Quantitative Evaluation of Methylmercury Bioaccumulation in Rotifer Brachionus plicatilis by AIEgen
Quantitative Evaluation of Methylmercury Bioaccumulation in Rotifer Brachionus plicatilis by AIEgen
Hangyu Lin1, Minhui Chen1, Hao Yang1, Xinying Xia1, Huaying Zhou1, Xi Song1 and Tao He1,2*
1College of fisheries, Southwest University, Chongqing, China, 400715.
2Key Laboratory of Freshwater Fish Reproduction and Development (Ministry of Education), Key Laboratory of Aquatic Science of Chongqing, China, 400715.
Hangyu Lin and Minhui Chen contributed equally to this study.
ABSTRACT
Mercury ion (Hg2+) can be transformed to methylmercury (MeHg) by a variety of aquatic microorganisms in water. MeHg accumulates in fish through the food chain and eventually poses a particular challenge to public health. In this study, we used a new tool named AIEgen (Aggregation-induced emission fluorogen) to quantify the process of MeHg bioaccumulation in vivo on the species of rotifers Brachionus plicatilis. We analysed the relationship between the ratio of photoluminescence (PL) intensities (I585/I480) and MeHg concentration (CMeHg) to create a master curve for calculating MeHg concentration based on the measurement of PL intensities. Quantitative results showed that the bioaccumulation of MeHg in the rotifers increased from 1.65 μg at 30 min to 2.33 μg at 180 min and reached a plateau concentration at the time of 180 min. After being transferred into artificial seawater without MeHg for 30 min, the rotifers released 0.38 μg MeHg into the medium, and the concentration of MeHg in water was kept relatively stable in the next 150 min. This study indicates that the bioaccumulation of MeHg in the body of zooplankton can be quantified by AIEgen effectively, and the main process of MeHg accumulation by rotifers in water may via passive transport.
Article Information
Received 23 Feabruary 2023
Revised 20 March 2023
Accepted 09 April 2023
Available online 21 July 2023
(early access)
Published 06 January 2025
Authors’ Contribution
HL presented the concept. MC, HY and XX performed investigation. MC formal analysis. HY and HZ curated data. XS performed formal analysis. TH planned methodology, contributed in editing, validation, funding
acquisition. HL and TH wrote the manuscript.
Key words
MeHg, Bioaccumulation, Rotifer, Aggregation-induced emission fluorogen, zooplankton
DOI: https://dx.doi.org/10.17582/journal.pjz/20230223030203
* Corresponding author: [email protected]
0030-9923/2025/0001-0159 $ 9.00/00
Copyright 2025 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Mercury (Hg), a toxic element, is widely spread in the environment (Ullrich et al., 2001). Hg exists in the environment in the forms of elemental Hg (Hg0), inorganic Hg (Hg2+), and organomercury compounds, such as monomethyl mercury (CH3Hg+, methylmercury, MeHg) (Ma et al., 2017). Besides, Hg2+ is convertible to organic mercury and MeHg is the most toxic organic form of mercury. MeHg is a potent toxin, bioaccumulated and concentrated through the aquatic food chain (Mergler et al., 2007). Atmospheric deposition of mercury (Hg) to surface water is one of the primary sources of Hg in aquatic environments and eventually drives MeHg toxin accumulation in fish (Luo et al., 2017), a greatly nutritious food, with known benefits for human health. Thus, methylmercury pollution poses human health risks via dietary fish consumption (Kucharzyk et al., 2015).
In 2001, the concept of aggregation-induced emission (AIE) was coined by Ben Zhong Tang (Luo et al., 2001). The fluorogens with AIE attribute have been referred to as AIEgens, which have attracted considerable attention due to their highly emissive behavior of aggregated illumination (Mei et al., 2015). AIEgen (m-TPE-RNS), a kind of Hg2+ sensor with high selectivity and high sensitivity has been developed (Chen et al., 2017; Ruan et al., 2015) and used to detect and quantify the Hg2+ bioaccumulation in algae, and Hg2+ release from algae after bioaccumulation (Jiang et al., 2016). In previous research, we found that the MeHg detection mechanism for a specific AIEgen is similar to the reaction between Hg2+ and AIEgen. Thus, we quantitatively evaluated the bioaccumulation of MeHg inside the waterflea Daphnia carinata by AIEgen (He et al., 2022).
