Protease and Synthetic Volatile Analogue Compounds from Bacillus amyloliquefaciens IIHR BA2 Exhibit Bio-Control Action Against Root Knot Nematode Meloidogyne incognita
Protease and Synthetic Volatile Analogue Compounds from Bacillus amyloliquefaciens IIHR BA2 Exhibit Bio-Control Action Against Root Knot Nematode Meloidogyne incognita
R. Umamaheswari*, P. Prabu, M.S. Rao, B.M. Kavya and G. N. Grace
Division of Crop Protection, ICAR – Indian Institute of Horticultural Research, Bengaluru, India-560089
ABSTRACT
Bio-control agents serve as effective and environment-friendly weapons to mitigate the menace due to diverse plant pathogens. Production of volatile organic compounds (VOCs), antibiotics, enzymes and other secondary metabolites produced by bio-control agents demonstrate their antagonistic action against a multitude of phytopathogenic microbes and nematodes. Bacillus amyloliquefaciens IIHR BA2 (Indian Institute of Horticultural Research, Bacillus amyloliquefaciens 2) is one such potential bacterial bioagent that demonstrated effective nematicidal activity against root knot nematode, Meloidogyne incognita, a major biotic constraint in successful crop production. This study aims to decipher the mechanism of action of B. amyloliquefaciens IIHR BA2 against M. incognita by studying the presence of nematicidal genes and production of VOCs. In vitro study recorded 88.34% of M. incognita Juvenile mortality treated with B. amyloliquefaciens IIHR BA2 cell free crude extract at 100% after 72 h. Neutral and alkaline protease genes which are responsible for nematicidal action were identified in B. amyloliquefaciens IIHR BA2. Through Head space solid-phase micro extraction gas chromatography- mass spectrophotometer 52 VOCs were detected. Among the different compounds, synthetic limonene exhibited the maximum juvenile mortality followed by α-Pinene and Carvone. From the result of this study, we concluded that the release of terpenoid volatiles from B. amyloliquefaciens IIHR BA2 increased its antagonistic action against nematodes.
Article Information
Received 06 January 2023
Revised 25 May 2023
Accepted 06 June 2023
Available online 29 July 2023
(early access)
Published 10 January 2025
Authors’ Contribution
RU and MSR presented the idea, designed the study and performed investigations. PP and BMK conducted instrument and molecular study, and interpreted the data. MSR and GNF analyzed the data and drafted the manuscript.
Key words
Nematodes, Protease, Volatiles, Limonene, Terpenoid
DOI: https://dx.doi.org/10.17582/journal.pjz/20230106040156
* Corresponding author: [email protected]
0030-9923/2025/0001-0231 $ 9.00/00
Copyright 2025 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Plant parasitic nematodes are one of the major limiting factors that hamper successful crop production throughout the world. Root-knot nematodes (Meloidogyne spp.) are the most ruinous plant parasitic nematodes and can cause a yield reduction of around 25- 50% over wide range of crops. The southern root knot nematode, Meloidogyne incognita is an economically important plant parasitic nematode species because of its entangled relationship with the host plants, wide host range and the level of damage caused by its infection (Samaliev and Stoyanov, 2007). They also interact synergistically with pathogenic fungi and bacteria, resulting in disease complexes that ultimately destroy the crop (Kloepper et al., 1999). Damage caused by this nematode species is more noticeable in tropical than in temperate climates due to appropriate conditions for nematode survival and multiplication. Stunting, wilting, reduction of photosynthetic pigments and nutrient uptake, root galling and browning of leaves results in delayed maturity, poor quality and yield losses in crops affected by root-knot nematodes.
Globally numerous strategies are employed to manage the problems due to nematodes. Many chemical pesticides, though popular among farmers for their instantaneous efficacy against nematodes, are in the verge of getting banned or withdrawn from the market in the recent years due to their toxicity to humans, non-target animals, and ecosystem (Kim et al., 2018; Xiang et al., 2018). As a result, there is an urgent need to identify environmentally safe and cost-effective nematicides.
Several non-chemical strategies comprising biocontrol agents (BCAs), botanicals, organic soil amendments, resistance breeding and cropping system approaches have demonstrated encouraging results in reducing root knot nematode population under field conditions. Microbial BCAs encompassing a diverse group of beneficial fungi and bacteria play a major role in suppressing phytopathogenic microbial and nematode population in soil and host plants. BCAs from Bacillus group are referred to as ‘microbial factories’ as they produce several biologically active molecules that exhibit broad spectrum antagonistic activity against various plant pathogens (Ongena and Jacques, 2008). Bacillus amyloliquefaciens is one such potential biocontrol bacterium that possesses antifungal, antibacterial and antinematodal activity (Chen et al., 2006). Prabu et al. (2019) reported the in vitro efficacy of B. amyloliquefaciens IIHR BA2 against a multitude of plant pathogens and root knot nematode, M. incognita. To explore further into the mechanism of nematicidal action of B. amyloliquefaciens IIHR BA2 against M. incognita, this study has been taken up to detect the protease activity, presence of protease genes and production of volatile organic compounds (VOCs) that contribute to nematicidal action.
MATERIALS AND METHODS
Microbial culture preparation
The native isolate of B. amyloliquefaciens IIHR BA2 (NAIMCC-TB2216) maintained in Nematology Laboratory, Division of Crop Protection, ICAR – Indian Institute of Horticultural Research, Bengaluru was used in this study. Fresh culture of B. amyloliquefaciens IIHR BA2 was grown in nutrient broth (NB) under constant shaking at 150 rpm for 48 h at room temperature (25 ± 2°C) and used for further experiments.
