Prevalence of Anaplasma marginale in Tropical Area of Khyber Pakhtunkhwa, Pakistan
Prevalence of Anaplasma marginale in Tropical Area of Khyber Pakhtunkhwa, Pakistan
1College of Veterinary Sciences and Animal Husbandry, Abdul Wali Khan University, Mardan
2Institute of Biotechnology and Genetic Engineering, The University of Agriculture, Peshawar
3Veterinary Research Institute, Peshawar
4Islamia College for Girls, Peshawar
5Department of Zoology, Abdul Wali Khan University, Mardan
ABSTRACT
Bovine anaplasmosis is an infectious tick-borne disease caused by a rickettsial parasite Anaplasma marginale. This study investigated the prevalence of A. marginale in cattle on different commercial farms in subtropical conditions of Khyber Pakhtunkhwa (KP). A total of 120 cattle from different farms were randomly sampled and tested for the presence of A. marginale between May to August 2016. The study revealed that 15.0%, 20.8% and 29.1% of sampled animals were found positive for A. marginale using microscopic examination, indirect ELISA and PCR, respectively. Two major surface protein genes (MSP1a and MSP4) sequence were compared with cattle isolates from different origins. Phylogenetic analysis of local isolates showed a close homology with the isolates from Australia, Brazil, Turkey and Japan. It was found that aged, exotic and crossbreed cattle were more susceptible to A. marginale infection in summer season compared to the younger and indigenous cattle breeds, respectively (P˂0.01). Moreover, the study revealed widespread prevalence of A. marginale in the subtropical areas of KP, Pakistan.
Article Information
Received 10 May 2018
Revised 15 December 2020
Accepted 12 January 2021
Available online 19 August 2021
Authors’ Contribution
SG and KK collected the data and conducted the experiment. TU wrote the paper. SA and SA designed the project and supervised the research. SG analyzed the data. All the authors read and approved the research paper.
Key words
Anaplasmosis, Cattle, ELISA, Major surface protein gene, PCR
DOI: https://dx.doi.org/10.17582/journal.pjz/20180510110525
* Corresponding author: talhasohail71@yahoo.com, tahircau@gmail.com, tahirusman@awkum.edu.pk
0030-9923/2021/0005-1977 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
Anaplasmosis is one of the most important infectious and hemoparasitic diseases of bovines and other ruminants (Dumlar et al., 2001). It is caused by an intraerythrocytic parasite of the genus Anaplasma (order: Rickettisale; family: Anaplasmataceae) (Dumlar et al., 2001). In the mammalian host, Anaplasma marginale produces antigenic variants by altering a surface coat, consisting of many proteins. Six major surface proteins (MSPs) have been identified on A. marginale derived from bovine erythrocytes (Kocan et al., 2000). The functional pseudogenes that belong to MSP2 super familyplay a vital role in surface coat antigenic variation (Kocan et al., 2000). MSPs involved in host-pathogen interactions evolve more rapidly than other nuclear genes because of selective pressures exerted by the host immune system. Three of these MSPs (MSP1a, MSP4 and MSP5) are from single genes and do not vary antigenically during the multiplication of the organism. The other three MSPs (MSP1b, MSP2 and MSP3) are from multigene families and may vary antigenically, most notably in persistently infected cattle (Bowie et al., 2002). MSP1a, encoded byMSP1αgene of A.marginale play a vital role in providing protective immunity in cattle (Fuente et al., 2003).
Ticks are obligate, blood feeding ectoparasites of vertebrates verified to transmit A. marginale (Kahn, 2005). Pakistan is located in a subtropical zone (30° N, 70° E) within South Asia and thus offers favorable environmental conditions for ticks to infest a variety of hosts and transmit diseases to livestock, companion animals and humans (Jabbar et al., 2015). Due to rapid extension of the dairy industry and an increased import of high milk producing exotic dairy cattle (Holstein Friesian and Jersey), it has become crucial to assess the status of tick born diseases (TBDs) in exotic and indigenous breeds of cattle. Exotic breeds are highly vulnerable to TBDs than indigenous breeds. Therefore, the present study was designed to investigate the prevalence and molecular identification of A. marginale in indigenous and exotic cattle breeds of subtropical areas of Khyber Pakhtunkhwa (KP), Pakistan.
