methane emission and N excretion per unit of BWG at high level feeding was lower than that of low level feeding. Luthfi et al. (2022) found that increasing the level of feeding significantly increases nutrient utilization, body weight gain and feed efficiency. Shah et al. (2022) found that a high of nitrogen and carbohydrate supply in the rumen increased the digestibility of DM and OM, so that N retention was efficiently used and improved feed utilization for increasing body weight of Kacang goats.
Conclusion
In conclusion, both young and mature Kacang goats demonstrated similar abilities to ferment feed effectively, resulting in comparable pH levels, VFA concentrations, and rumen ammonia levels. However, goats fed at higher levels showed increased concentrations of ruminal VFAs and ammonia. Furthermore, these goats exhibited lower methane emissions and nitrogen excretion per unit of body weight gain; higher feeding levels correlated with reduced methane emissions and nitrogen excretion per unit of body weight gain. Consequently, adopting higher feeding levels could potentially reduce the environmental impact of goat meat production, particularly in terms of methane emissions and nitrogen excretion. Therefore, raising Kacang goats with higher feeding levels is recommended to promote environmentally friendly animal production.
acknowledgements
Our sincere gratitude to the Ministry of Research, Technology and Higher Education of Indonesia for providing a scholarship to the author. Our greatest appreciation to Prof. Edy who always being the best teacher.
conflict of interest
The authors have declared no conflict of interest.
novelty statement
There have limited studies on the impact and efficiency of feeding based on the maintenance and production requirements of young and mature Kacang goats on its fermentation profile in the rumen and methane emission.
authors contribution
The authors confirm contribution to the paper as follows: study conception and design: Edy Rianto, Nadlirotun Luthfi, Nurul Mukminah; data collection: Nadlirotun Luthfi; Nurul Mukminah; analysis and interpretation of results: Endang Purbowati, Christina Maria Sri Lestari, Agung Purnomoadi; draft manuscript preparation: Edy Rianto, Nadlirotun Luthfi. All authors reviewed the results and approved the final version of the manuscript.
References
Abdelsattar M.M, Zhao W, Saleem A.M, Kholif A.E, Vargas-Bello-Pérez E, Zhang N. (2023). Physical, Metabolic, and Microbial Rumen Development in Goat Kids: A Review on the Challenges and Strategies of Early Weaning. Animals (Basel). 13(15):2420. doi: https://doi.org/10.3390/ani13152420.
Ahmad A. A., Yang C., Zhang J., Kalwar Q., Liang Z., Li C., et al. (2020). Effects of dietary energy levels on rumen fermentation, microbial diversity, and feed efficiency of Yaks (Bos grunniens). Front. Microbiol. 11:625. https://doi.org/10.3389/fmicb.2020.00625
Aluns M.S., N. Luthfi. (2018). The Productivity of Male Thin-Tailed Lambs and Sheep Fed Complete Feed. OP Conf. Ser.: Earth Environ. Sci. 119 012047.
Amin A.B., S. Mao. (2021). Influence of yeast on rumen fermentation, growth performance and quality of products in ruminants: A review: Anim. Nutrit. 7 (1): 31-41. https://doi.org/10.1016/j.aninu.2020.10.005.
Aprilliza M. N., A. Purnomoadi, E. Rianto, dan D. Pamungkas. (2020). Deposisi Energi dan Produksi Gas Metana pada Domba Lokal dengan Waktu Pemberian Pakan yang Berbeda. In Prosiding Seminar Nasional Teknologi Peternakan dan Veteriner. Pp: 514–519. In Bahasa Indonesia https://doi.org/10.14334/pros.semnas.tpv-2019-p.514-519.
Bach A., Calsamiglia S., Stern M.D. (2005). Nitrogen metabolism in the rumen. J. Dairy Sci. 88: 9–21.
Brouwer E. (1965). Report of sub-committee on constants and factors. Eaap Publication No 11. Academic Press. London.
Chen X. B, M.J. Gomes. (1992). Estimation of Microbial protein supply to sheep and cattle based on urinary excretion of Purine derivates: an overview of the technical details. International feed resources unit rowett research intitute, Bucksburn Aberdeen, UK.