Nematode culture maintenance
The culture of root knot nematode, M. incognita was maintained in susceptible tomato plants (cv. Arka Samrat) raised in earthen pots (3 kg capacity) with soil as substrate under glass house conditions at Division of Crop Protection, ICAR- Indian Institute of Horticultural Research, Bengaluru.
The egg masses of nematodes adhering to the tomato root surface were removed by agitating the root with sodium hypochlorite (0.05% solution) for 3 min. Further, the separated eggs were rinsed with sterile water and collected using nested 150 and 25 µm pore sieves (Richard and Kenneth, 1973). The collected eggs were left for hatching in sterile water. The second stage infective juveniles (J2) emerging out of the eggs were harvested at regular intervals up to 3 days (Southey, 1986). The collected juveniles were used for further studies.
Effect of cell free crude extract on juvenile (J2) mortality of M. incognita
The cell free crude extract of B. amyloliquefaciens IIHR BA2 were prepared freshly prior to use was collected from freshly cultivated broth culture of B. amyloliquefaciens IIHR BA2 (50 ml) were centrifuged in low temperature (4oC) for 15 min at 12,000 rpm and collected supernatant separately. Then the remaining bacterial cells were removed by filtering the supernatant through 0.22 μm syringe filter (WHATMANTM). The cell free supernatant contains crude biomolecules, which diluted into different concentrations as 25%, 50%, 75% and 100% with addition of sterile distilled water. Each concentration of crude extract was added separately into Petri plate. Approximately 100 J2 were added into each concentration and incubated at 25±2oC. The control Petri plates were maintained with sterile water with J2. All treatments had five replicates in CRD. The mortality of juveniles was counted after 24, 48 and 72 h using a stereo zoom microscope. After 72 h, inactive nematodes were picked and kept in sterile distilled water separately to check whether mortality of juveniles was permanent. The whole experiment was repeated twice. Per cent J2 mortality was calculated using the following formula (Ashoub and Amara, 2010).
J2 mortality (%) = (Number of J2 dead in culture filtrate/ total number of J2 in culture filtrate) x 100
Detection of protease enzyme activity
Proteolytic activity of B. amyloliquefaciens IIHR BA2 was tested on gelatine agar medium. Sterile NA media was prepared with incorporation of 2% gelatin and test culture was streaked on the media plates and incubated at 25±2oC for 48 h after which, culture plate was covered with mercuric chloride (15% HgCl2, 20% HCl and dis. H2O) solution for 5 min. The gelatin in the medium reacts with indicator solution to produce clear zone, where gelatin was hydrolyzed around the culture streak (Frazier, 1926).
Quantitative analysis of protease
B. amyloliquefaciens IIHR BA2 was cultured in nutrient broth (NB) media for 48h and centrifuged at 4oC for 15 min at 12,000 rpm. Supernatant was used as enzyme source for analysis. The reaction mixture of 1 ml culture filtrate and 0.5 ml gelatin substrate (0.65%) was incubated for 30 min at 37oC. Enzyme reaction was stopped by adding 5 ml of trichloroacetic acid (110 mmol/L) and centrifuged at 6000 rpm for 30 min. 2 ml supernatant was taken and mixed with 5 ml of sodium carbonate solution (500 mmol/L), Folin Ciocalteu phenol reagent (25%) and incubated at 25±2oC for 30 min and observation was taken at 650 nm. The protease activity was estimated according to (Pant et al., 2015). The total protein was estimated from cell free culture filtrate by using Bradford method (Bradford, 1976) and the enzyme activity was calculated from following formula.
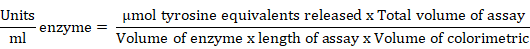
Detection of B. amyloliquefaciens IIHR BA2 protease genes
Using the modified sodium dodecyl sulfate (SDS) DNA extraction method, the genomic DNA (gDNA) was isolated from the 27 bacterial isolates (Ausubel et al., 1994). Genomic DNA was further used for identification of genes responsible for nematicidal properties.
The early isolated genomic DNA of selective B. amyloliquefaciens IIHR BA2 was used as a template for PCR amplification of neutral protease and alkaline protease. Primers were designed from the protease gene encoding B. amyloliquefaciens (Gene Bank accession no. K02497) (Lian et al., 2007).
Detection of neutral protease
The primer sequence was synthesized as forward primer np1 (5’ GGGGGATTTATTGTGGGTTT3’) and reverse primer np2 (5’ TACAATCCGACAGCATTCCA3’) to amplify the size of 1577bp neutral protease gene (15). PCR reaction mixture prepared with the total volume of 25 μl template DNA, 1xTaq A buffer with MgCl2, 0.2 mM each dNTP mixture, 20 pmol/primer, 2U of TaqDNA polymerase. With Thermocycler PCR reaction conditions 94oC for 5 min for initial denaturation, 35 cycle of 95°C for 45 sec, 50°C for 40 sec, 72°C for 90 sec and final extension at 72°C for 10 min.
Detection of alkaline protease
The amplification of alkaline protease (1149 bp) was carried out by using primers ap1 (5’GCGCCTAGGGTGAGAGGCAAAAAAGGTATG3’) and ap2 (5’CGCGGATCCTTACTGAGCTGCCGCCTGTAC 3’) (Ramarathnam, 2007). PCR reaction mixture was prepared with ~50 ng of template DNA, 1xTaq A buffer with MgCl2, 0.2 mM of each dNTP, 20 pmol/primer, 2U of Taq DNA polymerase. With thermocycler PCR reaction conditions 94oC for 5 min for initial denaturation, 35 cycle of 95°C for 45 sec, 50°C for 40 sec, 72°C for 90 sec and final extension at 72°C for 10 min.