Zooplankton play an important role in the biological carbon pump and serve as a trophic link between primary producers and predators (Petrik et al., 2022). Brachionus plicatilis is a widely-distributed small invertebrate in the global oceans, which is raised in the aquaculture as food for fish, shrimp and crab. Rotifers have been widely used as a model organism for toxicological studies due to their high sensitivity to various toxicants and ease of culture in the lab (Zheng et al., 2017).
In the present study, we investigated the toxic effects of MeHg on rotifer B. plicatilis. The main aim of this study was to use a novel AIEgen method to quantitatively evaluate the bioaccumulation of MeHg inside the rotifer. Thus, it would supplement the MeHg toxicity data to marine organisms and improve our understanding of the dynamics of MeHg transfer in aquatic systems.
Materials and Methods
Materials
Rotifers Brachionus plicatilis used in this study were resting eggs provided by College of Fisheries, Southwest University (Chongqing, China). The resting eggs were originally collected from Ningbo, China. B. plicatilis used in our research were hatching from the same resting eggs which represented the same strain. The rotifers were cultured with the density of 1000 ind mL-1 in artificial seawater (salinity of 20-35‰) under conditions with a L:D 12:12 h photoperiod at 3000 lx in an illumination incubator with the temperature maintained at 26 ℃. The artificial seawater comprised NaCl 21.1 g/L, Na2SO4 3.55 g/L, KCl 5.99 g/L, NaHCO3 2.94 g/L, KBr 86.0 mg/L, H3BO3 23.0 mg/L, NaF 3.00 mg/L, MgCl2·6H2O 9.96 g/L, CaCl2 10.1 g/L, SrCl2·6H2O 22.0 mg/L. All reagents were obtained from Aladdin (Shanghai, China) unless otherwise specified. The AIEgen (m-TPE-RNS) was provided by AIEgen Biotechnology (Hong Kong, China) and dissolved in acetonitrile (ACN). Stock solutions of AIEgen and CH3HgCl (MeHg) at a concentration of 0.1 mmol L-1 were prepared. The test solutions for MeHg determination contained 1 μmol L-1 m-TPE-RNS and different concentrations of MeHg. PL intensities were read on a fluorescent spectrometer (Edinburgh, England) with the excitation wavelength at 350 nm. The maximum photoluminescence (PL) intensity of m-TPE-RNS occurred in water/ACN mixtures with 60% water volume fractions (fw, vol%). Therefore, the fw was fixed at 60% in water/ACN mixtures in the subsequent experiments. The intense emission peak of PL was displayed at 480 nm and 585 nm in the reaction of m-TPE-RNS and MeHg and the PL intensity ratio was calculated at these two wavelengths (I585/I480) (He et al., 2022).
Survival rate of rotifer after incubation with MeHg
Rotifers were harvested from an aquarium tank with a 50-μm mesh net and introduced into a series of water samples containing different MeHg concentrations (0, 1, 3, 5 and 10 μmol L-1) with the rotifer density of 200 ind mL-1 for 1 h, followed by a period of recovery in artificial seawater without MeHg for 48 h. Survival was calculated based on the number of live and dead animals in each vial to determine the toxicity of MeHg to Brachionus plicatilis at each concentration.
Time optimization for staining MeHg with AIE
After mixing 10 μL of the m-TPE-RNS stock solution with 390 μL acetonitrile (ACN) and 590 μL Milli Q water, 10 μL of MeHg stock solution was added to reach the water: ACN ratio of 3:2. The photoluminescence (PL) intensities were measured at 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 and 60 min on the fluorescent spectrometer to determine value of the ratio of PL intensities (I585/I480) over time.
Development of the master curve for MeHg determination
To develop the master curve for MeHg determination, a series of MeHg concentrations (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.1, 1.2, 1.3, 1.4 and 1.5 μmol L-1) were detected at a constant AIE concentration (1 μmol L-1) over the abovementioned time periods. After the PL intensities were obtained, the PL master curve was developed to show the relationships between PL intensity ratio (I585/I480) and MeHg concentrations.
Rotifers density-dependent MeHg absorption
To determine the MeHg absorption by rotifer, a series of rotifer densities (0, 100, 200, 400, 600, 800 and 1000 ind mL-1) were prepared by diluting the stock rotifer culture with artificial seawater. MeHg was added to each rotifer sample to reach a MeHg concentration of 1 μmol L-1. After incubation for 30 min, the rotifers were removed by a mesh net. Then, the PL intensities of each residue solution were determined by fluorescence spectrometer and the amount of MeHg in each residue solution was calculated by the AIE method using the developed master curve.