Cheng L., G. Cantalapiedra-Hijar, S.J. Meale, I. Rugoho, A. Jonker, M.A. Khan, O. Al-Marashdeh, R.J. Dewhurst. 2021. Review: Markers and proxies to monitor ruminal function and feed efficiency in young ruminants. Animal, 15 (10): 100337
Choirunnisa, R., P. Ari, N. Luthfi, M. Arifin, Sutaryo A. Purnomoadi. (2016). Correlation between Crude Protein Levels in the Diets and Carcass Weight and Carcass Percentage in Thin Tailed Lambs. In: Enhancing Synergistic Roles of Stakeholders for Development of Sustainable Livestock Production : The 3rd Animal Production International Seminar and The 3rd ASEAN Regional Conference on Animal Production (3rd APIS & ARCAP 2016), 19-21 Oktober 2016, Batu, Malang, Indonesia.
Dieho K , J van,Baal, L Kruijt, A Bannink, JT Schonewille, D Carreño, WH Hendriks, J Dijkstra. (2017). Effect of supplemental concentrate during the dry period or early lactation on rumen epithelium gene and protein expression in dairy cattle during the transition period. J. Dairy Sci., 100 (2017): 7227-7245.
Dieho K., J. Dijkstra, J.T. Schonewille, A. Bannink. (2016). Changes in ruminal volatile fatty acid production and absorption rate during the dry period and early lactation as affected by rate of increase of concentrate allowance. J. Dairy Sci. 99 (7): 5370-5384. Doi: https://doi.org/10.3168/jds.2015-10819.
Fernández H.T., F. Catanese, G. Puthod, R.A. Distel, J.J. Villalba. (2012). Depression of rumen ammonia and blood urea by quebracho tannin-containing supplements fed after high-nitrogen diets with no evidence of self-regulation of tannin intake by sheep. Small Rumin. Res. 105 (1–3): 126-134. https://doi.org/10.1016/j.smallrumres.2012.03.013.
Han X., Yang Y., Yan H., Wang X., Qu L., Chen Y. (2015). Rumen bacterial diversity of 80 to 110-day-old goats using 16s rrna sequencing. PLoS ONE. 2015;10:e0117811. https://doi.org/10.1371/journal.pone.0117811.
Hristov A. N., J. Oh, J. L. Firkins, J. Dijkstra, E. Kebreab, G. Waghorn, H.P.S. Makkar, A.T. Adesogan, W.Yang, C. Lee, P.J. Gerbe, B. Henderson, J.M. Tricarico. (2013). Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. American Societ. Anim. Sci., 91: 5045–5069. https://doi.org/10.2527/jas2013-6583.
Huhtanen P., E.H. Cabezas-Garcia, S.J. Krizsan, K.J. Shingfield. (2015). Evaluation of between-cow variation in milk urea and rumen ammonia nitrogen concentrations and the association with nitrogen utilization and diet digestibility in lactating cows, J. Dairy Sci., 98 (5): 3182-3196. https://doi.org/10.3168/jds.2014-8215.
Humer E., Petri R.M., Aschenbach J.R., Bradford B.J., Penner G.B., Tafaj M., Südekum K.H., Zebeli Q (2018). Invited review: Practical feeding management recommendations to mitigate the risk of subacute ruminal acidosis in dairy cattle. J. Dairy Sci. 101:872–888. https://doi.org/10.3168/jds.2017-13191. 29153519.
Kawashima, T., Sumamal, W., Terada, F., Shibata, M. (2001). Respiration Trial System Using Ventilated Flow-through Method with a Face Mask. JIRCAS J. No.9: 53 74
Kenny D.A., C. Fitzsimons, S.M. Waters, M. McGee. (2018). Invited review: Improving feed efficiency of beef cattle – the current state of the art and future challenges. Animal. 12 (9): 1815-1826. https://doi.org/10.1017/S1751731118000976.
Li M, M., Srijan Sengupta, Mark D. Hanigan. (2019). Using artificial neural networks to predict pH, ammonia, and volatile fatty acid concentrations in the rumen. J. Dairy Sci. 102 (10): 8850-8861. https://doi.org/10.3168/jds.2018-15964.