The PCR amplified products were loaded on agarose gel (1.5 %) with 1kb DNA ladder (GeneRularTM) and confirmed for the size of the gene. Then the PCR product were purified and sequenced through Sanger’s dideoxy sequencing from Bioserve, Hyderabad. After gene sequencing the resultant products were received in chromas format from the Bioserve. The sequences were aligned and converted to FASTA format by the Bio Edit software. Once the gene was aligned properly, it was confirmed by blasting in NCBI for its respective regions.
Extraction of VOCs from B. amyloliquefaciens IIHR BA2 by HS-SPME
The volatiles produced by B. amyloliquefaciens IIHR BA2 were extracted by using head space solid-phase microextraction (HS-SPME) methods (Díaz-Maroto et al., 2004). The volatile extraction was repeated thrice and 9 ml NB broth was used as control.
Identification of VOCs by gas chromatography-mass spectrometry (GC-MS)
Volatiles were separated and identified by using GC-MS (GC- varian 3800 coupled with MS Varian 4000). The helium gas was used as carrier with 1 ml per min flow ratein split-splitles injection mode. The SPME volatile absorption fiber was directly inserted into the inlet of the gas chromatograph connected to a mass spectrometer and desorbed at 255°C for 10 min. The column temperature programmed was followed as initial temperature 40°C hold for 3 min, increased 40 to 160°C at the rate of 3°C/min for 2 min, then further increased to 230°C at the rate of 5°C/min and held for 1 min. The transfer line and ion trap temperatures were 250°C and 200°C, respectively and the external ionization with a full scan from 50 m/z to 400 m/z was used. The volatiles compounds were identified by comparing the mass spectrum volatiles and GCMS system standard database used NIST 2007 and WILEY 2005.
In vitro efficacy of chemical volatiles on M. incognita juvenile mortality
The effects of VOCs released by B. amyloliquefaciens IIHR BA2 were evaluated on the mortality of M. incognita juveniles. Three VOCs limonene, 1S-alpha-pinene (α-Pinene) and cyclohexen-1-one, 2-methyl-5-(carvone) were selected based on GC-MS results. Similar synthetic chemicals were purchased from Sigma Aldrich, Bangalore, India for analysis.
Each compound was diluted and evaluated at 125 µl/L, 250 µl/L and 500 µl/L, after which M. incognita J2 were added at ~100 J2 per plate and incubated at 27±2oC. The control was maintained with sterile water and juveniles with five replications. J2 mortality was observed after 24, 48 and 72 h under stereozoom microscope (Motic SMZ-180). After 72 h, inactive nematodes were picked and kept in sterile distilled water separately to check whether mortality of juveniles was permanent.
Results and Discussion
Effect of crude extract on mortality of M. incognita (J2)
Experimental results revealed that mortality of second-stage juveniles of M. incognita was significantly increased with increase in the crude extract concentrations and exposure time compared to untreated control. The highest juvenile mortality (88.34%) was observed in the crude extract of B. amyloliquefaciens IIHR BA2 at 100% concentration after exposure to 72 h followed by the concentration of 75%, 50% and 25% which shown the juvenile mortality 68.35%, 61.12% and 41.32%, respectively (Fig. 1).
Several earlier reports have proved the antagonistic action exhibited by Bacillus spp. towards plant parasitic nematodes by inhibiting their hatching of eggs and enhancing J2 mortality (Rao et al., 2017; Xiao et al., 2018; Kamalnath et al., 2019). Nematidical activity was exhibited owing to their capability to produce antibiotic compounds, nematicidal volatiles and enzymes (Ruiz et al., 2014). Cell free culture filtrate of B. subtilis possess the ability to produce various lytic enzymes such as protease and glucanase that directly attack nematode eggs and surface of the second stage juveniles (Chen et al., 2014) and produce lipopepetides such as bacilomycin that can affect the nematode behavior (Castaneda-Alvarez and Aballay, 2016).
Protease genes
B. amyloliquefaciens IIHR BA2 was subjected to PCR reaction for detection of nematicidal neutral protease and alkaline protease (Table I). Neutral protease gene amplified using specific primers showed amplicon size of 1577 bp (Fig. 2). Further amplicons were sequenced and blasted in NCBI proved 82% similarity with neutral protease which confirmed the presence of neutral protease gene. Alkaline protease gene amplified using specific primers showed amplicon size of 1149 bp (Fig. 3). Further amplicons were sequenced and blasted in NCBI proved 95% similarity with neutral protease which confirmed the presence of neutral protease gene.
Lian et al. (2007) amplified the neutral protease and alkaline protease from Bacillus spp. and reported that synergistic effect of these two different extracellular protease enzymes increased the rate of nematode mortality by 9%. Tian et al. (2007) reported that deletion of alkaline protease in nematotoxic B. laterosporus decreased up to 57 per cent of its nematicidal activity. Also, Siddiqui et al. (2005) confirmed that extracellular protease deletion from P. fluorescens CHA0 recorded reduced nematicidal activity. Hence, our research finding suggests that extracellular protease secretion by B. amyloliquefaciens IIHR BA2 might play a major role in its nematicidal activity.