Quantification of MeHg bioaccumulation in Brachionus plicatilis
Rotifers were harvested by the method above, and were adjusted to a density of 400 ind mL-1 with artificial seawater, to which MeHg was added to the final concentration of 1 μmol L-1. Group 1 was designed to determine the directional movement of MeHg dispersal in rotifers from the environment. The supernatant (600 μL) was collected from the B. plicatilis-MeHg solution at the times of 30, 60, 90, 120, 150 and 180 min, respectively. Subsequently, each of these supernatants was mixed with 400 μL AIEgen in the ACN solution at the water to ACN ratio of 3–2. The PL intensities of each stained solution were determined by fluorescence spectrometer. Group 2 was set up to quantify the release of MeHg from rotifers after incubation in artificial seawater containing MeHg (1 μmol L-1) for 60 min. The rotifers were rinsed twice with artificial seawater at the beginning of the release trial. After incubation for the same time regimes as in group one, 600 μL supernatant was collected from each rotifer solution for MeHg quantification. The MeHg concentration was determined by the PL intensities read on the fluorescence spectrometer.
Statistical analysis
All data were analyzed by Excel 2016 and SPSS18.0 using univariate ANOVA. The amount of bioaccumulation (At), bioaccumulation efficiency (Et) and bioaccumulation ratio (Rt) of MeHg in rotifer were calculated as (Jiang et al., 2016).
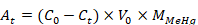
Where At is the amount of MeHg accumulated in rotifer at time t; C0 is the initial concentration of MeHg in the culture medium; Ct is the concentration of MeHg in the culture medium at time t; V0 is the volume of culture medium; MMeHg is the molar mass of MeHg.
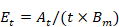
Where Et is the bioaccumulation efficiency of MeHg in rotifers at time t; t is the indicated time for MeHg absorption; Bm is the biomass of Brachionus plicatilis (1000 ind ≈ 0.6 mg) in the medium.
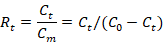
Where Rt is the bioaccumulation ratio of MeHg in rotifers at time t; Ct is the amount of MeHg absorbed by rotifers at the indicated time t. Cm is the amount of MeHg in the medium at the indicated time t. C0 is the initial amount of MeHg added with C0 = Ct + Cm.
ResultS
Survival rate of rotifer after incubation with MeHg
An acute toxic experiment was conducted to further determine the effect of MeHg toxicity on rotifer B. plicatilis. Figure 1 shows the survival rate of rotifer after 1-h incubation at different MeHg concentrations, followed by 48-h recovery in clean artificial seawater. After incubation with 1 µmol L-1 MeHg, 90% rotifers survived, which was not significantly different from the control group (CMeHg = 0 μmol L-1), but rotifer survival rates decreased to 65%, 40% and 0 (after 36 h recovery) after MeHg incubation at 3 µmol L-1, 5 µmol L-1 and 10 µmol L-1, respectively. Therefore, the MeHg concentration of 1 μmol L-1 was used in subsequent studies.
Development of the AIE method for MeHg determination
The effect of time elapse on PL intensity ratio associated with AIE and MeHg reaction is shown in Figure 2. With fixed AIE and MeHg concentrations at 1 µmol L-1 and time elapse at 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55 and 60 min, the PL intensity ratio increased with reaction time during the initial 20 min, and reached a plateau at 20 min to 40 min. A slight decrease was observed from 40 min to 60 min. Therefore, 30 min was used as the reaction time of mixtures with AIE and MeHg in the subsequent study.
After adding 1 µmol L-1 AIEgen to MeHg with different concentrations, we separately calculated the PL intensity ratio and developed the regression equation between the PL ratio (I585/I480) and MeHg concentration (CMeHg). As shown in Table I, a variety of function curves were fitted through SPSS, and the results showed that the power function equation had the highest confidence, R2 = 0.996, so the power function equation was used as the standard curve for detecting MeHg. As shown in Figure 3, the power function between CMeHg and I585/I480 was yielded as:
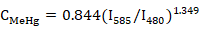
Table I. Fitting function equations between the PL ratio (I585/I480) and MeHg concentration (CMeHg).