Materials and methods
A total of both exotic 120, (Australian Friesian and Jersey) and indigenous (Sahiwal and Achai and cross bred cattle) animals, of both sexes were randomly selected from different commercial cattle dairy farms of KP from May-August 2017. The cattle were of different age group, maintained at loose housing system and were fed on the recommended feed. The study was approved by the ethical committee of the University of Agriculture Peshawar. Approximately 6mL of blood was collected from the jugular vein of each animal in two 6mL tubes one without anticoagulant for ELISA and the other with anticoagulant (Improvacuter®, Belgium) for DNA extraction. Serum was isolated after centrifugation and stored at -20°C. Thin blood smears were prepared with fresh blood in EDTA anticoagulant and Giemsa stained using an AMES HemaTek slide stainer (Bayer, Zurich, Switzerland).
Anaplasmosis indirect ELISA was performed using SVANOVIR A. marginale Ab (Company Boehringer Ingelheim Svanova, Sweden) following the manufacturer’s instruction. The optical density (OD) was measured at 405nm in microplate photometer. The percentage positivity (PP%) for the serum samples was calculated using the following equation:
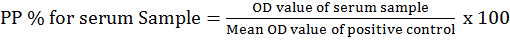
Serum sample showing PP% greater than 25% were considered positive for A. marginale while those less than 25% were considered negative according to the kit protocol of indirect ELISA kit (SVANOVIR).
For PCR amplification of MSP1and MSP4 genes, DNA was extracted from blood samples (200µL) using commercially available kits (Thermo Scientific; Gene JET Whole Blood Genomic DNA Purification Mini Kit k0781, no k0782) according to the manufacturer’s protocol.
The extracted DNA was used for PCR using primers specific for A. marginale MSP1α and MSP4 genes as described by Fuente et al. (2005). PCR reaction was performed in a final volume of 20μL containing 2μL of DNA, 6μL distilled water, 10μL PCR master mix (10xTaq buffer, MgCl2, 0.2Mm dNTPs, 1U Taq polymerase, Loading dye). Blood from cattle shown to be free of A. marginale was used as a negative control to observe contamination. Amplified PCR products were run on 1% agarose gel containing ethidium bromide and visualized by UV transillumination. A 2300bp and 872bp DNA fragments were identified by comparison with DNA molecular weight marker (100plus bp DNA Ladder, Promega) (Supplementary Fig. 1). For phylogenetic analysis, positive samples confirmed by PCR were loaded on gel. The samples were excised, purified and then sent to Macrogen, Inc. South Korea for nucleotide sequencing.
The MSP4 amplified region was sequenced completely while MSP1α amplified region was partially sequenced using big dye method. The sequences obtained were checked with Chromas v.1.45 and compared to sequence data from GenBank1, using the BLAST 2.2.15. program (http://www.ncbi.nlm.nih.gov/BLAST/). The sequences obtained in this study were aligned by Multiple Sequence Alignment using online software multalin (http://multalin.toulouse.inra.fr/multalin/) and a phylogenetic tree was constructed by maximum likelihood method using Mega 6 software.
The data was analyzed statistically by chi-square test using SPSS version 13.0. A P value of less than 0.01 was considered statistically significant. The kappa coefficient was calculated to estimate the degree of agreement between the Indirect ELISA and PCR assay.
Results
Out of 120 blood samples of different breed of cattle, microscopic examination detected 15% samples positive. The proportion of infected erythrocytes in blood smears was low (<4%) (results not presented) and contained 1-3 inclusion bodies in submarginal, marginal, or central position. There were no relevant clinical signs found in majority of the sampled animals. However, some showed nonspecific clinical signs. The prevalence rate was 20.8%, and 29.1% using ELISA and PCR, respectively. Comparing the two techniques in our study, a concordance of 87.6% was found between ELISA and PCR assays. The microscopic examination, although presents a similar result as PCR, yet the chances of sampling error are unavoidable, and thus can provide false negative results.
Table I shows the prevalence of A. marginale in animals with different age group, breed, gender and sampling-months using the total number of samples positive for either test. Statistically significant association was found between level of prevalence of A. marginale and different age groups, sampling-months and breeds (P˂0.01). Contrary, no significant difference was observed between the two genders regarding prevalence rate. Animals older than six years were more susceptible to A. marginale as compared to young animals (1-3 years). Pure and crossbred Holstein Friesian had a prevalence rate of 34% and 28%, respectively, whereas, the Achai and Sahiwal showed the lowest infestation rate of 8% and 6.2%, respectively (Table I). The A. marginale infection was found with an increasing trend from May to August. According to our study, female cattle were found more infected than male cattle with a ratio of 59% and 41%, respectively. The A. marginale MSP1α and MSP4 genes were successfully amplified from bovine samples. Sequence analysis of the PCR products showed that all of them correspond to A. marginale, showing 90-95% identity with the reference sequences of A. marginale found in GenBank. A. phagocytophilum was not noticed in analyzed samples. The phylogenetic analysis in our study was done using two genes of A. marginale i.e. MSP1α gene and MSP4 genes. Similar genes were also used by Fuente et al. (2003) for molecular characterization and phylogenetic studies of A. marginale. The phylogenetic analysis showed that our local isolates have high homology with the isolates of Australia, Turkey, Thailand and Japan with strong bootstrap value (Figs. 2, 3).