Table I. PCR amplified protease gene sequence NCBI blast result.
|
S. No |
Identified gene |
Description |
E value |
Identical % |
Accession No |
|
1. |
Alkaline protease |
Subtilisin like protease (Bacillus subtilis) |
0.0 |
95 |
AHN52401.1 |
|
2. |
Neutral protease |
neutral protease precursor (Bacillus subtilis) |
0.0 |
82 |
BAJ41480.1 |
Protease enzyme and VOCs produced by B. amyloliquefaciens IIHR BA2
The qualitative test of protease production performed on B. amyloliquefaciens IIHR BA2 using gelatin as substrate in agar media revealed a clear zone around culture streak by gelatin hydrolysis. This clearly demonstrated the protease enzyme production by B. amyloliquefaciens IIHR BA2 (Fig. 2). The extracellular protease enzyme activity was estimated as 156.80U/ml in B. amyloliquefaciens IIHR BA 2.
Many earlier studies have reported extracellular protease enzymes as a nematicidal virulence factor from bio-control bacteria and fungi (Chernin and Chet, 2002; Qiuhong et al., 2006; Tian et al., 2007). It was suggested that protease enzyme might play a role in the hydrolysis of proteinaceous outer membrane of nematode in order to help the bacteria to penetrate and kill the nematode (Leger et al., 1987; Ahman, 2000). In the present study, protease production by B. amyloliquefaciens IIHR BA2 was confirmed in gelatin agar media through clear zone around the grown culture. Subsequent quantitative analysis of B. amyloliquefaciens IIHR BA2 culture filtrate showed 156.80 U/ml of extracellular protease enzyme activity. Similarly, Alnahdi (2012) observed 246U/ml of protease activity in Bacillus spp. Qiuhong et al. (2006) reported protease enzyme extract of Bacillus sp. B16 showed effective control of nematodes. Hence in this present study, presence of extracellular proteases in B. amyloliquefaciens IIHR BA2 might possess a role in suppressing nematodes.
The headspace SPME/GC-MS analysis of B. amyloliquefaciens IIHR BA 2 revealed the presence of 52 VOCs (Table II) which includes phenols, alcohols, hydrocarbons, ketones, aldehydes, pyrazines, esters, organic acids and other molecules.
Table II. Volatile organic compounds identified from B. amyloliquefaciens IIHR BA2 using SPME/GC-MS.
|
S. No |
RT |
Peak name |
CAS No. |
Percentage abundance |
Probability |
|
1. |
4.143 |
Spiro (2.4)hepta-4,6-diene |
765-46-8 |
0.22 |
28.32 |
|
2. |
6.835 |
Ethylbenzene |
100-41-4 |
0.11 |
77.58 |
|
3. |
8.055 |
Styrene |
100-42-5 |
0.835 |
55.17 |
|
4. |
9.046 |
3-Amino-4-pyrazolecarbonitrile |
16617-46-2 |
0.397 |
62.32 |
|
5. |
9.255 |
1,2,5-Trimethylpyrrole |
930-87-0 |
0.073 |
40.51 |
|
6. |
9.529 |
1-amino-4-methyl- Pyridinium |
57156-85-1 |
0.069 |
33.5 |
|
7. |
10.579 |
1H-Pyrrolo (3,2-d) pyrimidine-2, 4(3H,5H)-dione |
65996-50-1 |
1.66 |
22.48 |
|
8. |
11.308 |
Ethanone, 2,2-dihydroxy-1-phenyl |
1075-06-5 |
2.67 |
5.97 |
|
9. |
11.819 |
Octamethylcyclotetrasiloxane |
556-67-2 |
1.661 |
35.45 |
|
10. |
12.216 |
1S-α –Pinene |
7785-26-4 |
2.735 |
12.25 |
|
11. |
13.397 |
Benzenamine, 2-methoxy-4-methyl |
39538-68-6 |
0.029 |
30.98 |
|
12. |
14.183 |
Limonene |
138-86-3 |
4.054 |
24.88 |
|
13. |
14.457 |
3-Carene |
13466-78-9 |
1.513 |
39.43 |
|
14. |
15.408 |
1-Methyl-1,4-cyclohexadiene |
99-85-4 |
0.129 |
45.46 |
|
15. |
16.678 |
4,5-Dimethyl-ortho-phenylenedia |
3171-45-7 |
0.087 |
33.61 |
|
16. |
17.845 |
2-(Phenylthio) propanenitrile |
76100-13-5 |
0.873 |
49.98 |
|
17. |
18.539 |
N-Ethyl-p-toluidine |
622-57-1 |
0.059 |
21.92 |
|
18. |
18.831 |
m-Ethylaniline |
587-02-0 |
0.19 |
9.82 |
|
19. |
18.944 |
2,2'-Bibenzothiazole |
4271-09-4 |
0.287 |
41.3 |
|
20. |
19.392 |
3,4-Diethyl-2-methyl-1H-pyrrole |
34874-30-1 |