|
Functional relationship |
Equation |
R2 |
Adjusted R2 |
Std. Error |
|
Power function |
y = 0.844x1.349 |
0.996 |
0.996 |
0.082 |
|
Ternary function |
y= -0.313x3+0.823x2 + 0.355x - 0.005 |
0.995 |
0.991 |
0.043 |
|
Binary function |
y = 0.119x2 + 0.78x - 0.060 |
0.993 |
0.991 |
0.044 |
|
Linear function |
y = 0.955x - 0.101 |
0.991 |
0.990 |
0.045 |
|
Exponential function |
y = 0.062e2.481x |
0.869 |
0.847 |
0.490 |
|
Logarithmic function |
y = 0.449ln(x) + 0.863 |
0.850 |
0.824 |
0.189 |
Quatitative evaluation of bioaccumulation of MeHg by the rotifer
As shown in Figure 4, with an increase in rotifer density, the amount MeHg bioaccumulation increased in the rotifer. The linear equation between rotifer density and the amount MeHg bioaccumulation (At) was yielded as: y = 0.008x + 0.7494, R² = 0.9968. In the solution with MeHg (1 µmol L-1), the bioaccumulation efficiency (Et) of MeHg decreased from 2.30 μg mg-1 h-1 at a low rotifer density of 100 ind ml-1 to 0.40 μg mg-1 h-1 at a high rotifer density of 1000 ind ml-1.
Figure 5 shows the amount of bioaccumulation (At) and bioaccumulation efficiency (Et) of MeHg inside rotifers over time. The bioaccumulation was a time- and MeHg concentration-dependent process. In the medium with 1 µmol L-1 MeHg, the amount of MeHg inside rotifers increased from zero to 1.65 µg (5.02 μg in the control group) within the first 30 min, followed to 2.33 µg at 180 min and reached a plateau concentration at the time of 180 min. The bioaccumulation efficiency (Et) of MeHg decreased from 0.91 μg mg-1 h-1 at 30 min to 0.22 μg mg-1 h-1 at 180 min. The bioaccumulation ratio (Rt) of MeHg in rotifer were calculated, the results showed that a total of 86.6% MeHg in the 1 µmol L-1 MeHg medium was transferred from the environment to the rotifers within 180 min.
After incubation for 60 min in the medium with 1 µmol L-1 MeHg, the amount of MeHg inside rotifers increased from zero to 1.97 µg. Figure 6 (Note: Control: The amount of MeHg bioaccumulation in rotifers within 60 min) shows the MeHg release from rotifers to water at different times. After being transferred into artificial seawater for 30 min, the rotifers released 0.38 µg MeHg into the clean water, and the MeHg concentration in water was kept relatively stable in the next 150 min (the amount of MeHg between 0.38 to 0.46 µg). Calculations showed that a total of 23.7% MeHg in rotifers was released to the environment within 180 min, and most MeHg retained in rotifers.
Discussion
Rotifers have been an effective indicator of environmental risk assessment because of their widely distributed and sensitive to environmental change (Duggan et al., 2002). The effects of mercury on the life parameters of rotifers have been reported. The estimated LC50 values for rotifer Brachionus koreanus at 24 h exposure was 2.964 μg L-1 MeHg (Lee et al., 2017). It was also reported that 0.005 mg L-1 Hg2+ would affect the survival rate and reproductive capacity of Brachionus rubens (Sarma et al., 2005). However, the non-lethal effect and inchoate biological response of low-concentration pollution are always not enough in the environmental risk assessment (Maier et al., 2004; Van Der Oost et al., 2003). In the present study, we found that high concentration MeHg (10 µmol L-1) in short term (60 min) can lead to irreversible damage to rotifers. For example, after incubation for 60 min with 10 μmol L-1 MeHg, the essential biological functions of rotifers were impaired, including the stop of movement and ingestion, and all rotifers were dead within 36 h after transfer to clean water. Nevertheless, after incubation for 60 min with 1 µmol L-1 MeHg, 90% rotifers survived, which was not significantly different from the control group (CMeHg = 0 μmol L-1).
Rotifer density is an important parameter influencing the process of bioaccumulation. In this study, the bioaccumulation of MeHg in rotifers increased with the climb of rotifer density, while the bioaccumulation efficiency showed a decreasing trend. This is similar to the results of the study on Hg2+ accumulation by rotifers Brachionus plicatilis (Jiang et al., 2017). In the solution with 1.355 μg mL−1 HgCl2 (5 μmol L-1), the bioaccumulation efficiency of Hg2+ decreased from 5.28 μg mg−1 h−1 at the rotifer density of 0.093 mg mL−1 to 2.61 μg mg−1 h−1 when the rotifer density increased to 0.375 mg mL−1.