Table I. Prevalence of Anaplasma marginale in 120 blood samples of different breeds of cattle maintained at various commercial farms in subtropical area of Khyber Pakhtunkhwa.
|
Variables |
Categories |
Prevalence (%) |
P values |
|
Age |
Calf |
20 |
0.002 |
|
Heifer |
17 |
||
|
Adults |
63 |
||
|
Sex |
Male |
41 |
0.06 |
|
Female |
59 |
||
|
Breed |
Sahiwal |
6.2 |
0.001 |
|
Achai |
8 |
||
|
HF |
34 |
||
|
HFX |
28 |
||
|
Jersey |
23.8 |
||
|
Summer season |
May |
15.4 |
0.002 |
|
June |
23.1 |
||
|
July |
26.9 |
||
|
August |
34.6 |
HF, Holstein Friesian; HFX, Holstein Friesian Cross.
Discussion
Prevalence of A. marginale was investigated using microscopic examination, indirect ELISA and further confirmation of antigen through PCR in cattle maintained at commercial dairy farms at KP. According to ELISA, the highest prevalence was recorded in aged animals followed by heifers and calves (Table I). Anaplasmosis is -more prevalent in aged cattle, younger animals might remain liable but show little visible signs (Singh et al., 2003). We found inclusion bodies characteristic of A. marginale in the RBCs of the majority of the diseased cows. In aged animals, immune system becomes weaker thus are more susceptible to the disease. Calves (3-6 months) on the other hand were less susceptible for anaplasmosis as they receive maternal antibodies and the resistance gradually wanes after one year of age and vulnerability to different infection increases (Kocan et al., 2000).
The highest prevalence (34.66%) of A. marginale was found in August. The hot (~ 44˚C) and humid environment of August favors the rapid multiplication of ticks. In closed housing system, higher humidity provides favorable environment for ovipositioning in the crevices and cracks in the floor, walls and roof of house (Muhammad et al., 2008). In the present study, highest prevalence of A. marginale was seen in exotic and cross bred cattle as compared to indigenous cattle breeds. The natural selection over the millennia made the indigenous cattle more resistant to the prevailing pathogen. This is manifested by lower incidence rate of ticks’ infestation in local cattle (Swai et al., 2007). Sing et al. (2003) found 12.5% and 51% samples positive for A. marginale using microscopic examination and nested PCR technique, respectively. His results showed that nested PCR ideally suited for diagnosis of carrier animals. Our results also showed that PCR is more sensitive technique for detecting A. marginale in susceptible and carrier animals. In Pakistan, the prevalence of A. marginale has been recorded as 7.36-75.71% using microscopic examination of blood smears (Khan et al., 2004; Rajput et al., 2005).
Sex influenced the extent and severity of the infection (Khan et al., 2004). Our findings showed higher susceptibility of female cattle compared to the male cattle. Higher prevalence of A. marginale in female population is due to the higher productivity, climatic and metabolic stress the females are facing which posedthem to a weakened immune system. Findings of the current study are in close agreement to those reported by Rajput et al. (2005). In the present study, two major surface protein genes of A. marginale i.e. MSP1α and MSP4 were identified as markers for PCR examination.
The sequence alignment and the phylogenetic analysis revealed that the two genes of A. marginale, MSP1α and MSP4 can be used as useful markers in evolutionary studies. The MSP4 and MSP2 genes were used previously as markers for the genetic characterization of A. marginale strains (Fuente et al., 2007). The high prevalence rate of A. marginale was due to poor management and unhygienic measure found in the present study. These results are in line with those suggested by Swai et al. (2005).