0.071 |
69.3 |
|
21. |
20.238 |
Cyclohexanol, 5-methyl-2-(1-methylethyl) |
23283-97-8 |
0.343 |
9.76 |
|
22. |
20.825 |
3-tert-Butyl-5-chloro-2-hydroxy |
52196-47-1 |
0.089 |
9.73 |
|
23. |
21.159 |
2-n-Propylthiolane, S, S-dioxide |
71053-02-6 |
0.244 |
15.75 |
|
24. |
22.278 |
Cyclohexanone, 2-methyl-5-(1-methylethenyl) |
5948-04-9 |
2.202 |
39.46 |
|
25. |
22.58 |
1-Pentadecyne |
765-13-9 |
0.147 |
5.73 |
|
Table continued on next page........... |
|||||
|
S. No |
RT |
Peak Name |
CAS No. |
Percentage abundance |
Probability |
|
26. |
23.837 |
5-Methoxy-2,3-dimethyl-1H-indole |
156785-76-1 |
0.171 |
75.71 |
|
27. |
24.455 |
2-Cyclohexen-1-one, 2-methyl-5-(1-methylethyl) |
99-49-0 |
3.376 |
66.85 |
|
28. |
25.288 |
1-Fluorododecane |
334-68-9 |
0.079 |
13.43 |
|
29. |
26.484 |
9,10-Anthracenedione, 1-methyl |
128-85-8 |
1.756 |
22.89 |
|
30. |
27.19 |
5-Acetyl-2-methylpyridine |
42972-46-3 |
1.122 |
28.6 |
|
31. |
27.352 |
Pyridine, 4-(1,1-dimethylethyl) |
3978-81-2 |
0.071 |
47.93 |
|
32. |
27.873 |
Cyclohexane, 1-ethenyl-1-methyl |
3242-08-8 |
0.439 |
20.74 |
|
33. |
28.505 |
Benzenamine, N, N-dibutyl |
613-29-6 |
0.11 |
63.58 |
|
34. |
28.818 |
4-Methyl Quinazoline |
700-46-9 |
0.387 |
35.92 |
|
35. |
29.541 |
2-Pentacosanone |
75207-54-4 |
1.247 |
7.53 |
|
36. |
29.717 |
Copaene |
3856-25-5 |
0.235 |
28.98 |
|
37. |
30.046 |
Cyclobuta (1,2:3,4) dicyclopenten |
5208-59-3 |
1.507 |
90.81 |
|
38. |
31.246 |
Benzenamine, 3,5-bis (1,1-dimethylethyl) |
2380-36-1 |
0.19 |
35.88 |
|
39. |
31.539 |
1,4-Methanoazulene, decahydro-4,8,8-trimethyl-9-methylene |
475-20-7 |
1.257 |
8.4 |
|
40. |
31.98 |
3-Butyl-2-methyl-6,7-dihydro-5H |
124790-36-9 |
0.247 |
62.21 |
|
41. |
32.202 |
4-(1-Butylpentyl) pyridine |
2961-47-9 |
0.052 |
22.69 |
|
42. |
33.203 |
6-Cyanoquinoline |
23395-72-4 |
0.293 |
24.53 |
|
43. |
34.083 |
1,2,3,4,4a,5,6,8a-Octahydro-naphthalene |
39029-41-9 |
0.45 |
46.27 |
|
44. |
34.879 |
2-Tridecanone |
593-08-8 |
0.503 |
16.76 |
|
45. |
35.117 |
α – Farnesene |
502-61-4 |
0.261 |
47.66 |
|
46. |
36.559 |
1H-Indole-3-methanamine, 5-ethyl |
74367-51-4 |
0.103 |
40.81 |
|
47. |
40.238 |
2-Buten-1-ol, 1,1-diphenyl |
63553-53-7 |
0.027 |
23.32 |
|
48. |
41.992 |
Murrayafolin a |
4532-33-6 |
0.047 |
48.24 |
|
49. |
42.064 |
2-Hexyl-1-octanol |
19780-79-1 |
0.079 |
5.15 |
|
50. |
42.742 |
Propaneisocyanide, 2-(6-methoxy-2-naphthyl) |
133097-33-3 |
0.049 |
34.57 |
|
51. |
43.123 |
3-Amino-2-methoxydibenzofuran |
5834-17-3 |
0.042 |
38.54 |
|
52. |
48.421 |
Butyl cyclohexyl phthalate |
84-64-0 |
0.544 |
16.25 |
Efficacy of chemical volatiles on M. incognita juvenile
Among the 52 VOCs identified, three major compounds which are synthetically available viz., limonene, α-pinene, and carvone were tested for their effect on juvenile mortality at different concentration (125 µl/L, 250 µl/L and 500 µl/L). Among the 3 VOCs, maximum juvenile mortality (75.43%) was observed in limonene after 72h at 500 µl/L concentration followed by carvone (63.40%) and α-pinene (55.14%) (Table III). It was observed that the immobile juveniles could not recover their mobility after transferring in fresh water indicating that the tested volatiles were nematicidal.
B. amyloliquefaciens exhibited antagonistic activities against a broad range of soil borne pathogens and nematodes by producing antibiotics and enzymes (Chen et al., 2006, 2007; Arguelles-Arias et al., 2009; Porwal et al., 2009). Many microbes released volatile organic compounds have been reported for enhancement of plant growth, toxic action on nematodes and induction of resistance systemically in plants (Ryu et al., 2003; Farag et al., 2006; Gu et al., 2007).