In this study, the amount of bioaccumulation and bioaccumulation efficiency of MeHg in rotifers changed continuously over time. The bioaccumulation of MeHg in rotifers increased with the passage of time, while the bioaccumulation efficiency showed a decreasing trend. This is similar to the results of Hg2+ accumulation in Brachionus plicatilis (Jiang et al., 2017) and Euglena gracilis (Jiang et al., 2016). There is evidence that passive transport seemed to be the major pathway for most phytoplankton to acquire MeHg (Lee and Fisher, 2016). The speed of passive transport decreases with the increase of concentration in vivo, which is similar to the result of MeHg accumulation by rotifers in this study, indicating that the main process of MeHg accumulation by rotifers in water may via passive transport. Rotifers could rapidly absorb MeHg from the environment, and nearly half of the supplied MeHg was absorbed within 180 min. This is similar to MeHg uptake by phytoplankton (Liu et al., 2011). The MeHg released into the water by rotifers became stable for a short time (30 min) and showed similar amount of release in the next 150 min. Only a small fraction (23.7%) of MeHg was excreted by rotifers. It is inferred that the MeHg absorbed by rotifers is likely to combine with proteins, forming MeHg-binding proteins and thus being accumulated by the rotifers.
Acknowledgement
This work was carried out at the College of Resources and Environment, Southwest University. The authors thank Dr. Songzhang Li for his technical support.
Funding
This research was supported by the Natural Science Foundation of Chongqing, China (No. cstc2021jcyj-msxmX0100) and National Training Program of Innovation and Entrepreneurship for Undergraduates (No. 202110635071).
IRB approval
All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Southwest University.
Ethical statement
All state and institutional guidelines for the care and use of animals were followed in this research.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Chen, Y., Zhang, W., Cai, Y., Kwok, R.T.K., Hu, Y., Lam, J.W.Y., Gu, X., He, Z., Zhao, Z., Zheng, X., Chen, B., Gui, C. and Tang, B.Z., 2017. AIEgens for dark through-bond energy transfer: Design, synthesis, theoretical study and application in ratiometric Hg2+ sensing. Chem. Sci. (Cambridge), 8: 2047-2055. https://doi.org/10.1039/C6SC04206F
Duggan, I.C., Green, J.D. and Shiel, R.J., 2002. Distribution of rotifer assemblages in North Island, New Zealand, lakes: Relationships to environmental and historical factors: New Zealand rotifer distribution. Freshw. Biol., 47: 195-206. https://doi.org/10.1046/j.1365-2427.2002.00742.x
He, T., Mao, X., Lin, H., Hassan, M.M., Zhu, S., Lu, Q., Qin, J. and Su, S., 2022. Methylmercury bioaccumulation in water flea Daphnia carinata by AIEgen. Ecotoxicol. Environ. Saf., 248: 114271. https://doi.org/10.1016/j.ecoenv.2022.114271
Jiang, Y., Chen, Y., Alrashdi, M., Luo, W., Tang, B. Z., Zhang, J., Qin, J. and Tang, Y., 2016. Monitoring and quantification of the complex bioaccumulation process of mercury ion in algae by a novel aggregation-induced emission fluorogen. RSC Adv., 6: 100318-100325. https://doi.org/10.1039/C6RA22190D
Jiang, Y., He, T., Chen, Y., Ruan, Y., Zhou, Y., Tang, B.Z., Qin, J. and Tang, Y., 2017. Quantitative evaluation and in vivo visualization of mercury ion bioaccumulation in rotifers by novel aggregation-induced emission fluorogen nanoparticles. Environ. Sci. Nano, 4: 2186-2192. https://doi.org/10.1039/C7EN00599G
Kucharzyk, K.H., Deshusses, M.A., Porter, K.A., Hsu-Kim, H. and Massachusetts Inst. of Technology, C.M.A., 2015. Relative contributions of mercury bioavailability and microbial growth rate on net methylmercury production by anaerobic mixed cultures. Environ. Sci. Process. Impacts, 17: 1568-1577. https://doi.org/10.1039/C5EM00174A