Currently, a number of different methods, including chemotherapy as well as prophylaxis, vaccination and chemical tick control are used worldwide to decrease economic damages resulting from TBDs (Muhammad et al., 2008). Owing to partial understanding of bovine TBDs, currently, no program for the chemoprophylaxis of these is in place in Pakistan. Grooming is the most commonly used strategy for tick control mostly at small-holding farms. Grooming includes the manual removal of ticks and burning them on the fire made with cattle dung cakes in Pakistan (Mondal et al., 2013). The present study showed that the indigenous breeds of cattle are well adapted and are resistant to a higher level towards the TBDs. Thus a wise strategy needs to be developed both at public and private farms for ticks control to avoid the financial losses caused by the TBDs in high yielding exotic dairy cattle especially in the hot and humid months of summer.
Conclusions
The current epidemiological study showed that the prevalence rate of A. marginale was approximately 29.1% in dairy cattle at commercial dairy farms maintained under subtropical environment of KP province of Pakistan. The molecular and serological techniques confirmed that exotic and crossbred cattle were highly susceptible to anaplasmosis compare to the indigenous cattle breeds.
Supplementary material
There is supplementary material associated with this article. Access the material online at: https://dx.doi.org/10.17582/journal.pjz/20180510110525
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abbas, R.Z., Zaman, M.A., Colwell, D.D., Gilleard, J. and Iqbal, Z., 2014. J. Vet. Parasitol., 1: 6-20. https://doi.org/10.1016/j.vetpar.2014.03.006
Bowie, M.V., Fuente, J.D.L., Katherine, M.K., Blouin, E.F. and Barbet, A.F., 2002. Gene, 282: 95-102. https://doi.org/10.1016/S0378-1119(01)00845-9
Dumler, J.S., Barbet, A.F., Bekker, C.P., Dasch, G.A., Palmer, G.H., Ray, S.C., Rikihisa Y. and Rurangirwa, F.R., 2001. Int. J. Syst. Evol. Microbiol., 51: 2145-2165.
Fuente, J.D.L., Ruybal, P., Mtshali, M.S., Naranjo, V., Shuqing, L., Mangold, A.J., Rodrı´guez, S.D., Jime´nez, R., Vicente, J., Moretta, R., Torina, A., Almaza´n, C., Mbati, P.M., Torioni de Echaide, S., Farber, M., Rosario-Cruz, R., Gortazar, C. and Kocan, K.M., 2007. Vet. Microbiol., 119: 382–390. https://doi.org/10.1016/j.vetmic.2006.09.015
Fuente, J.D.L., Alessandra, T., Sant, C., Giovanni, T., Roberto, F., Consuelo, A. and Katherine, M.K., 2005. Vet. Parasitol., 133: 357–362.
Fuente, J.D.L., Elizabeth, J., Golsteyn, T., Ronald, A., Van Den, B., Robert, G.H., Elaine, E.T., Susan, E.D. and Katherine, M.K., 2003. Appl. environ. Microbiol., 69: 5001–5005.
Jabbar, A., Abbas, T., Sandhu, Z.D., Saddiqi, H.A., Qamar, M.F. and Gasser, R.B., 2015. Parasites Vector, 8: 283. https://doi.org/10.1186/s13071-015-0894-2
Kahn, C.M., 2005. The merck veterinary manual. White House Station, N.J., USA. pp. 18-20.
Khan, M.Q., Zahoor, A., Jahangir, M. and Mirza, M.A., 2004. Pak. Vet. J., 24: 193-195.
Kocan, K.M., Blouin, E.F. and Barbet, A.F., 2000. Annls N. Y. Acad. Sci., 916: 501–509.
Mondal, D.B., Sarma, K. and Saravanan, M., 2013. Ticks Tick Borne Dis., 4: 1-10. https://doi.org/10.1016/j.ttbdis.2012.05.006
Muhammad, G., Naureen, A., Firyal, S. and Saqib, M., 2008. Pak. Vet. J., 28: 43-50.
Rajput, Z.I., Hu, S.H., Arijo, A.G., Habib, M. and Khalid, M.J., 2005. J. Zhejiang Univ. Sci., 6: 1057-1062. https://doi.org/10.1631/jzus.2005.B1057
Singh, J., Tuli, A. and Singla, L.D., 2003. Punjab Vet. J., 3: 73-74.
Swai E., Karimuribo E., Ogden N., French N., Fitzpatrick J., Bryant M. and Kambarage D., 2005. Trop. Anim. Hlth. Prod., 37: 599- 610.
Swai, E.S., Esrony, D.K., Kambarage, D.M., Moshy, W.E. and Mbise, A.N., 2007. Vet. J., 174: 390–396. https://doi.org/10.1016/j.tvjl.2006.08.004
To share on other social networks, click on any share button. What are these?