In this present study, the headspace SPME/GC-MS analysis showed diverse volatile compound profiles and a total of 52 VOCs were detected from B. amyloliquefaciens IIHR BA2 in different concentrations. Nematicidal activity evaluated on the synthetic compounds of three major volatiles viz., limonene, α-pinene and carvone showed significant juvenile mortality (55.14 - 75.43%). Similar trend in juvenile mortality was recorded by Abdel-Rahman et al. (2013) in limonene (79.8 %) carvone (30.1%) and α-pinene (13.3%) against C. elegans. Similarly, Eckert et al. (2018) reported Limonene as one of the major volatile components in B. subtilis. Various terpenoids were found to how nematicidal activity especially against M. incognita (Abdel-Rahman et al., 2013; Oka et al., 2000). VOCs affect the nematodes causing damage to nervous system (Warnock et al., 2017), intestine, surface coat (Geng et al., 2016), pharynx and other tissues. VOCs released by Bacillus spp. proved fungicidal against Fusarium oxysporum, Botryosphaeria berengeriana, Colletotrichum gloeosporioides and Penicillium spp. (Andersen et al., 1994; Minerdi et al., 2009; Zhang et al., 2010; Lee et al., 2012). Yuan et al. (2012) reported that B. amyloliquefaciens produces 36 VOCs out of which 11 compounds completely inhibited F. oxysporum. The results of above study suggest that the release of VOCs from B. amyloliquefaciens IIHR BA2 enhanced its antagonistic action against nematodes.
Table III. Effect of B. amyloliquefaciensIIHR BA2 volatiles organic compounds (VOCs) on M. incognita juveniles.
|
VOCs |
Concentration (C) |
% Juvenile mortality over control and exposure time |
||
|
24h |
48h |
72h |
||
|
Limonene |
125 µL/L |
55.40 (45.31) |
58.68 (47.86) |
62.71 (52.36) |
|
250 µL/L |
58.53 (44.04) |
63.87 (46.38) |
70.00 (49.55) |
|
|
500 µL/L |
61.07 (43.07) |
65.75 (45.25) |
75.43 (51.43) |
|
|
α- Pinene |
125 µL/L |
36.72 (44.85) |
41.72 (48.86) |
48.88 (50.87) |
|
250 µL/L |
40.11 (41.70) |
43.43 (45.17) |
50.13 (48.14) |
|
|
500 µL/L |
41.33 (49.66) |
45.25 (51.03) |
55.14 (53.40) |
|
|
Carvone |
125 µL/L |
40.08 (39.53) |
47.63 (41.39) |
51.40 (46.89) |
|
250 µL/L |
44.78 (46.06) |
53.20 (49.71) |
58.79 (55.13) |
|
|
500 µL/L |
52.93 (41.58) |
58.14 (45.54) |
63.40 (47.83) |
|
|
CD |
V – 1.78 C – 1.78 V x C – 3.08 |
V – 1.95 C – 1.95 V x C – 3.39 |
V – 1.74 C – 1.74 V x C – 3.02 |
|
|
S.ED (p=0.01) |
V - 0.65 C - 0.65 V x C – 1.13 |
V–0.72 C – 0.72 V x C – 1.24 |
V – 0.64 C – 0.64 V x C – 1.11 |
|
Note: Numericals within the parantheses are transformed values (Arc sine).
Conclusion
The study concluded that crude extract from B. amyloliquefaciens IIHR BA2 having higher larvicidal activity. Further investigation on active compound identification against M. incognita revealed that protease and terpenoid volatile organic compounds were responsible for larvicidal action. Thus, our study exposes B. amyloliquefaciens IIHR BA2 as a potential biocontrol agent against root knot nematode and decipheres its probable mode of action by producing nematicidal enzymes and volatiles organic compounds.
Acknowledgement
We thank Department of Science and Technology, India for providing fund to carry out this work. We also thank ICAR - Indian Institute of Horticultural Research, Bengaluru, India for providing lab and instrument facility.
Funding
The study was funded by Department of Science and Technology, India.
IRB approval
Not applicable.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abdel-Rahman, F.H., Alaniz, N.M. and Saleh, M.A., 2013. Nematicidal activity of terpenoids. J. Environ. Sci. Hlth. B, 48: 16-22. https://doi.org/10.1080/03601234.2012.716686
Ahman, J., 2000. Extracellular serine proteases as virulence factors in nematophagous fungi: Molecular characterization and functional analysis of the PII protease in Arthrobotrys oligospora, PhD thesis. Microbial Ecology, Lund University.
Alnahdi, H.S., 2012. Isolation and screening of extracellular proteases produced by new isolated Bacillus sp. J. appl. Pharma. Sci., 2: 71-74. https://doi.org/10.7324/JAPS.2012.2915
Andersen, R.A., Hamilton-Kemp, T.R., Hildebrand, D.F., McCracken, C.T., Collins, R.W. and Fleming, P.D., 1994. Structure-antifungal activity relationships among volatile C6 and C9 aliphatic aldehydes, ketones, and alcohols. J. Agric. Fd. Chem., 42: 1563-1568. https://doi.org/10.1021/jf00043a033
Arguelles-Arias, A., Ongena, M., Halimi, B., Lara, Y., Brans, A., Joris, B. and Fickers, P., 2009. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Fact., 8: 1-12. https://doi.org/10.1186/1475-2859-8-63
Ashoub, A.H. and Amara, M.T., 2010. Biocontrol activity of some bacterial genera against root-knot nematode, Meloidogyne incognita. J. Am. Sci., 6: 321-328.
Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A. and Struhl, K., 1994. Current protocols in molecular biology. Greene Publishing Associates/Wiley, New York.