Lee, C.S. and Fisher, N.S., 2016. Methylmercury uptake by diverse marine phytoplankton. Limnol. Oceanogr., 61: 1626-1639. https://doi.org/10.1002/lno.10318
Lee, Y.H., Kim, D.H., Kang, H.M., Wang, M., Jeong, C.B. and Lee, J.S., 2017. Adverse effects of methylmercury (MeHg) on life parameters, antioxidant systems, and MAPK signaling pathways in the rotifer Brachionus koreanus and the copepod Paracyclopina nana. Aquat. Toxicol., 190: 181-189. https://doi.org/10.1016/j.aquatox.2017.07.006
Liu, J., Pankhurst, L.J., Deacon, L.J., Abate, W., Hayes, E.T., Drew, G.H., Longhurst, P.J., Pollard, S., Longhurst, J., Tyrrel, S.F. and Jackson, S.K., 2011. Evaluation of inflammatory effects of airborne endotoxin emitted from composting sources. Environ. Toxicol. Chem., 30: 602-606. https://doi.org/10.1002/etc.434
Luo, H.W., Yin, X., Jubb, A.M., Chen, H., Lu, X., Zhang, W., Lin, H., Yu, H.Q., Liang, L., Sheng, G.P. and Gu, B., 2017. Photochemical reactions between mercury (Hg) and dissolved organic matter decrease Hg bioavailability and methylation. Environ. Pollut., 220: 1359-1365. https://doi.org/10.1016/j.envpol.2016.10.099
Luo, J., Xie, Z., Lam, J.W., Cheng, L., Chen, H., Qiu, C., Kwok, H.S., Zhan, X., Liu, Y., Zhu, D. and Tang, B.Z., 2001. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun. (Camb), pp. 1740-1741. https://doi.org/10.1039/b105159h
Ma, M., Du, H., Wang, D., Kang, S. and Sun, T., 2017. Biotically mediated mercury methylation in the soils and sediments of Nam Co Lake, Tibetan Plateau. Environ. Pollut., 227: 243-251. https://doi.org/10.1016/j.envpol.2017.04.037
Maier, A., Savage, R.E. and Haber, L.T., 2004. Assessing biomarker use in risk assessment. A survey of practitioners. J. Toxicol. environ. Hlth. A, 67: 687-695. https://doi.org/10.1080/15287390490428161
Mei, J., Leung, N.L.C., Kwok, R.T.K., Lam, J.W.Y. and Tang, B.Z., 2015. Aggregation-induced emisson: Together we shine, united we soar. Chem. Rev., 115: 11718-11940. https://doi.org/10.1021/acs.chemrev.5b00263
Mergler, D., Anderson, H.A., Chan, L.H.M., Mahaffey, K.R. and Murray, M., Sakamoto, M. and Stern, A.H., 2007. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio, 36: 3-11. https://doi.org/10.1579/0044-7447(2007)36[3:MEAHEI]2.0.CO;2
Petrik, C.M., Luo, J.Y., Heneghan, R.F., Everett, J.D., Harrison, C.S. and Richardson, A.J., 2022. Assessment and constraint of mesozooplankton in CMIP6 earth system models. Glob. Biogeochem. Cycles, 36: n/a-n/a. https://doi.org/10.1029/2022GB007367
Ruan, Z., Li, C., Li, J.-R., Qin, J. and Li, Z., 2015. A relay strategy for the mercury (II) chemodosimeter with ultra-sensitivity as test strips. Sci. Rep., 5: 15987-15987. https://doi.org/10.1038/srep15987
Sarma, S.S.S., Nunez-Cruz, H.F. and Nandini, S., 2005. Effects on the population dynamics of {\sl Brachionus rubens} (Rotifera) caused by mercury and cadmium administered through medium and algal food {\sl Chlorella vulgaris}. Dong Wu Xue Bao, 51: 46-52.
Ullrich, S.M., Tanton, T.W. and Abdrashitova, S.A., 2001. Mercury in the aquatic environment: A review of factors affecting methylation. Crit. Rev. environ. Sci. Technol., 31: 241. https://doi.org/10.1080/20016491089226
Van Der Oost, R., Beyer, J. and Vermeulen, N.P., 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol., 13: 57-149. https://doi.org/10.1016/S1382-6689(02)00126-6
Zheng, L., Pan, L., Lin, P., Miao, J., Wang, X., Lin, Y. and Wu, J., 2017. Evaluating the toxic effects of three priority hazardous and noxious substances (HNS) to rotifer Brachionus plicatilis. Environ. Sci. Pollut. Res. Int., 24: 27277-27287. https://doi.org/10.1007/s11356-017-0298-2
To share on other social networks, click on any share button. What are these?