Li J., Yan H., Chen J., Duan C., Guo Y., Liu Y., Zhang Y., Ji S. (2022). Correlation of Ruminal Fermentation Parameters and Rumen Bacterial Community by Comparing Those of the Goat, Sheep, and Cow In Vitro. Fermentation., 8: 427. https://doi.org/10.3390/fermentation8090427
Li X. (2021). Plant cell wall chemistry: implications for ruminant utilisation. J. Appl. Anim. Nutrit. 9 (1): 31-56. DOI: https://doi.org/10.3920/JAAN2020.0017
Liang J., M. Zubair, Le Chen, J. Chang, W. Fang, M. Nabi, W. Yang, Y. Zhang, Y. Li, P. Zhang, G. Zhang, A. Wang. (2023). Rumen microbe fermentation of corn stalk to produce volatile fatty acids in a semi-continuous reactor, Fuel, Volume 350, 2023, 128905. https://doi.org/10.1016/j.fuel.2023.128905.
Luo J., A.L Goetsch, T Sahlu, I.V Nsahlai, Z.B Johnson, J.E Moore, M.L Galyean, F.N Owens, C.L Ferrell. (2004). Prediction of metabolizable energy requirements for maintenance and gain of preweaning, growing and mature goats. Small Rumin. Res., 53 (3): 231-252. https://doi.org/10.1016/j.smallrumres.2004.04.006.
Luthfi N., V. Restitrisnani, M. Umar. (2018). The Optimation of Crude Fiber Content of Diet for Fattening Madura Beef Cattle to Achieve Good A:P Ratio and Low Methane Production. IOP Conf. Ser.: Earth Environ. Sci. 119 012056.
Luthfi N., Lestari C. M. S., Purnomoadi A. (2014). Ruminal fermentation and blood glucose at low and high level intake of growing and mature Kacang goat. J. Indonesian Trop. Anim. Agricult., 39(3): 152-158.
Luthfi N., Solkhan M., Suryani H. F., Hindratiningrum N. (2023). The Determination of Nutrient Intake on Productivity and Potential Methane Emission of Fat-Tailed Sheep Fed Odot Grass as a Source of Crude Fibre. J. Sain Peternakan Indonesia., 18(2): 88–92. https://doi.org/10.31186/jspi.id.18.2.88-92
Manoni M, M. Terranova, S. Amelchanka, L. Pinotti, P. Silacci, M. Tretola. (2023). Effect of ellagic and gallic acid on the mitigation of methane production and ammonia formation in an in vitro model of short-term rumen fermentation. Anim. Feed Sci. Technol. 305: 115791. https://doi.org/10.1016/j.anifeedsci.2023.115791.
Mariam M., Asmuddin Natsir, Syahriani Syahrir. (2023). Consumption and Digestibility Studies in Goats Fed with Complete Feed Corn Tumpi and Red Dragon Fruit Peel Flour (HylocereusSp.). Hasanuddin J. Anim. Sci. 5 (1): 10-17.
Meng Y., J. Mumme, H. Xu, K.J. Wang. (2016). A biologically inspired variable-pH strategy for enhancing short-chain fatty acids (SCFAs) accumulation in maize stalk fermentation Bioresour. Technol, 201 (2016): 329-336
Min B.R., S. Solaiman, Heidi M. Waldrip, David Parker, Richard W. Todd, David Brauer. (2020). Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options, Anim. Nutrit., 6 (3): 231-246. https://doi.org/10.1016/j.aninu.2020.05.002.
Nabila F., Restitrisnani V., Adiwinarti R., Purnomoadi A. (2021). Feed protein utilization and nitrogen emission of young and mature Kejobong goats fed different ratios of concentrate and forage. J. Ilmu-Ilmu Peternak., 31(2): 160-167. https://doi.org/10.21776/ub.jiip.2021.031.02.09
Pokharel K, Peippo J, Honkatukia M, Seppälä A, Rautiainen J, Ghanem N, Hamama TM, Crowe MA, Andersson M, Li MH, Kantanen J. (2018). Integrated ovarian mRNA and miRNA transcriptome profiling characterizes the genetic basis of prolificacy traits in sheep (Ovis aries). BMC Genomics. 19(1):104. https://doi.org/10.1186/s12864-017-4400-4.
Rianto E., C.J. Thwaites dan J.V. Nolan. (2002). Feed Digestibility and Microbial Nitrogen Production in Pair-fed Lambs at 20oc and 50o/42oc when Water Intake was Restricted at the Higher Temperature. Proceedings The Third International Seminar on Tropical Animal Production: Animal Production and Total Management of Local Resources. Yogyakarta, 15-16 October, 2002. Hal. 71-77.