Bradford, M.M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248-254. https://doi.org/10.1016/0003-2697(76)90527-3
Castaneda-Alvarez, C. and Aballay, E., 2016. Rhizobacteria with nematicide aptitude: Enzymes and compounds associated. World J. Microbiol. Biotechnol., 32: 1-7. https://doi.org/10.1007/s11274-016-2165-6
Chen, D., Liu, X., Li, C., Tian, W., Shen, Q. and Shen, B., 2014. Isolation of Bacillus amyloliquefaciens S20 and its application in control of eggplant bacterial wilt. J. environ. Manage., 137: 120-127. https://doi.org/10.1016/j.jenvman.2014.01.043
Chen, X.H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., Morgenstern, B., Voss, B., Hess, W.R., Reva, O. and Junge, H., 2007. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol., 25: 1007-1014. https://doi.org/10.1038/nbt1325
Chen, X.H., Vater, J., Piel, J., Franke, P., Scholz, R., Schneider, K., Koumoutsi, A., Hitzeroth, G., Grammel, N., Strittmatter, N. and Gottschalk, G., 2006. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. J. Bact., 188: 4024-4036. https://doi.org/10.1128/JB.00052-06
Chernin, L. and Chet, I., 2002. Microbial enzymes in the biocontrol of plant pathogens and pests. Book Chapter. Enzymes Environ. Activity, Ecol. Appl., 26: 171-226. https://doi.org/10.1201/9780203904039.ch7
Díaz-Maroto, M.C., Sánchez-Palomo, E. and Pérez-Coello, M.S., 2004. Fast screening method for volatile compounds of oak wood used for aging wines by headspace SPME-GC-MS (SIM). J. agric. Fd. Chem., 52:6857-6861. https://doi.org/10.1021/jf049032m
Eckert, K.E., Carter, D.O. and Perrault, K.A., 2018. Sampling dynamics for volatile organic compounds using headspace solid-phase microextraction arrow for microbiological samples. Separations, 5: 45. https://doi.org/10.3390/separations5030045
Farag, M.A., Ryu, C.M., Sumner, L.W. and Paré, P.W., 2006. GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry, 67: 2262-2268. https://doi.org/10.1016/j.phytochem.2006.07.021
Frazier, W.C., 1926. A method for the detection of changes in gelatin due to bacteria. J. Infect. Dis.,71: 302-309. https://doi.org/10.1093/infdis/39.4.302
Geng, C., Nie, X., Tang, Z., Zhang, Y., Lin, J., Sun, M. and Peng, D., 2016. A novel serine protease, Sep1, from Bacillus firmus DS-1 has nematicidal activity and degrades multiple intestinal-associated nematode proteins. Sci. Rep., 6: 1-12. https://doi.org/10.1038/srep25012
Gu, Y.Q., Mo, M.H., Zhou, J.P., Zou, C.S. and Zhang, K.Q., 2007. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem., 39: 2567-2575. https://doi.org/10.1016/j.soilbio.2007.05.011
Kamalnath, M., Rao, M.S. and Umamaheswari, R., 2019. Rhizophere engineering with beneficial microbes for growth enhancement and nematode disease complex management in gherkin (Cucumis anguria L.). Sci. Hortic., 257: 108681. https://doi.org/10.1016/j.scienta.2019.108681
Kim, T.Y., Jang, J.Y., Yu, N.H., Chi, W.J., Bae, C.H., Yeo, J.H. and Kim, J.C., 2018. Nematicidal activity of grammicin produced by Xylaria grammica KCTC 13121BP against Meloidogyne incognita. Pest Manage. Sci., 74: 384-391. https://doi.org/10.1002/ps.4717
Kloepper, J.W., Rodriguez-Kabana, J.W., Zehnder, G.W., Murphy, J.F., Sikora, E. and Fernandez, C., 1999. Plant root bacterial interactions in biological control of soil borne diseases and potential extension to systemic and foliar diseases. Austral. Pl. Pathol., 28: 21-26. https://doi.org/10.1071/AP99003
Lee, H.J., Park, K.C., Lee, S.H., Bang, K.H., Park, H.W., Hyun, D.Y., Kang, S.W., Cha, S.W. and Chung, I.M., 2012. Screening of antifungal Bacillus spp. against Alternaria blight pathogen (Alternaria panax) and anthracnose pathogen (Colletotrichum gloeosporioides) of ginseng. Korean J. Med. Crop Sci., 20: 339-344. https://doi.org/10.7783/KJMCS.2012.20.5.339
Leger, R.J., Richard, M.C. and Charnley, A.K., 1987. Production of cuticle degrading enzymes by the entomopathogen Metarhizium anisopliae during infection of cuticles from Calliphora vomitoria and Manduca sexta. J. Gen. Microbiol., 133: 1371–1382. https://doi.org/10.1099/00221287-133-5-1371
Lian, L.H., Tian, B.Y., Xiong, R., Zhu, M.Z., Xu, J. and Zhang, K.Q., 2007. Proteases from Bacillus: A new insight into the mechanism of action for rhizobacterial suppression of nematode populations. Lett. appl. Microbiol., 45: 262-269. https://doi.org/10.1111/j.1472-765X.2007.02184.x
Minerdi, D., Bossi, S., Gullino, M.L. and Garibaldi, A., 2009. Volatile organic compounds: A potential direct long-distance mechanism for antagonistic action of Fusarium Oxysporum strain Msa 35. Environ. Microbiol., 11: 844–854. https://doi.org/10.1111/j.1462-2920.2008.01805.x
Oka, Y., Nacar, S., Putievsky, E., Ravid, U., Yaniv, Z. and Spiegel, Y., 2000. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology, 90: 710-715. https://doi.org/10.1094/PHYTO.2000.90.7.710
Ongena, M. and Jacques, P., 2008. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol., 16: 115-125. https://doi.org/10.1016/j.tim.2007.12.009
Pant, G., Prakash, A., Pavani, J.V.P., Bera, S., Deviram, G.V.N.S., Kumar, A., Panchpuri, M. and Prasuna, R.G., 2015. Production, optimization and partial purification of protease from Bacillus subtilis. J. Taibah Univ. Sci., 9: 50-55. https://doi.org/10.1016/j.jtusci.2014.04.010
Porwal, S., Lal, S., Cheema, S. and Kalia, V.C., 2009. Phylogeny in aid of the present and novel microbial lineages: Diversity in Bacillus. PLoS One, 4: 38-44. https://doi.org/10.1371/journal.pone.0004438
Prabu, P., Umamaheswari, R. and Rao, M.S., 2019. Deciphering the biocontrol mechanism of Bacillus amyloliquefaciens IIHR BA2: detection of nematicidal, fungicidal and bactericidal lipopeptides. Asian J. Microbiol. Biotechnol. Environ. Sci., 21: 204-212.
Qiuhong, N., Xiaowei, H., Baoyu, T., Jinkui, Y., Jiang, L., Lin, Z. and Keqin, Z., 2006. Bacillus sp. B16 kills nematodes with a serine protease identified as a pathogenic factor. Appl. Microbiol. Biotechnol., 69: 722-730. https://doi.org/10.1007/s00253-005-0019-5
Ramarathnam, R., 2007. Phyllosphere bacterial biological control of Leptosphaeria maculans, the blackleg pathogen of canola (Brassica napus L.): Screening for potential antibiotic producers, investigation of the mechanism of control, biochemical detection of the antifungal compounds, and establishment of the role of antibiosis. Ph.D. thesis, University of Manitoba, Winnipeg, Manitoba.
Rao, M.S., Kamalnath, M., Umamaheswari, R., Rajinikanth, R., Prabu, P., Priti, K., Grace, G.N., Chaya, M.K. and Gopalakrishnan, C., 2017. Bacillus subtilis IIHR BS-2 enriched vermicompost controls root knot nematode and soft rot disease complex in carrot. Sci. Hortic., 218: 56-62. https://doi.org/10.1016/j.scienta.2017.01.051
Richard, S.H. and Kenneth, R.B., 1973. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Pl. Dis. Rep., 57: 1025-1028.
Ruiz, S.E., Cristóbal, A.J., Reyes, R.A., Tun, S.J., García, R.A. and Pacheco, A.J., 2014. In vitro antagonistic activity of Bacillus subtilis strains isolated from soils of the Yucatan peninsula against Macrophomina phaseolina and Meloidogyne incognita. Fyton, 83: 45-47. https://doi.org/10.32604/phyton.2014.83.045
Ryu, C.M., Farag, M.A., Hu, C.H., Reddy, M.S., Wei, H.X., Paré, P.W. and Kloepper, J.W., 2003. Bacterial volatiles promote growth in Arabidopsis. Proc. natl. Acad. Sci., 100: 4927-4932. https://doi.org/10.1073/pnas.0730845100
Samaliev, H. and Stoyanov, D., 2007. Parasitic nematodes of crop plants and their control. Agricultural Academic Press Plovdiv, pp. 328-341.
Siddiqui, I.A., Haas, D. and Heeb, S., 2005. Extracellular protease of Pseudomonas fluorescens CHA0, a biocontrol factor with activity against the root-knot nematode, Meloidogyne incognita. Appl. environ. Microbiol., 71: 5646–5649. https://doi.org/10.1128/AEM.71.9.5646-5649.2005
Southey, J.F., 1986. Laboratory methods for work with plant and soil nematodes. New Delhi.
Tian, B., Yang, J., Lian, L., Wang, C., Li, N. and Zhang, K.Q., 2007. Role of an extracellular neutral protease in infection against nematodes by Brevibacillus laterosporus strain G4. Appl. Microbiol. Biotechnol., 74: 372-380. https://doi.org/10.1007/s00253-006-0690-1
Warnock, N.D., Wilson, L., Patten, C., Fleming, C.C., Maule, A.G. and Dalzell, J.J., 2017. Nematode neuropeptides as transgenic nematicides. PLoS Pathog., 13: e1006237. https://doi.org/10.1371/journal.ppat.1006237
Xiang, N., Lawrence, K.S. and Donald, P.A., 2018. Biological control potential of plant growth- promoting rhizobacteria suppression of Meloidogyne incognita on cotton and Heterodera glycines on soybean. A review. J. Phytopathol., 166: 449–458. https://doi.org/10.1111/jph.12712
Xiao, L., Wan, J.W., Yao, J.H., Feng, H. and Wei, L.H., 2018. Effects of Bacillus cereus strain Jdm1 on Meloidogyne incognita and the bacterial community in tomato rhizosphere soil. 3 Biotech, 8: 1–8. https://doi.org/10.1007/s13205-018-1348-2
Yuan, J., Raza, W., Shen, Q. and Huang, Q., 2012. Antifungal activity of Bacillus amyloliquefaciens Njn-6 volatile organic compounds against Fusarium Oxysporum f. sp. Cubense. Appl. environ. Microbiol., 78: 5942–5944. https://doi.org/10.1128/AEM.01357-12
Zhang, L., Chang, Y., Ding, F., Liu, Y., Liu, C. and Chen, Z., 2010. Inhibition effect of 2 Bacillus subtilis on Botryosphaeria berengeriana f. sp. piricola in vitro. J. Fruit Sci., 27: 823-827.
To share on other social networks, click on any share button. What are these?