Riaz M.Q., K.-H. Südekum, M. Clauss, A. Jaya-negara. (2014). Voluntary feed intake and digestibility of four domestic ruminant spe-cies as influenced by dietary constituents: A meta-analysis, Lives. Sci.162: 76-85
Shah A.M., Qazi I.H., Matra M., Wanapat M (2022). Role of Chitin and Chitosan in Ruminant Diets and Their Impact on Digestibility, Microbiota and Performance of Ruminants. Fermentation. 8: 549. https://doi.org/10.3390/fermentation8100549.
Sun X, Chen A, Pacheco D, Hoskin SO, Luo D. (2019). Sheep Rumen Fermentation Characteristics Affected by Feeding Frequency and Feeding Level When Fed Fresh Forage. Animals (Basel). 10(1):7. https://doi.org/10.3390/ani10010007.
Tan P., H. Liu, J. Zhao, X. Gu, X. Wei, X. Zhang, N. Ma, Lee J. Johnston, Y. Bai, W. Zhang, C. Nie, X. Ma. (2021). Amino acids metabolism by rumen microorganisms: Nutrition and ecology strategies to reduce nitrogen emissions from the inside to the outside. Sci. Total Environ., Volume 800. 149596. https://doi.org/10.1016/j.scitotenv.2021.149596.
Tan P., H. Liu, J. Zhao, X. Gu, X. Wei, X. Zhang, N. Ma, Lee J. Johnston, Y. Bai, W. Zhang, C. Nie, X. Ma. (2021). Amino acids metabolism by rumen microorganisms: Nutrition and ecology strategies to reduce nitrogen emissions from the inside to the outside, Sci. Total Environ., Volume 800. 149596, https://doi.org/10.1016/j.scitotenv.2021.149596.
Wanapat M., O. Pimpa. (1999). Effect of ruminal NH3-N levels on ruminal fermentation, purine derivatives, digestibility and rice straw intake in swamp buffaloes. Asian Austral. J. Anim., 12 (6): 904-907,
Wang P., Z. Tan. (2013). Ammonia assimilation in rumen bacteria: a review. Anim. Biotechnol., 24 (2): 107-128.
Wang Q, Zeng Y, Zeng X, Wang X, Wang Y, Dai C, Li J, Huang P, Huang J, Hussain T, Zhu M, Yang H. (2021). Effects of Dietary Energy Levels on Rumen Fermentation, Gastrointestinal Tract Histology, and Bacterial Community Diversity in Fattening Male Hu Lambs. Front Microbiol. 12:695445. https://doi.org/10.3389/fmicb.2021.695445.
Wang XY, Shi BG, Zuo Z, Qi YP, Zhao SJ, Zhang XP, Lan LJ, Shi Y, Liu X, Li SB, Wang JQ, Hu J. (2023). Effects of two different straw pellets on yak growth performance and ruminal microbiota during cold season. Animals. 13:335. https://doi.org/10.3390/ani13030335.
Weatherburn M. W. (1967). Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39 971–974. Weatherburn M. W. (1967). Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39 971–974. https://doi.org/10.1021/ac60252a045
Weimer, P. J. (2015). Redundancy, resilience, and host specificity of the ruminal microbiota: implications for engineering improved ruminal fermentations. Front. Microbiol. 6:296. https://doi.org/10.3389/fmicb.2015.00296
Wu J, X. Zhang, R. Wang, M. Wang, Z. He, Z. Tan, J. Jiao. (2020). Replacing corn grain with corn gluten feed: Effects on the rumen microbial protein synthesis, functional bacterial groups and epithelial amino acid chemosensing in growing goats. Anim. Feed Sci. Technol., 270. 114684. https://doi.org/10.1016/j.anifeedsci.2020.114684.
Wu Q., Zeng D., Niu Z., Yang X., Liu Y., Yin F., Zhao Z., Chai J., Gao Z. (2024). Investigations of morphological development, digestive enzymes and microbiome of the gastrointestinal tract in goat kids from birth to adulthood. J. Appl. Anim. Res., 52(1). https://doi.org/10.1080/09712119.2023.2295456.
Yin X, Ji S, Duan C, Tian P, Ju S, Yan H, Zhang Y, Liu Y (2021). Age-Related Changes in the Ruminal Microbiota and Their Relationship With Rumen Fermentation in Lambs. Front. Microbiol. 12:679135. Doi: https://doi.org/10.3389/fmicb.2021.679135
To share on other social networks, click on any share button. What are these?






